Key Points
Anti-BCMA-Exo-Btz precisely targets myeloma cells, effectively inhibiting MM and mitigating bone destruction.
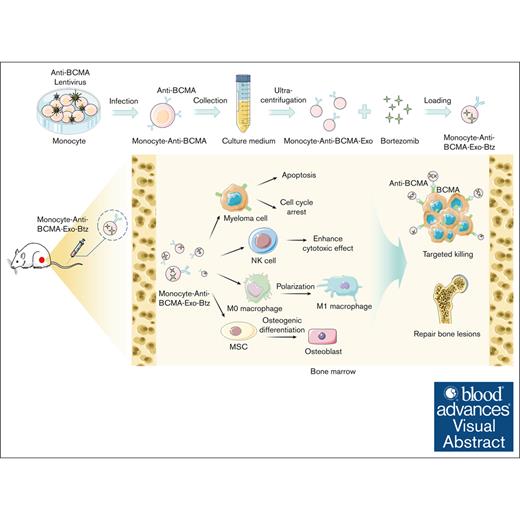
Visual Abstract
Exosomes have emerged as promising vehicles for delivering therapeutic cargoes to specific cells or tissues, owing to their superior biocompatibility, reduced immunogenicity, and enhanced targeting capabilities compared with conventional drug delivery systems. In this study, we developed a delivery platform using exosomes derived from monocytes, specifically designed for targeted delivery of bortezomib (Btz) to multiple myeloma (MM) cells. Our approach involved the genetic modification of monocytes to express antibodies targeting B-cell maturation antigen (anti-BCMA), because BCMA selectively expresses on myeloma cells. This modified anti-BCMA was then efficiently incorporated into the monocyte-derived exosomes. These adapted exosomes effectively encapsulated Btz, leading to enhanced drug accessibility within MM cells and sustained intracellular accumulation over an extended period. Remarkably, our results demonstrated that anti-BCMA–modified exosome-loaded Btz (anti-BCMA-Exo-Btz) outperformed free Btz in vitro, exhibiting a more potent myeloma-suppressive effect. In orthotopic MM xenograft models, anti-BCMA-Exo-Btz exhibited a significant antitumor effect compared with free Btz. Furthermore, it demonstrated remarkable specificity in targeting Btz to myeloma cells in vivo. Importantly, we observed no significant histological damage in mice treated with anti-BCMA-Exo-Btz and a slight effect on peripheral blood mononuclear cells. In addition, our study highlighted the multifunctional potential of monocyte exosomes, which induced cell apoptosis, mediated immune responses, and enhanced the osteogenic potential of mesenchymal stromal cells. In conclusion, our study suggests that exosomes modified with targeting ligands hold therapeutic promise for delivering Btz to myelomas, offering substantial potential for clinical applications.
Introduction
Multiple myeloma (MM) is a hematologic malignancy marked by the abnormal proliferation of plasma cells, resulting in myeloma formation in the bone marrow, leading to osteolytic bone disease, anemia, acute kidney injury, and various clinical manifestations.1
Bortezomib (Btz), a first-generation proteasome inhibitor, has been approved for the treatment of patients with MM, and it significantly prolongs their survival time.2 Btz-based combination therapy has gradually become the first-line regimen for patients with MM.2,3 However, the treatment efficacy of Btz is hindered by issues such as lack of specificity, poor permeability, limited bioavailability, and drug resistance with long-term use.4 Although increasing the Btz dose slightly improves therapeutic effects, it also leads to significantly increased toxicity.5 Therefore, a novel approach is required to address these limitations and further enhance the efficacy of Btz in myeloma treatment.
