Key Points
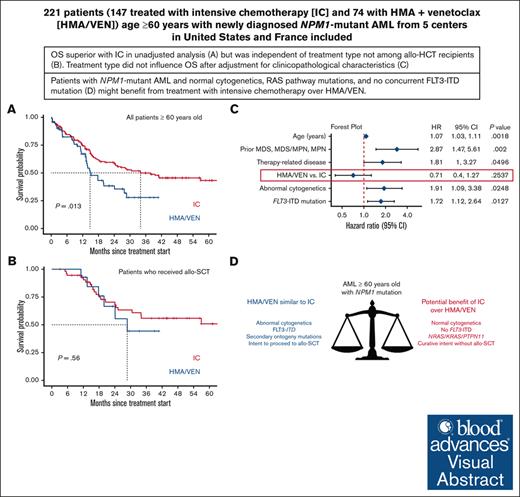
OS of patients aged ≥60 years with NPM1-mutant AML was similar with IC vs HMA/VEN after adjustment for clinicopathologic characteristics.
Patients with normal cytogenetics and without a concurrent FLT3 internal tandem duplication mutation might benefit from treatment with IC over HMA/VEN.
Visual Abstract
Although intensive induction chemotherapy (IC) remains the standard of care for younger patients with acute myeloid leukemia (AML), hypomethylating agents + venetoclax (HMA/VEN) can lead to durable remission among older patients with nucleophosmin 1 (NPM1) mutations. Whether IC or HMA/VEN is superior in patients aged ≥60 years with NPM1-mutant AML is unknown. We performed an international, multicenter retrospective cohort study of 221 patients (147 IC and 74 HMA/VEN) with previously untreated NPM1-mutant AML. Composite complete remission (cCR) (defined as CR + CR with incomplete count recovery) rate was similar for IC and HMA/VEN (cCR, 85% vs 74%; P = .067). Although overall survival (OS) was favorable with IC in unselected patients compared with HMA/VEN (24-month OS, 59% [95% confidence interval (CI), 52-69%] vs 38% [95% CI, 27-55%]; P = .013), it was not statistically different among patients aged 60-75 years (60% [95% CI, 52-70%] vs 44% [95% CI, 29-66%]; P = .069) and patients who received an allogeneic stem cell transplant (70% [95% CI, 58-85%] vs 66% [95% CI, 44-100%]; P = .56). Subgroup analyses suggested that patients with normal cytogenetics (24-month OS, 65% [95% CI, 56-74%] with IC vs 40% [95% CI, 26-60%] with HMA/VEN; P = .009) and without FLT3 internal tandem duplication mutations might benefit from IC compared with HMA/VEN (24-month OS, 68% [95% CI, 59-79%] vs 43% [95% CI, 29-63%]; P = .008). In multivariable analysis, OS was not statistically different between patients treated with IC and HMA/VEN (hazard ratio for death with HMA/VEN vs IC, 0.71; 95% CI, 0.40-1.27; P = .25).
Introduction
Mutations in the nucleophosmin 1 (NPM1) gene are detected in approximately one-third of all patients with acute myeloid leukemia (AML), including 50% to 60% of AML cases with a normal karyotype.1,2 Although the standard of care for younger adults with AML remains intensive induction chemotherapy, only a subset of patients is eligible to receive it because of age or significant comorbidities.3-5 For patients considered ineligible for intensive chemotherapy, the addition of the BCL2 inhibitor venetoclax (VEN) to hypomethylating agents (HMA) prolongs overall survival (OS) compared with HMA monotherapy.6 HMA/VEN is especially active in NPM1-mutated AML, with rates of complete remission (CR) or CR with incomplete hematologic recovery (CRi) and 24-month OS of 93% and 71.8%, respectively.7
Because there have been no randomized clinical trials comparing intensive induction chemotherapy with HMA/VEN in patients with NPM1-mutant AML, the optimal frontline treatment for patients eligible for both therapeutic modalities remains unclear. Small retrospective series suggest comparable efficacy of intensive chemotherapy and HMA/VEN in older patients with AML.8,9 Although most clinicians would use intensive induction chemotherapy for patients with NPM1-mutant AML who are aged <60 years and HMA/VEN for patients aged >75 years, it remains unclear whether patients with NPM1-mutant AML aged 60 to 75 years should be treated with intensive induction chemotherapy or HMA/VEN. In addition, it is an open question whether there are molecularly defined subgroups that benefit more from one treatment modality compared with the other. Hence, we performed a large international, multicenter retrospective cohort study comparing the efficacy of intensive induction chemotherapy vs HMA/VEN in patients aged ≥60 years with newly diagnosed NPM1-mutated AML.
