Key Points
SES was associated with transplant outcomes after diagnosis of aGVHD and cGVHD.
In patients with aGVHD, non-Hispanic Black race was associated with increased TRM and overall mortality.
Visual Abstract
Socioeconomic status (SES) and race/ethnicity have been associated with the outcomes of allogeneic hematopoietic stem cell transplantation (allo-HCT). Certain aspects of graft-versus-host disease (GVHD) management, such as the need for long-term care, prolonged immunosuppressive treatment, and close follow-up for complications, may exacerbate disparities. Adults (≥18 years) reported to the Center for International Blood and Marrow Transplant Research who underwent a first allo-HCT for acute leukemia, myelodysplastic syndrome, or myeloproliferative neoplasm between 2008 and 2018 were included. End points for those developing GVHD included overall survival (OS), transplant-related mortality (TRM), and disease relapse. Models were adjusted for patient- and transplant-related variables. A 2-sided P value < .01 was considered significant. Among the 14 825 allo-HCT recipients, 6259 (42.2%) and 6675 (45.0%) patients developed acute GVHD (aGVHD) and chronic GVHD (cGVHD), respectively. Among patients with aGVHD, non-Hispanic Black patients had increased TRM and overall mortality compared with non-Hispanic White patients; this association disappeared when severity of aGVHD was included in the model. Lower SES was associated with increased risk of disease relapse but not OS or TRM. In patients who developed cGVHD, race and ethnicity were not associated with OS, TRM, or disease relapse. However, the highest quartile of annual household income (≥$80 000) had improved OS and reduced TRM compared with the lowest quartile, after adjusting for race and ethnicity. In summary, race/ethnicity and SES are associated with outcomes after GVHD. Optimizing the health care resources available to low SES patients and strategies to minimize the risk of severe GVHD in non-Hispanic Black patients may improve long-term outcomes.
Introduction
There has been an increase in the number of allogeneic hematopoietic stem cell transplants (allo-HCT) and improvement in post-HCT outcomes for most hematologic diseases over the past 2 decades. Despite this success, racial and ethnic disparities in access and outcomes of HCT have been well documented, and efforts are underway to mitigate these disparities.1-3
An earlier Center for International Blood and Marrow Transplant Research (CIBMTR) study investigating the association of race/ethnicity and socioeconomic status (SES) with HCT outcomes among 6207 matched unrelated donor HCT recipients between 1995 and 2004 revealed worse overall survival (OS) and higher transplant-related mortality (TRM) in Black patients.4 Moreover, across all racial groups, patients with lower SES had inferior outcomes. However, in that study, the inferior outcomes among Black patients were not fully explained by SES. Another CIBMTR study evaluated transplant outcomes among ethnic minorities receiving a single umbilical cord blood transplantation between 1995 and 2006, and found inferior OS of Black patients compared with White patients. The higher mortality among the Black patients was attributed to greater human leukocyte antigen (HLA) disparity and suboptimal cell doses in the donor grafts. In contrast to the findings of the above 2 studies, a recent CIBMTR study of 93 790 allo-HCT recipients from 2009 to 2020 revealed an increase in the relative proportions of racial and ethnically diverse patients undergoing allo-HCT over the last decade, largely through the use of alternative donor platforms.5 In this study, similar survival rates were observed among racial and ethnic groups, although graft-versus-host disease (GVHD) was a more common cause of death for Black patients than for other races following matched unrelated transplantation.
It remains unknown whether racial, ethnic, and SES disparities in outcomes still persist or may even be exacerbated in allo-HCT recipients with GVHD. Certain aspects of GVHD management, such as the need for long-term care, prolonged immunosuppressive treatment, and close follow-up for complications, may exacerbate disparities. Given that racial and ethnic minority groups have a greater proportion of people with lower SES, complications leading to poor HCT outcomes after diagnosis of GVHD may also be accentuated. To address these gaps in knowledge, our study investigated racial/ethnic and SES outcome disparities in HCT recipients with acute GVHD (aGVHD) and chronic GVHD (cGVHD). This study sought to (1) determine the association of ethnicity/race and SES with the cumulative incidence of aGVHD and cGVHD, and (2) investigate whether ethnicity/race and SES are associated with important transplant outcomes after the diagnosis of aGVHD and cGVHD.