The utilization of exosomes as drug delivery systems has gained significant attraction in the field of delivering chemotherapeutic drugs.6,7 Unlike free drug molecules, exosomes encapsulation improves drug stability, reducing degradation and inactivation in the circulation, thereby enhancing overall efficacy.8 Due to their capacity to reshape the microenvironment and attract other cell populations to inhibit tumor progression, exosomes derived from immune cells are ideal candidates as delivery vehicles for chemotherapy drugs.9 Exosomes derived from immune cells, particularly monocyte-derived exosomes, show reduced immunogenicity and are seldom cleared by the mononuclear phagocyte system, making them ideal vehicles for drug delivery.10
Targeting modified exosomes exhibit remarkable cell specificity, facilitating interactions with specific cells through surface proteins or other biomarkers, enabling precise drug delivery and minimizing potential adverse effects on normal cells.6,11 B-cell maturation antigen (BCMA) serves as a prime target antigen for myeloma immunotherapy due to its highly selective expression on malignant plasma cells and absence in other tissues, rendering it an ideal candidate for immunotherapeutic intervention in MM compared with CD38 andsignaling lymphocytic activation molecule family 7 (SLAMF7).12 Significant breakthroughs have been made in using BCMA as a target for immunotherapy. BCMA-directed chimeric antigen receptor T-cell therapy has demonstrated outstanding efficacy in the treatment of MM.13
In this study, monocyte-derived exosomes demonstrated a high capacity to modulate the tumor microenvironment, as evidenced by their ability to regulate macrophage polarization, enhance the cytotoxicity of natural killer (NK) cells, and promote osteogenic differentiation of mesenchymal stromal cells (MSCs). Based on the advantages mentioned above, monocyte-derived exosomes were modified with anti-BCMA and used as the drug carrier to deliver Btz, named anti-BCMA–modified exosome-loaded Btz (anti-BCMA-Exo-Btz). These exosomes were then used for targeted imaging and treatment of myeloma cells and orthotopic xenograft. Results showed that anti-BCMA-Exo-Btz could accumulate in bone marrow, facilitating accurate myeloma recognition, improving the curative effect, and repairing bone lesions. Overall, our results indicated that the proposed drug delivery system represents a viable approach against myeloma, overcoming the limitations of conventional chemotherapy.
Methods
Preparation of anti-BCMA–modified monocytes
For the genetic modification of anti-BCMA to monocytes, anti-BCMA lentivirus with green fluorescent protein (GFP) labeling was added to the cells. Then, polybrene with a final concentration of 8 μg/mL was added. After 48 hours of culture, GFP+ cells were isolated by flow cytometry and further collected and cultured to obtain anti-BCMA–expressing cells.
Synthesis of anti-BCMA-Exo-Btz
Anti-BCMA-Exo (200 μg) obtained by differential centrifugation was gently mixed with Btz (100 μg) and incubated in a constant temperature shaking bed at 37°C for 48 hours. Phosphate-buffered saline (PBS) solution was added to the mixture and centrifuged (130 000g; 2 hours) to remove the free Btz. The pellet was resuspended in PBS and subsequently centrifuged at 130 000g for another 2 hours. The pellets were resuspended in PBS and stored at –80°C for further use.
Loading capacity and in vitro release
The content of Btz in exosomes was determined by high-performance liquid chromatography (HPLC). Anti-BCMA-Exo-Btz was mixed with 1 mL acetonitrile and thoroughly mixed; after ultrasound, it was centrifuged at 16 500g for 20 minutes. The supernatant was detected by HPLC. The supernatant was filtered with a 0.22 μm syringe filter, and 20 μL aliquots were transferred into HPLC autosampler vials. The loading efficiency represents the ratio of loaded Btz dose to exosome dose, whereas encapsulation efficiency denotes the ratio of loaded Btz dose to the initially used dose of Btz for loading. To measure Btz release, anti-BCMA-Exo-Btz was loaded in a dialysis bag in cell buffers at pH 7.35 and pH 7.45. Samples were taken at different time points and analyzed using HPLC, with results expressed as the percentage of Btz released divided by total Btz.
Intracellular Btz accumulation
To quantify the amount of Btz accumulation in MM cells, anti-BCMA-Exo-Btz was added to and incubated with MM cells for 12 hours or 24 hours. Then, the cells were washed with PBS and lysed with Triton X-100, and ultrasound was performed on ice. The lysed cell fluid was centrifuged at 16 500g for 20 minutes, and the supernatant (20 μL) was determined by HPLC.