Methods
Patient selection
We included consecutive patients, aged ≥60 years, with NPM1-mutant AML diagnosed between January 2010 and May 2023 who were treated at 5 academic centers in the United States (Dana-Farber Cancer Institute, Memorial Sloan Kettering Cancer Center, Yale Cancer Center, and City of Hope National Medical Center) and France (Institut Paoli-Calmettes, Marseille). Patients received frontline treatment with intensive induction chemotherapy (either conventional 7 + 3 or liposomal cytarabine/daunorubicin [CPX-351]) or HMA/VEN. Patients could receive up to 2 cycles of intensive induction chemotherapy in the intensive induction chemotherapy group. Detection of NPM1 mutations was performed at the participating sites either by polymerase chain reaction or next-generation sequencing per local standards. We also collected information on concurrent molecular alterations and cytogenetic abnormalities at baseline. Patients in the intensive induction chemotherapy group could have received concurrent treatment with midostaurin or gemtuzumab ozogamicin in line with the US Food and Drug Administration approval. Treatment with other investigational combination therapies in either group was an exclusion criterion. Patients were eligible for enrollment regardless of whether they were candidates for an allogeneic stem cell transplantation (allo-SCT). Approval by the institutional review boards at all participating institutions was obtained before the start of the study.
Outcomes
We defined composite CR (cCR) as a composite of CR and CRi per the European LeukemiaNet (ELN) 2022 criteria.4 Response assessment was performed at the end of induction treatment for patients in the intensive induction chemotherapy group and at the time of best response in the HMA/VEN group. OS was measured from the time of treatment initiation to death from any cause. Patients lost to follow-up were censored on the date at which they were last known to be alive.
Statistical analyses
All statistics were performed using R, version 4.3, and a 2-sided P value of < .05 was considered statistically significant in all analyses. For descriptive statistics, percentages and counts were summarized for categorical variables, and comparisons of counts were made by Fisher exact tests. Median and range were used to summarize continuous variables, and Wilcoxon rank sum tests were used for comparisons. For both categorical and continuous variables, 2-sided 95% confidence intervals (CIs) were provided for point estimates. The method of Kaplan-Meier was applied for OS, and the log method provided the 95% CI. The log-rank test was used to compare time-to-event end points. Follow-up time was calculated using the reverse Kaplan-Meier method. Comparison of time-to-event end points and responses to induction treatment were made in prespecified subgroups of interest: patients aged 60 to 75 years, patients who received an allo-SCT, patients with abnormal cytogenetics, and patients with mutations in FLT3 internal tandem duplication (FLT3-ITD) or in at least 1 gene defining secondary ontogeny (SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR, or STAG2). Because there were substantial differences in treatment assignment based on patient age, we restricted subgroup analyses of patients with FLT3-ITD or secondary ontogeny mutations to patients aged 60 to 75 years to minimize the confounding impact of patient age on OS and to perform a dedicated analysis of patients who may be eligible for both treatments.
Cox proportional hazards models were used to estimate the effect of different covariates on OS in univariable and multivariable analyses, with time-to-allo-SCT as a time-varying covariate. Starting from a full model with covariates with a P value of < .1 from the univariate models, the backward elimination method was used to select the best multivariable model containing the treatment group as a predefined covariate. Subgroup analyses to compare OS in the treatment groups included patient age groups (60-75 years and >75 years), baseline clinical characteristics (prior disease and prior therapies of interest), and cytogenetic and molecular characteristics. The subgroup analysis stratified by patient age groups was unadjusted, whereas other subgroup analyses were adjusted by age groups. Only patients for whom no selected variables were missing were included in the final multivariable Cox model.
Results
Patient and disease characteristics
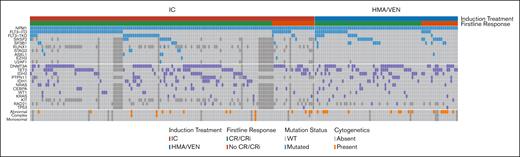
We included 221 patients aged ≥60 years with newly diagnosed NPM1-mutant AML in the analysis (Table 1). Among these patients, 67% (n = 147) received intensive induction chemotherapy and 33% (n = 74) received HMA/VEN. Patients in the HMA/VEN group received a median of 5 cycles of treatment (range, 1-22 cycles). A subset of patients with a concurrent FLT3 mutation (n = 25; 17% patients of the entire cohort and 29.8% with FLT3 mutations) in the intensive induction chemotherapy group also received midostaurin, in contrast to no patients in the HMA/VEN group. Patients who received intensive induction chemotherapy vs HMA/VEN were younger (median age, 65.9 years [range, 60-79] vs 74.9 years [63-89]; P < .001) and less likely to have a prior myelodysplastic syndrome (MDS) or myeloproliferative neoplasm (MPN; 5.4% vs 18%; P = .006), or a concurrent SF3B1 (4.7% vs 15%; P = .017) or SRSF2 (9.2% vs 22%; P = .019) mutation. Patients treated with intensive induction chemotherapy were more likely to have normal cytogenetics compared with patients who received HMA/VEN (90% vs 77%; P = .020). There was no statistically significant difference in the rate of FLT3-ITD mutations (39% vs 30%; P = .18) or any secondary mutations (any of SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR, or STAG2; 30% vs 36%; P = .50) between the groups. Clinical and molecular disease characteristics by treatment group are summarized in Figure 1.
Molecular and cytogenetic profile and response for patients with NPM1-mutant AML treated with intensive chemotherapy and HMA/VEN. An oncoprint shows the molecular and cytogenetic profile and response for patients with NPM1-mutant AML treated with intensive induction chemotherapy and HMA/VEN. Only mutations present in at least 2% of the patient cohort and evaluated in at least 66% of patients were included.