Methods
Data source
The CIBMTR is a research collaboration between the National Marrow Donor Program/Be The Match and the Medical College of Wisconsin. CIBMTR includes ∼420 centers worldwide that contribute detailed data on HCT and cellular therapies. Studies conducted by the CIBMTR follow all applicable US federal regulations pertaining to the protection of human research participants. The CIBMTR collects data before transplantation and at 100 days and 6 months after transplantation, annually until posttransplantation year 6 and biannually thereafter until death. Data quality is monitored by computerized error checks, physician data review, and on-site audits.
Study population
The study population included adults (aged ≥18 years at transplantation) who underwent first allo-HCT in the United States for treatment of acute myeloid leukemia, acute lymphoblastic leukemia, myelodysplastic syndrome, or myeloproliferative neoplasm between 2008 and 2018. All graft sources, donor sources, and conditioning regimens were included. Because of the small numbers, American Indian/Alaskan Native, Hawaiian/Pacific Islander, and mixed-race patients, and those who received grafts from multiple sources were excluded. Although the entire study population was analyzed to determine the cumulative incidence of aGVHD or cGVHD, the main analyses focused on the separate populations who developed either aGVHD or cGVHD to explore transplant outcomes specifically for these cohorts.
Statistical analysis
Patients were classified as non-Hispanic White, non-Hispanic Black, Hispanic, and Asian. Median household income by ZIP code was used to assess SES, and patients were categorized into the following 4 quartiles: <$48 000, $48 000 to $60 999, $61 000 to $79 999, and >$80 000. Patient-, disease-, and transplant-related factors were compared among the 4 groups using the χ2 test for categorical variables and the Mann-Whitney U test for continuous variables.
The primary objective of this study was to evaluate the association of race/ethnicity (non-Hispanic Black, Hispanic, or Asian vs non-Hispanic White [reference cohort]) and SES with OS, relapse, and TRM after diagnosis of aGVHD or cGVHD. aGVHD was graded using the Glucksberg grading system (grade I-IV, based on organ involvement and organ stage).6 The grading of cGVHD (limited or extensive) was based on the Seattle criteria7 because the National Institutes of Health consensus criteria for cGVHD was implemented on CIBMTR forms after 2017. The CIBMTR clinical definition for cGVHD severity (mild, moderate, severe) was also used for this analysis.8 Therefore, cGVHD severity reflects the maximum severity documented over longitudinal follow-up as determined by best clinical judgment. OS was defined as time from HCT to death from any cause. TRM was defined as time from HCT to death without evidence of relapse or progression of primary disease. Relapse was defined as time to recurrence of malignancy with TRM as a competing event. Patients were censored at the date of last follow-up for all outcomes defined above. Probability of OS was calculated using the Kaplan-Meier estimator.
Cox proportional hazards multivariable models (MVA) were used to assess the association between the variables of interest and outcomes. All the clinical variables were tested for the affirmation of the proportional hazards assumption using a time-dependent covariate approach. Factors violating the proportional hazards assumption were adjusted through stratification. A stepwise backward variable selection procedure was used in developing models for each outcome with a threshold of 0.05 for retention in the model. The main variables of interest (race/ethnicity and SES) were then forced into the models. For cGVHD MVA, aGVHD and severity of cGVHD were included as covariates. Interactions between race/ethnicity and SES and the adjusted covariates were tested at the significance level of .01. No significant interactions between the main variables and the adjusted covariates were detected in any of the models. All models were adjusted for center effects. To adjust for multiple testing, a significance level of .01 was used to determine statistical association of the main variables with outcomes. All statistical analyses were performed using SAS 9.4 software.