In vivo studies
Animals were housed and maintained in accordance with the institutional guidelines for the use of laboratory animals and after acquiring permission from the ethics committee of Soochow University for animal experimentation. Four-week-old female NSG (NOD-Prkdcscid Il2rgtm1/Bcgen) mice were purchased from a Biocytogen company (Beijing, China) and were acclimated 1-week before tumor cell inoculation. A total of 1 × 106 luciferase–labeled LP-1 cells were injected via the lateral tail vein. Tumors were allowed to grow for a week, and then mice were injected with primary monocytes (PC)-Exo, PC-Anti-BCMA-Exo, and monocyte cell line (CL)-Anti-BCMA-Exo, PC-Anti-BCMA-Exo-Btz, CL-Anti-BCMA-Exo-Btz, Btz, and PBS as control, 3 times a week for 2 weeks. The treatment doses for each group were 2.23 mg/kg exosomes in PC-Exo, PC-Anti-BCMA-Exo, and CL-Anti-BCMA-Exo group, 2.23 mg/kg exosomes loaded with 0.29 mg/kg Btz in PC-Anti-BCMA-Exo-Btz and CL-Anti-BCMA-Exo-Btz group, and 0.29 mg/kg Btz in Btz group. Four- to 6-week-old C57BL/KaLwRij mice were housed at the laboratory animal center of Suzhou medical college. A total of 1 × 106 luciferase–labeled 5TGM1 cells were injected via the lateral tail vein. Tumors were allowed to grow for a week, and then mice were injected with CL-Exo (2.23 mg/kg) and PBS as control, 3 times a week for 2 weeks.
Mice were imaged after injection of 75 mg/kg of D-luciferin (Promega, Madison, WI) using IVIS Lumina II optical imaging system (Caliper Life Sciences, Hopkinton, MA). Tumor burden was assessed by serial bioluminescence imaging every 3 days. After injection of DiR-labeled exosomes for 6 hours, the biodistribution of DiR-labeled exosomes in mice and organs was monitored by the IVIS Lumina II optical imaging system at an excitation wavelength of 740 nm and an emission wavelength of 780 nm.
Micro-CT imaging
Tomography scans were performed in a SkyScan 1172 micro computed tomography (micro-CT) system (Bruker, Kontich, Belgium). The spectrum was filtered with a 1.0 mm aluminum filter. After scanning, CTAn and Mimics software were used for micro-CT analysis and 3-dimensional reconstruction of the scapula.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 8.0. All data were expressed as the mean ± standard deviation. For normally distributed data, the significance of mean differences was determined using unpaired Student t test between 2 groups or analysis of variance followed by Newman-Keuls multiple comparison test among multiple groups. For all tests, a P value <.05 was considered to be statistically significant.
The animal experimentation acquired permission from the ethics committee of Soochow University. All human primary monocytes were collected from The Second Affiliated Hospital of Soochow University after receiving permission from the ethics committee of The Second Affiliated Hospital of Soochow University. Informed written consent was obtained from each donor or each donor's guardian. The study was conducted in accordance with the Declaration of Helsinki.
Other detailed assays are available in the supplemental Methods.
Results
Engineering exosomes for targeted drug delivery in MM
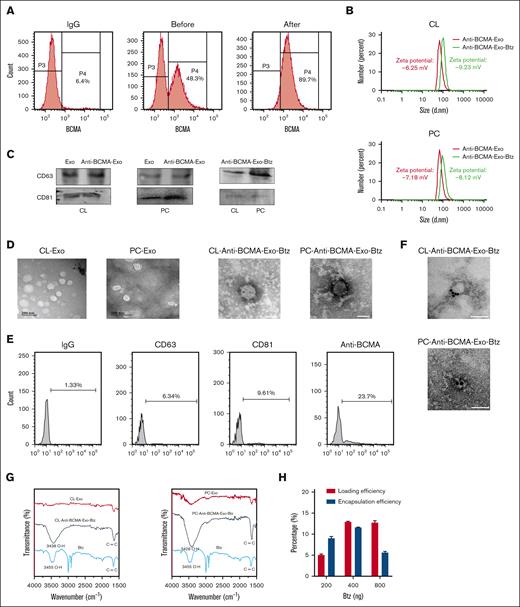
This approach aimed to use the natural targeting properties of exosomes derived from monocytes to specifically deliver therapeutic cargo to myeloma cells expressing BCMA. Monocytes were obtained from either the human monocyte cell line THP-1 (CL-monocytes) or primary monocytes derived from peripheral blood mononuclear cells (PBMCs; PC-monocytes). The myeloma-targeting capability of exosomes was conferred by engineering the monocytes to express anti-BCMA. We introduced a lentiviral vector containing the anti-BCMA construct into either CL-monocytes or PC-monocytes and then used flow cytometry–based sorting to identify and isolate the significant fraction of cells expressing anti-BCMA (Figure 1A). We then collected exosomes secreted by the engineered monocytes and characterized them using dynamic light scattering analysis, western blotting, and transmission electron microscopy to confirm their size, presence of exosome markers, and morphology (Figure 1B-D). Anti-BCMA was strongly expressed in engineered monocytes, incorporated into the monocyte-derived exosomes, and identified on the external surface of the exosomes based on flow cytometry (Figure 1E).