Molecular and cytogenetic profile and response for patients with NPM1-mutant AML treated with intensive chemotherapy and HMA/VEN. An oncoprint shows the molecular and cytogenetic profile and response for patients with NPM1-mutant AML treated with intensive induction chemotherapy and HMA/VEN. Only mutations present in at least 2% of the patient cohort and evaluated in at least 66% of patients were included.
In our prespecified subgroup analysis of patients aged 60 to 75 years, we included 178 patients (137treated with intensive induction chemotherapy and 41 with HMA/VEN). Baseline patient characteristics of this subgroup are shown in supplemental Table 1. In this subgroup, patients treated with HMA/VEN compared with those who received intensive induction chemotherapy only differed by age (median age, 70.6 vs 65.6 years; P < .001), sex (female, 76% vs 50%; P = .004), and rate of prior MDS or MPN (20% vs 5.1%; P = .008).
A total of 69 patients (31% of all patients aged ≥60 years) underwent an allo-SCT. Baseline patient and disease characteristics of patients who underwent an allo-SCT are shown in supplemental Table 2. Rates of proceeding to allo-SCT were higher among patients treated with intensive induction chemotherapy compared with HMA/VEN (n = 55 [37%] vs n = 14 [19%]; P = .006).
Response rates
Among patients treated with intensive induction chemotherapy, 5.4% (n = 8) received reinduction chemotherapy. The median time to best response in the HMA/VEN group was 1.5 cycles (range, 1-7). Treatment with intensive induction chemotherapy and HMA/VEN resulted in similar rates of cCR (intensive induction chemotherapy: 85% [n = 125/147] vs 74% [55/74] with HMA/VEN; P = .067). However, CR rates were higher with intensive induction chemotherapy than with HMA/VEN (83% [122/147] vs 51% [38/74]; P < .001). Figure 1 provides a summary of concurrent mutations and cytogenetic abnormalities and response to treatment among patients treated with intensive induction chemotherapy and HMA/VEN.
When restricting the analysis to patients aged 60 to 75 years (n = 178 patients; 137 patients treated with intensive induction chemotherapy and 41 with HMA/VEN), cCR rates were not statistically significantly different between patients treated with intensive induction chemotherapy and those treated with HMA/VEN (86% [118/137] vs 78% [32/41]; P = .23). However, there was a statistically significantly higher CR rate among patients aged 60 to 75 years who received intensive induction chemotherapy than among those treated with HMA/VEN (84% [115/137] vs 54% [22/41]; P < .001).
OS
After a median follow-up of 38 months (95% CI, 30-44), the median OS for the entire cohort of 221 patients aged ≥60 years with NPM1-mutant AML was 26 months (95% CI, 18-41). OS was longer among patients treated with intensive induction chemotherapy (median OS, 34 months [95% CI, 24 to not reached]; 24-month OS, 59% [95% CI, 52-69]) than among those treated with HMA/VEN (median OS, 15 months [95% CI, 13-28]; 24-month OS, 38% [95% CI, 27-55]; P = .013; Figure 2A).
OS by treatment type, patient age, and transplant status. (A) OS from the time of treatment initiation for the entire cohort of patients with newly diagnosed NPM1-mutant AML aged ≥60 years by treatment type (intensive induction chemotherapy [IC; red] vs HMA/VEN [blue]). Because there were statistically significant differences in treatment assignment by age, we performed a (B) subgroup analysis restricted to patients aged 60 to 75 years . (C) OS for patients who underwent an allo-SCT by type of initial AML treatment.
OS by treatment type, patient age, and transplant status. (A) OS from the time of treatment initiation for the entire cohort of patients with newly diagnosed NPM1-mutant AML aged ≥60 years by treatment type (intensive induction chemotherapy [IC; red] vs HMA/VEN [blue]). Because there were statistically significant differences in treatment assignment by age, we performed a (B) subgroup analysis restricted to patients aged 60 to 75 years . (C) OS for patients who underwent an allo-SCT by type of initial AML treatment.
For patients aged 60 to 75 years (n = 178), the median OS was 29 months (95% CI, 20 to not reached) at a median duration of follow-up of 43 months (95% CI, 38-47). There was no statistically significant difference in OS between patients treated with intensive induction chemotherapy (median OS, 38 months [95% CI, 24 to not reached]; 24-month OS, 60% [95% CI, 52-70]) and those treated with HMA/VEN (median OS, 20 months [95% CI, 13 to not reached]; 24-month OS, 44% [95% CI, 29-66]; P = .069; Figure 2B).
The median OS for patients who received an allo-SCT (n = 69) was not reached, with a 24-month OS rate of 70% (95% CI, 59-83). Among allo-SCT recipients, there was no difference in OS by treatment type (24-month OS, 70% [95% CI, 58-85] for intensive induction chemotherapy vs 66% [95% CI, 44-100] for HMA/VEN; P = .56; Figure 2C).