Results
A total of 14 825 patients who received first allo-HCT for acute myeloid leukemia, acute lymphoblastic leukemia, myelodysplastic syndrome, or myeloproliferative neoplasm between 2008 and 2018 in the United States were included in the analysis. Demographics and transplant characteristics of the entire population are shown in supplemental Table 1. Among the 14 825 patients, 6259 and 6675 patients developed aGVHD and cGVHD, respectively. In the univariable analysis, there was no significant difference in the cumulative incidence of grade 2 to 4 aGVHD at 100 days after transplantation based on race/ethnicity (P = .173) and SES (P = .415). However, the cumulative incidence of severe aGVHD (grade 3-4) was higher among non-Hispanic Black patients than among other racial/ethnic cohorts (P = .015; supplemental Table 2A-B).
In the univariable analysis, there was no significant difference in the cumulative incidence of cGVHD at 1, 3, and 5 years after transplantation based on race/ethnicity (P = .489) and SES (P = .441; supplemental Table 3A-B).
aGVHD results
Characteristics of patients who developed aGVHD
Among the 6259 patients with aGVHD, 40.5% (n = 2535) had severe (grade 3-4) aGVHD. Baseline characteristics of the study population with the diagnosis of aGVHD are listed in Table 1. Patients were reported to be non-Hispanic White (n = 5015), non-Hispanic Black (n = 403), Hispanic (n = 564), and Asian (n = 277). Non-Hispanic White patients were more likely to be older compared with the other racial/ethnic groups. A higher proportion of the non-Hispanic Black and Hispanic cohorts received alternative graft sources compared with non-Hispanic White cohort. There were notable differences in baseline SES characteristics, with the non-Hispanic Black and Hispanic cohorts having a higher proportion of median income <$48 000 compared with the non-Hispanic White cohort. Moreover, non-Hispanic Black and Hispanic patients were more likely to have Medicaid and have lower educational status compared with non-Hispanic White and Asian patients. In terms of severity of aGVHD, a higher proportion of the non-Hispanic Black cohort had grade 3 or 4 aGVHD (51.1%) compared with non-Hispanic White (36.0%), Hispanic (38.2%), and Asian cohorts (37.8%).
OS after diagnosis of aGVHD
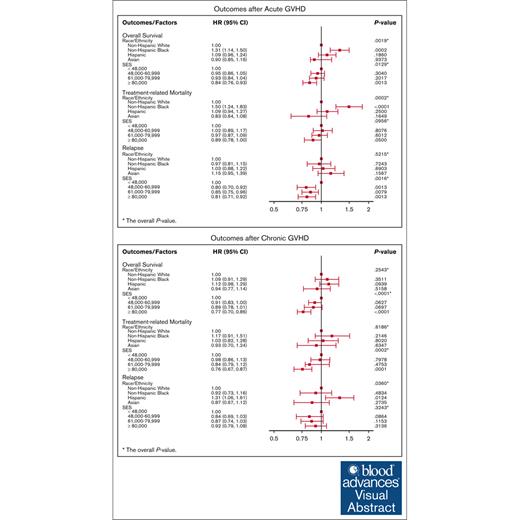
In an MVA adjusting for patient-, disease-, and transplant-related characteristics, race/ethnicity was significantly associated with OS (P = .0019; Table 2). Specifically, non-Hispanic Black race was associated with increased overall mortality compared with the non-Hispanic White cohort (hazard ratio [HR], 1.31; 95% confidence interval [CI], 1.14-1.50; P = .0002). However, after accounting for aGVHD severity as a covariate in the MVA, race/ethnicity was no longer significantly associated with OS (P = .1131; Table 3), suggesting that aGVHD severity partially explains the association of race with OS. When evaluating SES independent of race and ethnicity, there was a trend toward an association between SES and OS, although this did not reach statistical significance (P = .0129).
TRM after diagnosis of aGVHD
In an MVA including both SES and race/ethnicity in the model, race/ethnicity was significantly associated with TRM (P = .0002; Table 2). The non-Hispanic Black cohort had significantly higher TRM compared with the non-Hispanic White cohort (HR, 1.5; 95% CI, 1.24-1.83; P = .0001). However, after including aGVHD severity in the model as a covariate, race/ethnicity was no longer significantly associated with TRM (P = .0392; Table 3), suggesting that aGVHD severity partially explains the association of race with TRM. SES was not associated with TRM (P = .0956) irrespective of aGVHD severity. Other factors significantly associated with TRM are listed in Table 3.