Characterization of anti-BCMA-Exo-Btz. (A) Flow sorting of anti-BCMA+ monocytes. (B) Size distribution of anti-BCMA-Exo and anti-BCMA-Exo-Btz determined by dynamic light scattering. (C) CD81 and CD63 expressions of anti-BCMA-Exo and anti-BCMA-Exo-Btz by western blot. (D) Representative transmission electron microscopy image of anti-BCMA-Exo and anti-BCMA-Exo-Btz. (E-F) The expression of anti-BCMA on the surface of anti-BCMA-Exo-Btz membrane was detected by nano-flow cytometry (E) and colloidal gold immunoelectron microscopy (F). Scale bar, 100 nm. (G) The loading of Btz into exosomes was detected by fourier transform infrared spectrometer. (H) The loading efficiency and encapsulation efficiency of Btz into exosomes.
Characterization of anti-BCMA-Exo-Btz. (A) Flow sorting of anti-BCMA+ monocytes. (B) Size distribution of anti-BCMA-Exo and anti-BCMA-Exo-Btz determined by dynamic light scattering. (C) CD81 and CD63 expressions of anti-BCMA-Exo and anti-BCMA-Exo-Btz by western blot. (D) Representative transmission electron microscopy image of anti-BCMA-Exo and anti-BCMA-Exo-Btz. (E-F) The expression of anti-BCMA on the surface of anti-BCMA-Exo-Btz membrane was detected by nano-flow cytometry (E) and colloidal gold immunoelectron microscopy (F). Scale bar, 100 nm. (G) The loading of Btz into exosomes was detected by fourier transform infrared spectrometer. (H) The loading efficiency and encapsulation efficiency of Btz into exosomes.
Based on the properties of Btz, we used coincubation to load Btz into anti-BCMA-Exo. This loading process did not alter the exosomes' physical attributes, as confirmed through dynamic light scattering, western blotting, and transmission electron microscopy analyses (Figure 1B-D). Meanwhile, the zeta potential of the anti-BCMA-Exo was determined to be –6.25 mV and –7.18 mV, whereas the anti-BCMA-Exo-Btz exhibited a zeta potential of –9.23 mV and –8.12 mV, with no significant alterations observed (Figure 1B). The retention of anti-BCMA on the exosomal surface was evidenced by colloidal gold immunoelectron microscopy (Figure 1F). Furthermore, infrared spectroscopy detected the presence of hydroxyl groups and benzene ring frameworks characteristic of Btz within the anti-BCMA-Exo-Btz (Figure 1G). Loading efficiency and encapsulation efficiency are commonly used parameters to evaluate the efficiency of drug loading into exosomes or other drug delivery systems. The anti-BCMA-Exo-Btz achieved a loading efficiency of 13.24 ± 1.2% and an encapsulation efficiency of 12.05 ± 0.50% when the dosage was 400 ng/mL, as measured by HPLC (Figure 1H). Collectively, these outcomes solidify the confirmation of Btz's effective encapsulation within anti-BCMA-Exo.