Impact of baseline cytogenetics, FLT3 mutation, and secondary ontogeny mutation status on survival
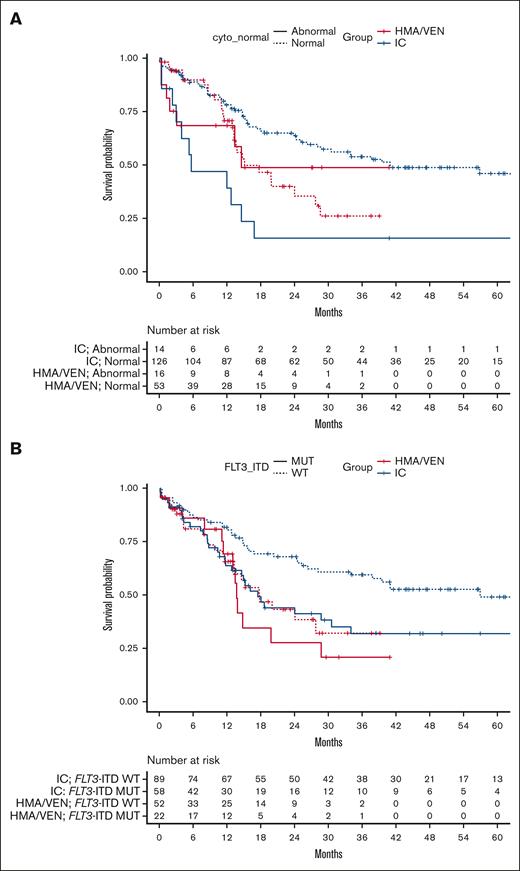
We next analyzed whether patient subgroups stratified by their baseline cytogenetics would benefit from one treatment type over the other (Figure 3A). When comparing outcomes of patients with normal cytogenetics by treatment type (n = 179; 126 patients treated with intensive induction chemotherapy and 53 treated with HMA/VEN), we found that these patients with normal cytogenetics had better 24-month OS when treated with intensive induction chemotherapy compared with HMA/VEN (65% [95% CI, 56-74] vs 40% [95% CI, 26-60]; P = .009). However, when comparing patients with abnormal cytogenetics (n = 30; 14 patients treated with intensive induction chemotherapy and 16 treated with HMA/VEN), there was no treatment type–dependent difference in 24-month OS (16% [95% CI, 4.4-56] for intensive induction chemotherapy vs 49% [95% CI, 27-87] for HMA/VEN; P = .16). When evaluating the impact of cytogenetic status (normal vs abnormal) within a treatment type, we found that the presence of normal cytogenetics as compared with abnormal cytogenetics was associated with improved 24-month OS among patients treated with intensive induction chemotherapy (P < .001) but not among those treated with HMA/VEN (P = .93).
Comparison of OS by treatment assignment in patients with abnormal cytogenetics and with and without concurrent FLT3-ITD mutations. (A) OS from the time of treatment initiation in patients with AML aged ≥60 years with normal and abnormal cytogenetics by treatment type (IC vs HMA/VEN). (B) OS from the time of treatment initiation in patients with AML aged ≥60 years with and without concurrent FLT3-ITD mutations. Cyto, cytogenetics; MUT, mutant; WT, wild-type.
Comparison of OS by treatment assignment in patients with abnormal cytogenetics and with and without concurrent FLT3-ITD mutations. (A) OS from the time of treatment initiation in patients with AML aged ≥60 years with normal and abnormal cytogenetics by treatment type (IC vs HMA/VEN). (B) OS from the time of treatment initiation in patients with AML aged ≥60 years with and without concurrent FLT3-ITD mutations. Cyto, cytogenetics; MUT, mutant; WT, wild-type.
When evaluating the impact of FLT3-ITD mutation status, we found that among patients treated with intensive induction chemotherapy, the presence of an FLT3-ITD mutation was associated with inferior 24-month OS compared with patients without an FLT3-ITD mutation (FLT3-ITD mutant [n = 58], 44% [95% CI, 32-61] vs FLT3-ITD wild-type [n = 89], 68% [95% CI, 59-79]; P = .013). However, the prognostic impact of FLT3-ITD mutations was not observed among patients treated with HMA/VEN (FLT3-ITD mutant [n = 22], 28% [95% CI, 12-63] vs FLT3-ITD wild-type [n = 52], 43% [95% CI, 29-63]; P = .56). When we stratified by FLT3-ITD mutation status (present vs absent) and compared between treatments, we found that patients without FLT3-ITD mutations had better 24-month OS when treated with intensive induction chemotherapy compared with HMA/VEN (68% [95% CI, 59-79] vs 43% [95% CI, 29-63], respectively; P = .008). Treatment type did not impact 24-month OS among patients with concurrent FLT3-ITD mutations (intensive induction chemotherapy, 44% [95% CI, 32-61] vs HMA/VEN, 28% [95% CI, 12-63]; P = .46; Figure 3B).
Finally, we compared 24-month OS by treatment type among 52 patients with secondary ontogeny mutations (n = 25 patients treated with intensive induction chemotherapy; n = 27 patients treated with HMA/VEN). Patients with secondary ontogeny mutations who were treated with intensive induction chemotherapy had better 24-month OS compared with those treated with HMA/VEN (intensive chemotherapy, 52% [95% CI, 34-80] vs HMA/VEN, 20% [95% CI, 7.4-53]; P = .032).