Relapse after diagnosis of aGVHD
In an MVA adjusting for patient-, disease-, and transplant-related characteristics that included both race/ethnicity and SES, SES was significantly associated with risk of relapse (P = .0016; Table 2). Median household incomes of $48 000 to $60 999 (HR, 0.80; 95% CI, 0.70-0.92; P = .0013), $61 000 to $79 999 (HR, 0.85; 95% CI, 0.75-0.96; P = .0079), and >$80 000 (HR, 0.81; 95% CI, 0.71-0.92; P = .0013) were significantly associated with lower risk of relapse compared with the lowest household income quartile (<$48 000), even after including aGVHD grade as a covariate in the MVA (P = .0013; Table 3).
cGVHD results
Characteristics of patients who developed cGVHD
Baseline characteristics of the study population with a diagnosis of cGVHD are described in Table 4. Patients were reported to be non-Hispanic White (n = 5360), non-Hispanic Black (n = 420), Hispanic (n = 590), and Asian (n = 305). There were significant differences in median household income by race, with a greater proportion of non-Hispanic Black (44%) and Hispanic (37%) recipients with median annual household income of <$48 000 compared with non-Hispanic White (21%) and Asian (13%) recipients. Non-Hispanic Black (21%) and Hispanic patients (32%) were more likely to be on Medicaid compared with non-Hispanic White (9%) and Asian patients (17%). Non-Hispanic White patients were also more likely to be married and have a higher education status. There were also significant differences in the 4 race/ethnicity cohorts by donor type and GVHD prophylaxis. A greater proportion of non-Hispanic Black and Hispanic patients received haploidentical transplantation and posttransplant cyclophosphamide as GVHD prophylaxis compared with non-Hispanic White and Asian patients. In terms of severity of cGVHD, a higher proportion of the non-Hispanic Black (62%) and non-Hispanic White (64%) cohorts had moderate to severe cGVHD compared with Hispanic (54%) and Asian (55%) cohorts.
OS after diagnosis of cGVHD
In the MVA, after adjusting for patient-, disease-, and transplant-related characteristics, there was no significant difference in OS (P = .2543) among the 4 race/ethnicity groups in patients with cGVHD (Table 5). In contrast, SES was significantly associated with OS (P < .0001). Specifically, the highest quartile of household income (≥$80 000) was associated with improved OS (HR, 0.77; 95% CI 0.69-0.85; P < .0001) compared with the lowest household income quartile (<$48 000).
TRM after diagnosis of cGVHD
In the MVA, after adjusting for patient-, disease-, and transplant-related characteristics, there was no significant difference in TRM (P = .6186) among the 4 race/ethnicity groups in patients with cGVHD. SES was significantly associated with TRM (P = .0002; Table 5). Specifically, the highest quartile of household income (≥$80 000) was associated with lower TRM (HR, 0.86; 95% CI, 0.67-0.87; P < .0001) compared with the lowest household income quartile (<$48 000). SES remained significantly associated with TRM after accounting for cGVHD severity, education, and insurance status as covariates in the MVA (data not shown), suggesting that these factors do not explain the SES association.
Relapse after diagnosis of cGVHD
According to the MVA, neither race/ethnicity (P = .0360) nor SES (P = .3243) were significantly associated with disease relapse (Table 5). Similar results were observed after including cGVHD severity as a covariate in the MVA.