Evaluation of anti-BCMA-Exo-Btz for myeloma cell uptake and controlled drug release
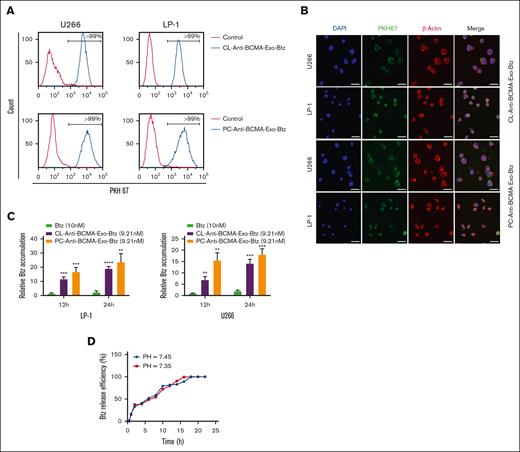
We assessed the BCMA expression levels of ARH-77, U266, and LP-1 cell lines, with 293T cells as a control. U266 and LP-1 cells exhibited significantly higher BCMA expression levels than ARH-77 cells (supplemental Figure 1A). Therefore, U266 and LP-1 cell lines were selected for further experiments. To assess the potential uptake of anti-BCMA-Exo-Btz by myeloma cells, we introduced PKH67–labeled anti-BCMA-Exo-Btz to LP-1 and U266 cells. The myeloma cells' ability to internalize anti-BCMA-Exo-Btz was confirmed via flow cytometry (Figure 2A) and immunofluorescence assays (Figure 2B). Cells expressing high levels of BCMA demonstrated significantly greater uptake of exosomes, underscoring the specificity of BCMA targeting (supplemental Figure 1B-C). For a comprehensive assessment of cellular uptake and drug delivery, we conducted a quantitative comparison of drug accumulation within cells, both for the free drug and the exosome-loaded drug, using HPLC. In LP-1 cells (Figure 2C), at 12 hours, the intracellular Btz concentration was notably higher for CL-Anti-BCMA-Exo-Btz (11.62-fold) and even more so for PC-Anti-BCMA-Exo-Btz (16.82-fold) than the free Btz group (1.36-fold). This trend persisted and intensified after 24 hours for the anti-BCMA-Exo-loaded Btz. Similar results were observed in U266 cells. These findings indicated that Btz, when encapsulated within anti-BCMA-Exo, exhibited enhanced accessibility to MM cells and sustained intracellular accumulation over an extended duration.
The uptake, Btz intracellular accumulation, and release of anti-BCMA-Exo-Btz in vitro. U266 cells and LP-1 cells were treated with anti-BCMA-Exo-Btz. Internalization was measured by flow cytometry (A) and fluorescence microscopy (B). Scale bar, 20 μm. (C) Intracellular Btz accumulation of U266 cells and LP-1 cells treated with free Btz and anti-BCMA-Exo-Btz. (D) In vitro release of Btz from anti-BCMA-Exo-Btz in pH 7.45 and pH 7.35 by HPLC. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. DAPI, 4',6-diamidino-2-phenylindole.
The uptake, Btz intracellular accumulation, and release of anti-BCMA-Exo-Btz in vitro. U266 cells and LP-1 cells were treated with anti-BCMA-Exo-Btz. Internalization was measured by flow cytometry (A) and fluorescence microscopy (B). Scale bar, 20 μm. (C) Intracellular Btz accumulation of U266 cells and LP-1 cells treated with free Btz and anti-BCMA-Exo-Btz. (D) In vitro release of Btz from anti-BCMA-Exo-Btz in pH 7.45 and pH 7.35 by HPLC. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. DAPI, 4',6-diamidino-2-phenylindole.
Assessing drug release from exosomes provides valuable information about drug delivery kinetics and the potential for sustained drug release, which is essential for the development of effective drug delivery systems.14 In our study, we placed Btz-loaded exosomes in a dialysis membrane using filtration techniques to separate the exosomes from the release medium. The quantification of Btz released over time was achieved by periodically collecting and analyzing the release medium. We used pH values of 7.35 and 7.45 to simulate physiological human body pH levels. As depicted in Figure 2D, both pH conditions exhibited no significant influence on the release of Btz. The release pattern of Btz from anti-BCMA-Exo was time dependent. At 6 hours, ∼50% of Btz was released, and this figure approached nearly 100% release after 17 hours. Therefore, the release experiments indicated that the anti-BCMA-Exo-Btz construct developed in this study can achieve a gradual, time-dependent release of Btz.