Impact of concurrent FLT3-ITD and secondary ontogeny mutations on OS among patients aged 60 to 75 years by treatment type
We next analyzed the impact of concurrent mutations on OS in our prespecified subgroup of patients aged 60 to 75 years. Among patients with a concurrent FLT3-ITD mutation (n = 66; 52 patients treated with intensive induction chemotherapy and 14 with HMA/VEN), 24-month OS was not statistically significantly different (P = .58) between patients treated with intensive chemotherapy (49% [95% CI, 35-67]) and those treated with HMA/VEN (33% [95% CI, 13-82]; supplemental Figure 1A). When comparing patients without a concurrent FLT3-ITD mutation (n = 112; 85 patients treated with intensive induction chemotherapy and 27 treated with HMA/VEN), 24-month OS was not statistically significantly different between patients treated with intensive induction chemotherapy (66% [95% CI, 57-78]) and patients who received HMA/VEN (49% [95% CI, 31-77]; P = .066; supplemental Figure 1B).
Given that secondary ontogeny mutations have recently been introduced as a distinct AML subgroup in the ELN AML 2022 criteria, and the optimal treatment for patients who also harbor a NPM1 mutation is unclear,4 we next compared OS among patients aged 60 to 75 years with any concurrent secondary ontogeny mutations by treatment type. Among the 34 patients with a concurrent secondary ontogeny mutation (n = 23 patients treated with intensive induction chemotherapy and n = 11 treated with HMA/VEN), 24-month OS was not statistically significantly different between patients treated with intensive induction chemotherapy (46% [95% CI, 28-77]) and those treated with HMA/VEN (27% [95% CI, 8.5-87]; P = .36; supplemental Figure 1C).
Multivariable OS Cox regression analysis adjusted for clinical and molecular covariables
Next, we sought to identify the impact of treatment modality as a covariable in predicting survival outcomes when used in conjunction with clinical and molecular patient characteristics.
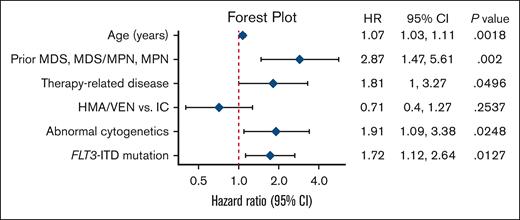
In univariable Cox regression analysis among all patients, treatment (HMA/VEN vs intensive induction chemotherapy), age, therapy-related disease, abnormal cytogenetics, prior MDS, MPN, or MDS/MPN, and SF3B1, FLT3-ITD, and RNA splicing factor mutations were all associated with worse OS (Table 2). Of note, the receipt of allo-SCT as a time-varying covariate was not statistically significant in univariate analyses.
In the multivariable Cox regression analysis, age at diagnosis (hazard ratio [HR], 1.07; 95% CI, 1.03-1.11; P = .002), prior MDS, MPN, or MDS/MPN (HR, 2.87; 95% CI, 1.47-5.61; P = .002), therapy-related disease (HR, 1.81; 95% CI, 1.00-3.27; P = .05), abnormal cytogenetics (HR, 1.91; 95% CI, 1.09-3.38; P = .025), and FLT3-ITD mutations (HR, 1.72; 95% CI, 1.12-2.64; P = .013) remained associated with worse OS. However, treatment type (HMA/VEN vs intensive induction chemotherapy) was no longer statistically significantly associated with OS (HR, 0.71; 95% CI, 0.40-1.27; P = .25; Figure 4).
Multivariable regression model of OS. Forest plot shows HRs for death with associated 95% CIs of variables included in the final multivariable regression model. The type of frontline treatment (IC vs HMA/VEN) was not statistically significantly associated with OS in this model.
Multivariable regression model of OS. Forest plot shows HRs for death with associated 95% CIs of variables included in the final multivariable regression model. The type of frontline treatment (IC vs HMA/VEN) was not statistically significantly associated with OS in this model.
Subgroup OS analysis for optimal treatment modality
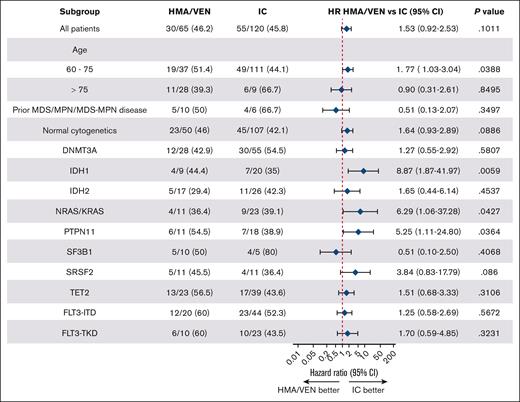
Finally, we performed subgroup analyses by patient and mutational/cytogenetic disease characteristics to evaluate whether there was a benefit of 1 treatment modality over the other in a specific subgroup of patients. Among all subgroups analyzed, patients aged 60 to 75 years (n = 148 patients; HR, 1.77; 95% CI, 1.03-3.04; P = .039) and those with IDH1 (n = 29; HR, 8.87; 95% CI, 1.87-44.97; P = .006), NRAS/KRAS (n = 34; HR, 6.29; 95% CI, 1.06-37.28; P = .043), and PTPN11 mutations (n = 29; HR, 5.25; 95% CI, 1.11-24.80; P = .036) demonstrated better OS when treated with intensive chemotherapy compared with HMA/VEN after adjusting for their age groups (Figure 5). There were no statistically significant differences in OS in any of the other subgroup analyses when comparing patients treated by HMA/VEN or intensive induction chemotherapy.