Discussion
The effect of SES and race/ethnicity on HCT outcomes has been the subject of evaluation over the past few decades. However, to our knowledge, this is the first study aiming to explore SES and race/ethnicity influence on outcomes after diagnosis of aGVHD and cGVHD using contemporary data. Our study highlights the strong association of SES, independent of race/ethnicity, with transplant outcomes after diagnosis of acute and chronic GVHD. Low SES was significantly associated with increased risk of relapse after diagnosis of acute GVHD and increased TRM and reduced OS after diagnosis of cGVHD. The association of SES with TRM and OS in patients with cGVHD was significant even after adjusting for other potential factors such as insurance status and educational level. In this study, we did not observe a statistically significant association of SES with OS or TRM in the aGVHD population. Acute GVHD mostly occurs within the first 100 days of transplantation. During this time, patients are residing near and receive well-coordinated care through the transplantation center, and have access to the transplant center’s assistance in financial and living resources, which may mitigate the impact of SES on outcomes during the acute phase of HCT. Conversely, cGVHD mostly occurs beyond 100 days after HCT, during the period when patients are frequently discharged back to their local physicians, especially if they reside a considerable distance from the transplant center, and are subsequently comanaged with varying levels of involvement by the transplant center. Similar coordinated resources may not be available. Declines in quality of life and functional status and chronic health conditions observed in patients with cGVHD demand intensive and prolonged health care utilization and closer follow-up.9 Therefore, cGVHD patients with lower SES and potentially poorer access to health care and resources are likely to be at higher risk for longer-term complications, which may lead to worse outcomes for those who have fewer resources to address them.10
In contrast to our results, a recent multicenter observational study by the Chronic GVHD Consortium evaluating the association between SES and HCT outcomes in 421 adults demonstrated a lack of correlation between SES and mortality in the cGVHD cohort, despite a higher symptom burden with low SES.11 There are several differences between the 2 studies that may account for the discrepancies in results. The Chronic GVHD Consortium used patient-reported income and other sociodemographic factors rather than ZIP code of residence as an indicator of SES. Moreover, the sample size was smaller and consisted of more socioeconomically advantaged individuals (51% with household income >$75 000 vs only ∼25% with household income of ≥$80 000 in our study); such differences may explain the different conclusions.
Although health disparities among patients with varying SES are in part attributable to differences in health behaviors, social determinants of health may also have biological ramifications. Social environment affects the stress-related gene expression profile known as the conserved transcriptional response to adversity, which involves upregulation of proinflammatory genes and downregulation of genes involved in type I interferon response and antibody synthesis.12,13 Low SES and extended exposure to life adversity, leading to upregulation of conserved transcriptional response to adversity gene expression, has been associated with adverse outcomes after unrelated HCT, including increased risk of disease relapse. This phenomenon may explain our finding of an association between relapse and lower SES.
Our study also demonstrated a significant association of non-Hispanic Black race with worse posttransplant outcomes after the diagnosis of aGVHD, despite adjustment for SES and other transplant characteristics. Worse OS and higher TRM observed in the non-Hispanic Black population could be explained by a higher incidence of severe aGVHD compared with other racial/ethnic groups given that the associations were attenuated after adjusting for aGVHD severity. Although controversial, the association of race and ethnicity with the incidence and severity of GVHD has been reported in prior studies.14,15 Mielcarek et al reported a higher risk of severe aGVHD among Black patients after unrelated donor HCT, associated with higher overall mortality compared with White patients.16 Greater genetic disparity at both the HLA locus and at minor transplantation antigens17 and frequencies of cytokine polymorphisms18 among non-Hispanic Black populations compared with other races and ethnicities may contribute to the greater severity of aGVHD.19,20 Several prior studies have shown differences in response to medications including chemotherapeutic agents and immunosuppressants between and within racial/ethnic groups due to frequency of polymorphisms in genes encoding drug-metabolizing enzymes.21,22 Unfortunately, racial/ethnic minorities have historically been underrepresented in clinical trials. Lack of diversity in clinical trials is an obstacle to understanding the safety and efficacy of novel therapies for prophylaxis and treatment of GVHD and compromises the generalizability of clinical research findings.23
Despite the association of Black race with more severe aGVHD, we did not observe an association between race/ethnicity and transplant outcomes after diagnosis of cGVHD. One possible explanation is the poor overall long-term survival rate of 10% to 25% in patients with severe aGVHD, observed more frequently in the non-Hispanic Black than in the non-Hispanic White cohort in our study. Although posttransplant cyclophosphamide-based GVHD prophylaxis has overcome many of the barriers associated with HLA mismatches and reduced the incidence of moderate to severe cGVHD,24,25 it is unlikely to have affected the results of our study, which only included patients up until the end of 2018.