Identifying the function of monocyte-exosome
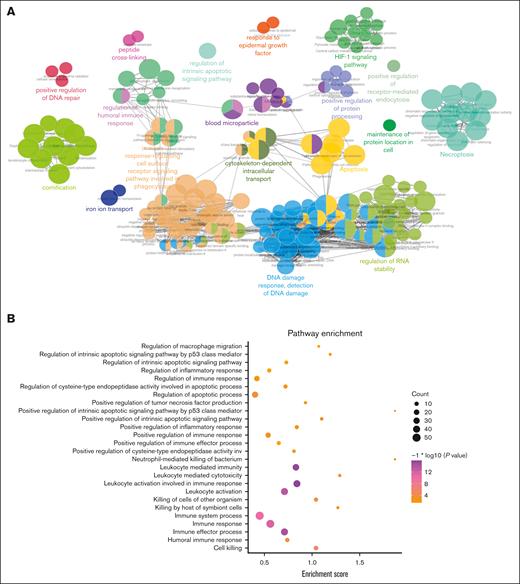
To evaluate the safety of exosomes as drug carriers and their cellular affect, we used a liquid chromatograph mass spectrometer to identify and characterize exosomal proteins, resulting in the identification of 400 proteins. Protein interaction analysis was then conducted using the GeneMANIA database (Figure 3A). The involvement of exosomal proteins in apoptosis and immune response pathways was revealed by Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis (Figure 3B).
Proteomic analysis of monocyte-derived exosomes. (A) The GeneMANIA database was adopted for protein interaction analysis of the protein in monocyte-derived exosomes. (B) The protein in monocyte-derived exosomes were enriched in pathways.
Proteomic analysis of monocyte-derived exosomes. (A) The GeneMANIA database was adopted for protein interaction analysis of the protein in monocyte-derived exosomes. (B) The protein in monocyte-derived exosomes were enriched in pathways.
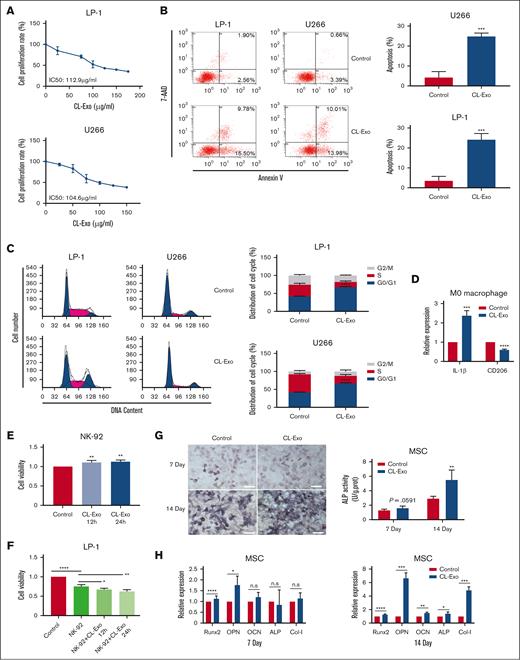
We next proceeded to experimentally validate these essential functions. Firstly, monocyte exosomes inhibited myeloma cell proliferation (Figure 4A). Analysis of 7-AAD/Annexin V-PE double staining indicated an elevated proportion of apoptotic myeloma cells after treatment with monocyte exosomes (Figure 4B). In addition, monocyte exosomes induced G0/G1 cell cycle arrest in myeloma cells (Figure 4C).
The function of monocyte-exosome. (A) Cell viability of MM cells in response to treatment with CL-Exo. (B-C) The CL-Exo treatment at the IC50 concentration induced apoptosis (B) and cell cycle arrest (C) in MM cells. (D) The relative mRNA expression level of IL-1β and CD206 in macrophage after treated with 100 μg/mL CL-Exo. (E) Cell viability of NK-92 cells in response to treatment with 100 μg/mL CL-Exo. (F) The CL-Exo treatment at a concentration of 100 μg/mL enhanced the antitumor activity of NK-92 cells to MM cells. (G) The calcium deposition and ALP activity was determined after 7-day and 14-day treatment of 100 μg/mL CL-Exo in MSCs. Scale bar, 100 μm. (H) The relative mRNA expression levels of Runx2, OPN, OCN, ALP, and Col-l were determined by qPCR after 7-day and 14-day treatment of 100 μg/mL CL-Exo in MSCs. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ALP, alkaline phosphatase ; IL-1β, interleukin-1β; mRNA, messenger RNA; n.s, no significance; qPCR, quantitative real-time PCR.