Subgroup analysis of OS for HMA/VEN vs intensive chemotherapy. Forest plot shows a comparison of OS for patients treated with HMA/VEN vs intensive induction chemotherapy (IC) by molecular subgroups. Cox proportional hazards models were adjusted for treatment (IC vs HMA/VEN) and age category (60-75 years and >75 years), except for the subgroups defined by age category, which are based on unadjusted Cox proportional hazards models.
Subgroup analysis of OS for HMA/VEN vs intensive chemotherapy. Forest plot shows a comparison of OS for patients treated with HMA/VEN vs intensive induction chemotherapy (IC) by molecular subgroups. Cox proportional hazards models were adjusted for treatment (IC vs HMA/VEN) and age category (60-75 years and >75 years), except for the subgroups defined by age category, which are based on unadjusted Cox proportional hazards models.
Discussion
In this large, international, multicenter, retrospective analysis of patients aged ≥60 years with newly diagnosed NPM1-mutant AML, treatment with intensive induction chemotherapy was associated with similar cCR rates but longer OS when compared with HMA/VEN in unadjusted analyses. However, after initial adjustment for age of 60 to 75 years in the multivariable analysis, treatment type was no longer associated with OS. This indicates that other patient and disease characteristics have a greater influence on outcomes among patients with newly diagnosed, NPM1-mutant AML than the assignment of treatment modality. Although patients with NPM1-mutant AML frequently have de novo AML and a normal karyotype, we found that older age, abnormal cytogenetics, prior MDS, MPN, or MDS/MPN, FLT3-ITD comutation, and therapy-related disease were associated with adverse outcomes, which supports prior observations that the favorable outcomes of NPM1-mutant disease are limited to younger patients with normal cytogenetics and de novo AML.10
Although subgroup analyses from various clinical trials showed high response rates with HMA/VEN among patients with NPM1-mutant, it is unclear how these results apply to patients aged <75 years. Additionally, there are no prospective data from randomized clinical trials comparing intensive induction chemotherapy with HMA/VEN in patients with newly diagnosed AML eligible for intensive induction chemotherapy. Thus, we focused our analysis on patients with NPM1-mutant AML aged ≥60 years, with patients aged 60 to 75 years constituting a key subgroup of interest. In this age cohort, HMA/VEN was comparable to intensive chemotherapy both in terms of cCR rate and OS. This is consistent with prior, smaller studies comparing intensive induction chemotherapy with HMA/VEN in patients with NPM1-mutant AML aged >65 years.8,9 In addition, among patients who proceeded to allo-SCT after initial therapy, OS was not different between intensive induction chemotherapy and HMA/VEN, suggesting a balance in effectiveness between the 2 treatment modalities as long as a subsequent consolidation treatment strategy with allo-SCT is pursued.
The question remains whether there are specific clinical scenarios for which intensive induction chemotherapy still remains the preferred option over HMA/VEN. In our analysis, we found that patients with NPM1-mutant AML and normal cytogenetics and those without a concurrent FLT3-ITD mutation might benefit from treatment with intensive induction chemotherapy over HMA/VEN. Additionally, patients with NPM1-mutant, FLT3-ITD wild-type AML can be cured with chemotherapy alone and do not require an allo-SCT in first CR.11 This is in contrast to treatment with HMA/VEN, which is generally administered on an ongoing basis until disease progression because the curative potential of this combination therapy remains unclear.
Given that patients aged <60 years were excluded from our study, we cannot comment on the comparative efficacy of intensive induction chemotherapy and HMA/VEN in patients of this age group. Thus, intensive induction chemotherapy should remain the standard of care in patients aged <60 years with NPM1-mutant, FLT3-ITD wild-type AML until more definitive data comparing intensive induction chemotherapy and HMA/VEN in this specific subgroup of patients with AML are available.
In our analyses, we found that the presence of a concurrent FLT3-ITD mutation was associated with adverse outcomes in this patient population, which is consistent with prior studies showing a higher risk status for patients with NPM1-mutant AML and a concurrent FLT3-ITD mutation.11,12,13 These findings are also reflected in the most recent ELN risk stratification of patients with AML.4 Emerging data suggest that the addition of an FLT3 inhibitor to HMA/VEN can be an effective option, although duration of follow-up is short and such triplet combinations can be limited by significant myelosuppression.14 Given that patients with NPM1-mutant, FLT3-ITD-mutant AML have a higher risk of disease relapse without a subsequent allo-SCT, our data suggest that both therapeutic strategies can successfully allow patients to proceed to allo-SCT with comparable long-term outcomes.
Given the recent conflicting reports regarding the prognostic impact of concurrent secondary ontogeny mutations in NPM1-mutated AML, we analyzed whether these mutations lead to better OS outcomes with one treatment type over the other.15,16 We found that patients aged 60 to 75 years with NPM1-mutant AML and secondary ontogeny mutations had similar outcomes when treated with intensive induction chemotherapy and HMA/VEN. Acknowledging the limitations of the small sample size in this subgroup analysis, we conclude that regardless of whether secondary ontogeny mutations impact prognosis in NPM1-mutated AML, they should not impact treatment selection in patients with NPM1-mutated AML aged 60 to 75 years.