We acknowledge several limitations of this analysis, including the retrospective design and degree of precision available for some variables of interest. The ZIP code of residence was used as a surrogate for SES rather than individual patient-reported income26 or other validated definitions such as neighborhood deprivation indices that encompass other measures of socioeconomic disadvantage, including income, education, employment, and housing quality27 because we lacked patient-level data on these factors. Moreover, distance to HCT center was not included in the analysis; although, the adverse impact of rural residence on outcomes has been shown to disappear when adjusted for SES parameters such as income and education level.28,29 Another limitation was the lack of data about longitudinal change in SES, which may influence transplant outcomes over time. Furthermore, we used race/ethnicity as reported by centers in this study, which is not as accurate as self-report or genetic assessment. In admixed populations such as the Hispanic population (admixed with European, African, and Native American ancestry), self-reported race/ethnicity may not accurately represent the population genetically.30 Moreover, further studies are needed to evaluate the impact of newer GVHD prevention strategies and therapeutic agents on closing the gaps in access and outcomes related to race and ethnicity.
In summary, this analysis shows worse outcomes with lower SES and non-Hispanic Black race among patients with GVHD. Although many studies have reported worse outcomes based on these factors in the general post-HCT population, our findings of worse OS in non-Hispanic Black patients with aGVHD and higher relapse with lower SES were not reported in prior CIBMTR analyses. Efforts to decrease severe aGVHD in the non-Hispanic Black population and to better support patients with lower SES who develop GVHD may improve outcomes.
Acknowledgments
The Center for International Blood and Marrow Transplant Research is primarily supported by the Public Health Service U24CA076518 from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases; 75R60222C00011 from the Health Resources and Services Administration; and N00014-23-1-2057 and N00014-24-1-2057 from the Office of Naval Research. Support is also provided by the Medical College of Wisconsin, National Marrow Donor Program, Gateway for Cancer Research, Pediatric Transplantation and Cellular Therapy Consortium, and the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc; Adaptive Biotechnologies Corporation; ADC Therapeutics; ADIENNE SA; Alexion; Allogene; AlloVir, Inc; Amgen, Inc; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics; BeiGene; BioLineRX; Blue Spark Technologies; bluebird bio, Inc; Blueprint Medicines; Bristol Myers Squibb Co; CareDx Inc; CSL Behring; CytoSen Therapeutics, Inc; DKMS; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida Cell, Ltd; Gift of Life Biologics; Gift of Life Marrow Registry; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc; Karius; Kashi Clinical Laboratories; Kiadis Pharma; Kite, a Gilead company; Kyowa Kirin; Labcorp; Legend Biotech; Mallinckrodt Pharmaceuticals; Med Learning Group; Merck & Co; Mesoblast; Millennium, the Takeda Oncology Co; Miller Pharmacal Group, Inc; Miltenyi Biotec, Inc; MorphoSys; MSA-EDITLife; Neovii Pharmaceuticals AG; Novartis Pharmaceuticals Corporation; Omeros Corporation; OptumHealth; Orca Biosystems, Inc; OriGen BioMedical; Ossium Health, Inc; Pfizer, Inc; Pharmacyclics, LLC, an AbbVie company; PPD Development, LP; REGiMMUNE; Registry Partners; Rigel Pharmaceuticals; Sanofi; Sarah Cannon; Seagen Inc; Sobi, Inc; STEMCELL Technologies; Stemline Therapeutics; STEMSOFT; Takeda Pharmaceuticals; Talaris Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc; WellSky; and Xenikos BV.
Authorship
Contribution: N.F., N.R., and S.J.L. designed the study, analyzed data, interpreted analyses, and wrote the manuscript; K.C., J.D., and T.W. performed the statistical analysis; and K.B., A.B., V.R.B., B.K.H., P.H., S.M.G., S.R. Solomon, N.E.J., C.J.L., M.L.M., H.G.R., H.S., A.S., S.R. Spellman, and J.R.W. critically reviewed and revised the design, interpreted analyses, reviewed the manuscript, and approved the final version.
