In this issue of Blood Advances, Farhadfar et al1 reported the impact of racial, ethnic, and socioeconomic diversity on outcomes of allogeneic hematopoietic cell transplant (allo-HCT) recipients who develop graft-versus-host disease (GVHD).
The number of recipients receiving allo-HCT has steadily increased over the past 2 decades.2 This increase can be attributed to many factors, including better supportive care, advent of reduced intensity conditioning, and better strategies for GVHD prevention. Posttransplant cyclophosphamide (PTCy) as a GVHD prevention strategy has not only improved outcomes of allo-HCT,3 but its role in haploidentical and mismatch unrelated donor transplants has increased access to allo-HCT, especially in ethnic variant patients with less likelihood of finding an HLA-matched donor.4,5
Cancer outcomes are adversely affected by race, ethnicity, and socioeconomic status (SES), and this is no different in allo-HCT.6 A recent Center for International Blood and Marrow Transplant Research study revealed an improvement in access to allo-HCT and posttransplant outcomes among ethnic minorities, largely through the use of alternative donor platforms incorporating PTCy. However, Farhadfar et al posed an important question on whether outcomes of allo-HCT after diagnosis of GVHD are affected by race, ethnicity, and SES. Over a 10-year period from 2008 to 2018, they found nearly half of the 14 825 allo-HCT recipients developed acute GVHD (n = 6259) and chronic GVHD (n = 6675), with underrepresented minorities (Hispanic, non-Hispanic Black, and Asian) comprising 19.9% and 19.7% of acute and chronic GVHD cases, respectively. They found lower SES was predictive of worse overall survival (OS) and increased treatment-related mortality in patients with chronic GVHD. Importantly, they found non-Hispanic Black patients had a higher reported rate of severe acute GVHD. In those with acute GVHD, non-Hispanic Black patients had worse OS than non-Hispanic White patients; although when acute GVHD was used as a covariate in multivariate analysis, race and ethnicity were no longer significantly associated with OS. This suggested that acute GVHD severity accounts for the association of race and OS.
The next logical question to ask is why these factors influence allo-HCT outcomes after diagnosis of GVHD. Several factors can be hypothesized, including biologic, socioeconomic, or some combination of both. Biologically, how much is influenced by genetic disparity at the HLA locus and minor transplantation antigens or by drug-metabolizing enzymes conferring less effective response to GVHD therapies? Does SES impact manifest in access to transplant centers for GVHD care or in affordability of effective therapies for GVHD? These represent a few questions raised based on the findings of this study. Are there factors that intersect both biologic and SES factors, such as conserved transcriptional response to adversity gene expression profile,7 or other factors yet undiscovered that affect outcomes of GVHD?
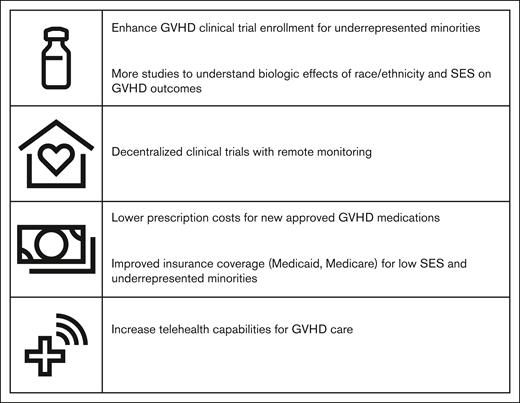
It is important to note PTCy as a GVHD prophylaxis was only accounted for 15% of patients reported in the study of Farhadfar et al, which analyzed patients with GVHD until 2018. This should not detract from the fact that this study has identified a vulnerable population within those with GVHD. There exists a need to improve outcomes for this population and proposed solutions will need to encompass both biologic and socioeconomic factors (see figure).
Understanding race and ethnicity impact in GVHD can be achieved through clinical trial participation, whether through biomarker sampling or genomic or pharmacokinetic studies. However, minorities remain underrepresented in GVHD clinical trials. Of the new US Food and Drug Administration (FDA)-approved therapies in both acute and chronic GVHD, minority enrollment, when reported, only accounted for 1% to 7% of the total trial population.8-12
Additionally, telehealth has become an established practice after the COVID-19 pandemic and is becoming implemented in GVHD and HCT survivorship programs.13,14 Telehealth allows for easier access for patients, especially those from lower SES, who otherwise were hindered by geographical distance to transplant center and associated travel costs. Enhancing telehealth capabilities and promoting decentralized clinical trials with remote monitoring can help in giving patients better access to GVHD care and clinical trials, without hinderance from travel costs and time away from work.
Finally, improvement in costs and access to newer GVHD therapies, particularly for those from lower SES, is crucial to improving outcomes for this vulnerable population. The 30-day cost of recent FDA-approved therapies for GVHD can exceed $20 000. Choice and access to newer therapies can be cost prohibitive and determined by SES and insurance coverage rather than by need or effectiveness.
The National Marrow Donor Program (NMDP) has taken initiatives to address disparate outcomes associated with patient race and lower SES in HCT. The 15-MMUD and ACCESS clinical trials both have demonstrated high accrual in racial/ethnic minorities. The NMDP-ACCESS initiative, launched in 2022, seeks to focus on awareness, poverty, and racial and ethnic inequity through collaboration with physicians as well as program administrators, health policy and health equity experts, health service researchers, participants from commercial payer organizations, and federal stakeholders.15 These efforts are commendable and important first steps. However, there is still more to be done to rectify the disparate outcomes associated with patient race and lower SES, not only in access to HCT, but in GVHD outcomes and survival.
Conflict-of-interest disclosure: H.S.M. reports advisory board with Bristol Myers Squibb, Jazz Pharmaceuticals, Incyte, and Senti Bio; and also serves as medical monitor for Blood and Marrow Transplant Clinical Trial Network.