Over the recent years, measurable residual disease (MRD) status as assessed by either flow cytometry, quantitative polymerase chain reaction, or next-generation sequencing has emerged as an important predictor of OS in multiple studies of patients with NPM1-mutant AML treated with both intensive induction chemotherapy and HMA/VEN.17-20 However, comparison across studies is limited by differences in modality and timing of assessment and the influence of treatment types. Although only limited data exist for patients treated with HMA/VEN, the prognostic implications of NPM1 MRD status seem to apply similarly as for patients treated with intensive induction chemotherapy.20 Furthermore, emerging data also suggest that treatment with VEN–based lower-intensity combinations (HMA or low-dose cytarabine) can be an option for MRD eradication among patients with NPM1-mutant AML.21,22 Because our study was an international, retrospective cohort study that enrolled patients dating back to 2010, MRD status was not uniformly available for our patients, and we cannot comment on the rates of MRD-negative remission and the impact on OS in our patients.
We focused on the comparison of cCR rates rather than CR rates because HMA/VEN is given on a continuous basis, leading to ongoing myelosuppression, which is not the case with intensive induction chemotherapy. Although rates of CR were higher with intensive induction chemotherapy than with HMA/VEN in our study, it is important to note that achieving cCR following treatment with HMA/VEN has been shown to be associated with improved OS leading to increased recognition of near-CR end points (eg, CRi and CR with partial hematologic recovery) as surrogate markers for therapeutic benefit.6,23 This might become even more relevant in the context of an increasing number of HMA/VEN-based triplet therapies for patients with NPM1-mutant AML, including combinations with menin inhibitors (eg, NCT05453903 and NCT05360160), or future trials of quadruplet therapies of HMA/VEN in combination with menin and FLT3 inhibitors. Similar combination trials are also ongoing for patients receiving intensive induction chemotherapy (eg, NCT06313437 and NCT05453903).
Finally, it is important to note from the patient perspective that HMA/VEN is generally administered indefinitely until disease progression, whereas induction chemotherapy followed by consolidation therapy with either chemotherapy or an allo-SCT offers the possibility of time-limited therapy. Thus, more data on quality-of-life aspects with both therapeutic approaches and longer follow-up with HMA/VEN capturing information on, for example, infectious complications from intermittent myelosuppression are needed. However, quality-of-life data were not available in our study and are difficult to capture accurately in retrospective studies in general. Thus, future clinical trials should focus on these aspects to provide a more comprehensive comparison of the entire treatment course between intensive chemotherapy and HMA/VEN.
Our analysis was limited by its retrospective nature. Namely, residual confounding cannot be excluded given the significant differences in baseline patient and disease characteristics between the 2 groups. Given that patients aged <60 years were excluded from the analysis, our results should not be extrapolated to patients in this age group. Additionally, the numbers of patients in some of the subgroup analyses (eg, based on concurrent mutations) were small, limiting the statistical power of our analysis and necessitating subsequent larger studies. Given that this was a retrospective, real-world analysis, we cannot comment on the specific reasons for selecting intensive chemotherapy vs HMA/VEN or whether to proceed to an allo-SCT for an individual patient. Only a randomized clinical trial with clear, prespecified criteria can exclude any confounding effect of differences in baseline patient characteristics and treatment assignment. Finally, information on subsequent lines of therapy including the specific consolidation regimen and maintenance therapy with oral azacitidine was not available. Thus, we cannot exclude an impact of subsequent crossover between treatment arms on OS.
In summary, in this large, international, multicenter, retrospective analysis of patients with newly diagnosed NPM1-mutant AML, treatment with intensive induction chemotherapy and HMA/VEN resulted in similar OS in multivariable analyses after adjustment for important patient and disease characteristics. Prospective randomized clinical trials are needed to ultimately determine the optimal frontline therapy for patients with NPM1-mutant AML.
Acknowledgments
B.B. reports funding from the National Institutes of Health (NIH; K12 grant 5K12CA001727-27). This work was supported by the Dana-Farber Cancer Institute Hematologic Malignancies Data Repository team. This research was funded in part through NIH/National Cancer Institute Cancer Center Support grants P30 CA008748 and 5P50CA254838-03.
Authorship
Contribution: J.P.B., S.S., and M.S. conceptualized the study; J.P.B., S.S., R.M. Shallis, G.B., S.G., B.B., and M.S. collected data; J.P.B., S.S., Y.L., and M.S. analyzed the data; J.P.B. wrote the initial draft of the manuscript; and all authors reviewed and contributed to subsequent versions of the manuscript.