Conflict-of-interest disclosure: N.F. has been an advisory board member for Incyte; received speaker fees from Incyte; is on the data safety monitoring committee for Chronic Graft-versus-Host Disease Consortium; and is the medical monitor for the Blood and Marrow Transplant Clinical Trials Network. K.B. reports that her institution receives research funding from Stemline Therapeutics. V.R.B. reports participating in the safety monitoring committee for Protagonist; serving as an Associate Editor for the Current Problems in Cancer Journal; is a contributor for BMJ Best Practice; received consulting fees from Taiho, Sanofi, Imugene, Genentech, Incyte, Servier Pharmaceuticals LLC, and AbbVie; reports research funding (institutional) from MEI Pharma, Actinium Pharmaceutical, Sanofi US Services, AbbVie, Pfizer, Incyte, Jazz, and the National Marrow Donor Program; and drug support (institutional) from Chimerix for a trial. B.K.H. has served on advisory committees or as a consultant for Nkarta, Sanofi, Incyte, and Equillium; is a part of the data and safety monitoring board for Angiocrine; and serves on the adjudication committee for CSL Behring. A.S. reports receiving consulting fees from Spotlight Therapeutics, Medexus Pharmaceuticals, Inc, Vertex Pharmaceuticals, Sangamo Therapeutics, and Editas Medicine; serving as a medical monitor for the Resource for Clinical Investigations in Blood and Marrow Transplantation CSIDE clinical trial, for which he receives financial compensation; received research funding from CRISPR Therapeutics; and honoraria from Vindico Medical Education. A.S. is the St. Jude Children’s Research Hospital site principal investigator of clinical trials for genome editing of sickle cell disease sponsored by Vertex Pharmaceuticals/CRISPR Therapeutics (NCT03745287), Novartis Pharmaceuticals (NCT04443907), and Beam Therapeutics (NCT05456880). The industry sponsors provide funding for the clinical trial, which includes salary support paid to A.S.’s institution. A.S. has no direct financial interest in these therapies. J.R.W. reports consulting fees from Ansun, Cidara, Celgene, F2G, Takeda, and Orca. C.J.L. reports membership on the advisory boards of Sanofi, Kite, Kadmon, Bristol Myers Squibb, and Incyte; research funding from Incyte; steering board committee membership for Incyte; speaker’s panel participation for Kite; and consulting for Fresenius Kabi, Sanofi, and Mallinckrodt. H.G.R. reports being a medical monitor for CD33 chimeric antigen receptor T cell (National Marrow Donor Program) (uncompensated, ie, honorary). H.S. reports having received personal fees from Incyte, Janssen, Novartis, Sanofi, and the Belgian Hematology Society (BHS); research grants from Novartis and the BHS, all paid to her institution and not directly related to this work; and nonfinancial support (travel grants) from Gilead, Pfizer, the European Society for Blood and Marrow Transplantation, and the Center for International Bone Marrow Transplantation Research. S.J.L. reports receiving consulting fees from Mallinckrodt, Equillium, Kadmon, Novartis, Sanofi, and Incyte; research funding from AstraZeneca, Pfizer, Sanofi, and Syndax; drug supply from Janssen; being on clinical trial steering committees for Incyte and Sanofi; and serving on the board of directors of the National Marrow Donor Program (uncompensated). The remaining authors declare no competing financial interests.
Correspondence: Nosha Farhadfar, Sarah Cannon Transplant & Cellular Program at Methodist Hospital, San Antonio, TX 78229; email: nosha.farhadfar@hcahealthcare.com.
References
Author notes
The Center for International Blood and Marrow Transplant Research (CIBMTR) supports accessibility of research in accordance with the National Institutes of Health Data Sharing Policy and the National Cancer Institute Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases deidentified data sets that comply with all relevant global regulations regarding privacy and confidentiality.
The full-text version of this article contains a data supplement.