Conflict-of-interest disclosure: R.M. Shallis provided consultancy for Bristol Myers Squibb (BMS), Curio Science, Gilead Sciences, Servier, and Rigel. A.M.Z. received research funding (institutional) from Celgene/BMS, AbbVie, Astex, Pfizer, MedImmune/AstraZeneca, Boehringer Ingelheim, Cardiff Oncology, Incyte, Takeda Pharmaceuticals, Novartis, Aprea Therapeutics, and ADC Therapeutics; participated in advisory boards, provided consultancy for, and received honoraria from AbbVie, Otsuka Pharmaceutical, Pfizer, Celgene/BMS, Jazz Pharmaceuticals, Incyte, Agios Pharmaceuticals, Boehringer Ingelheim, Novartis, Acceleron Pharma, Astellas Pharma, Daiichi Sankyo, Cardinal Health, Taiho, Seattle Genetics, BeyondSpring, Cardiff Oncology, Takeda Pharmaceuticals, Ionis, Amgen, Janssen, Epizyme, Syndax, Gilead, Kura, Chiesi, ALX Oncology, BioCryst, Notable Labs, Orum, and Tyme; and served on clinical trial committees for Novartis, AbbVie, Gilead, BioCryst, AbbVie, ALX Oncology, Geron, and Celgene/BMS. A.D.G. participated in advisory boards and provided consultancy for AbbVie, Astellas Pharma, Daiichi Sankyo, Genentech, BMS, and Molecular Partners; received honoraria from DAVA Oncology; received research funding from AbbVie, Aprea, Aptose Biosciences, AROG Pharmaceuticals, Celularity, Kura, Pfizer, and Prelude; and served on safety monitoring committees for AbbVie and Kura. E.M.S. received research funding from Bayer, Syndax, Daiichi Sankyo, Celgene Pharmaceuticals, and Novartis; served as a consultant for Amgen, AbbVie, Seattle Genetics, Biotheryx, Syndax, Astellas Pharmaceutical, Agios Pharmaceuticals, Genentech, Daiichi Sankyo, Celgene Pharmaceuticals, and Novartis; was a member of the board of directors or advisory committee for PTC Therapeutics, Syros, Astellas Pharmaceutical, Agios Pharmaceuticals, Genentech, Daiichi Sankyo, Celgene Pharmaceuticals, and Novartis; and is a current equity holder in privately held Auron Therapeutics. R.C.L. provided consultancy for Takeda Pharmaceuticals, bluebird bio, Qiagen, Sarepta Therapeutics, Verve Therapeutics, Jazz Pharmaceuticals, and Vertex Pharmaceuticals. E.C.C. received research funding from AbbVie and provided consultancy for Rigel Pharmaceuticals. A.S. declares stock ownership in Sanofi. D.J.D. received honoraria from Amgen, Autolus, Blueprint, Gilead, Incyte, Jazz, Kite, Novartis, Pfizer, Servier, and Takeda Pharmaceuticals, and research funding from AbbVie, Novartis, Bluprint, and Glycomimetics. D.S.N. declares equity ownership in Madrigal Pharmaceuticals. R.M. Stone provided consultancy for AbbVie, CTI Biopharma, GlaxoSmithKline, Hermavant, Ligand Pharma, Lava Therapeutics, Amgen, AvenCell, BerGenBio, Celularity, Jazz Pharmaceuticals, Kura One, and Rigel, and participated in drug and safety monitoring boards for Aptevo, Epizyme, Takeda Pharmaceuticals, and Syntrix. S.G. declares a consulting or advisory role with AbbVie, Astellas, BMS/Celgene, Jazz Pharmaceuticals, and Servier, and received travel grants from Gilead. B.B. served on advisory boards for BMS, Rigel, and Oncovalent Therapeutics. M.S. served on advisory boards for Novartis, Kymera, Sierra Oncology, GlaxoSmithKline, Rigel, BMS, and Taiho; consulted for Boston Consulting and Dedham Group; and participated in CME activities for Novartis, Curis Oncology, Haymarket Media, and Clinical Care Options. The remaining authors declare no competing financial interests.
Correspondence: Maximilian Stahl, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; email: maximilian_stahl@dfci.harvard.edu.
References
Author notes
J.P.B., S.S., and R.M.S. are joint first authors and contributed equally to this study.
S.G., B.B., and M.S. are joint senior authors and contributed equally to this study.
Presented in abstract form at the 65th annual meeting of the American Society of Hematology, San Diego, CA, 10 December 2023.
Deidentified original data are available on request from the corresponding author, Maximilian Stahl (maximilian_stahl@dfci.harvard.edu).
The full-text version of this article contains a data supplement.

![OS by treatment type, patient age, and transplant status. (A) OS from the time of treatment initiation for the entire cohort of patients with newly diagnosed NPM1-mutant AML aged ≥60 years by treatment type (intensive induction chemotherapy [IC; red] vs HMA/VEN [blue]). Because there were statistically significant differences in treatment assignment by age, we performed a (B) subgroup analysis restricted to patients aged 60 to 75 years . (C) OS for patients who underwent an allo-SCT by type of initial AML treatment.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/18/10.1182_bloodadvances.2024012858/2/m_blooda_adv-2024-012858-gr2.jpeg?Expires=1769410900&Signature=O~N28eMzzD-Tw2UzhV9FTIpw-UILoMZwPr21wlAFbRdewbDd6aQNqp~GRbkZt5F8aPVagZYzHmhx6csQ0XeryPod3zLyspKZLwNAW8CbzmtJqYQEqHtS2KHHD8lao6qD7Z90c~pK-725cAwpiKYPHFgEz4n14nlrZXLl~lzHmuVkYaiJlfRT2QhtisDLasFI1CJ1EWbOL0qo7X43c8ReiS-WshbDUwi8ssss3z1IYLQ5l2RmCpye9ofI8otnurXwgpdkFobiI~Gd3Rblf90H1xGiNkbCR0GQwvKI2ucO5srzTnm0wyWjp7Tb~Kznwv~HBbn7uOxpNjCczeO2~j3bGQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)