The function of monocyte-exosome. (A) Cell viability of MM cells in response to treatment with CL-Exo. (B-C) The CL-Exo treatment at the IC50 concentration induced apoptosis (B) and cell cycle arrest (C) in MM cells. (D) The relative mRNA expression level of IL-1β and CD206 in macrophage after treated with 100 μg/mL CL-Exo. (E) Cell viability of NK-92 cells in response to treatment with 100 μg/mL CL-Exo. (F) The CL-Exo treatment at a concentration of 100 μg/mL enhanced the antitumor activity of NK-92 cells to MM cells. (G) The calcium deposition and ALP activity was determined after 7-day and 14-day treatment of 100 μg/mL CL-Exo in MSCs. Scale bar, 100 μm. (H) The relative mRNA expression levels of Runx2, OPN, OCN, ALP, and Col-l were determined by qPCR after 7-day and 14-day treatment of 100 μg/mL CL-Exo in MSCs. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ALP, alkaline phosphatase ; IL-1β, interleukin-1β; mRNA, messenger RNA; n.s, no significance; qPCR, quantitative real-time PCR.
Next, we assessed the impact of monocyte exosomes on macrophage polarization. Monocyte exosomes were found to enhance the expression of the M1 marker interleukin-1β (IL-1β), while concurrently diminishing the expression of the M2 marker CD206 in macrophages (Figure 4D). This shift suggested that monocyte exosomes have the potential to repolarize M2 macrophages into an active M1 phenotype.
Furthermore, we investigated the influence of monocyte exosomes on NK cells. Monocyte exosomes promoted NK-cell proliferation (Figure 4E). To assess the impact of monocyte exosomes on NK-cell cytotoxicity, we cocultured NK cells with luciferase-labeled LP-1 cells. As depicted in Figure 4F, monocyte exosomes significantly enhanced NK-cell cytotoxic activity. Moreover, we established a syngeneic tumor model of MM in immunocompetent mice to examine the impact of CL-Exo on immune cells in vivo (supplemental Figure 1D). After CL-Exo treatment, we analyzed macrophages and NK cells isolated from the bone marrow. Our findings revealed that CL-Exo treatment resulted in elevated levels of CD107a+ NK cells and decreased proportion of M2-type macrophages. The CL-Exo group showed an increase in the M1/M2 ratio compared with the control groups. These results highlight the immunomodulatory role of CL-Exo in MM treatments (supplemental Figure 1E).
MM is characterized by the impaired osteogenic differentiation of MSCs.15 We found that monocyte exosomes led to enhanced calcium deposition in MSCs, as assessed by Von Kossa staining on days 7 and 14 (Figure 4G). Furthermore, the expression of osteogenic differentiation markers, including runt-related transcription factor 2 (Runx2), osteopontin (OPN), osteocalcin (OCN), alkaline phosphatase (ALP), and Collagen type I (Col-I), was increased after treatment with monocyte exosomes in MSCs. Consequently, it could be inferred that monocyte exosomes increased the osteogenic potential of MSCs.
In vitro and in vivo antimyeloma efficacy of anti-BCMA-Exo-Btz
We next evaluated the ability of anti-BCMA-Exo-Btz to inhibit myeloma cell proliferation. Myeloma cell lines LP-1 and U266 were treated with Exo, anti-BCMA-Exo-Btz, and an equivalent dose of free Btz (Figure 5A). Anti-BCMA-Exo–loaded Btz exhibited a more potent myeloma-suppressive effect than free Btz. Exosomes alone also displayed a certain level of myeloma inhibition. Furthermore, we evaluated the antitumor effect of anti-BCMA-Exo-Btz on CD138+ cells derived from patients with MM. The results revealed that CL-Exo and CL-Anti-BCMA-Exo-Btz significantly inhibited the cell viability of CD138+ cells, consistent with the observations in MM cell lines (supplemental Figure 1F).
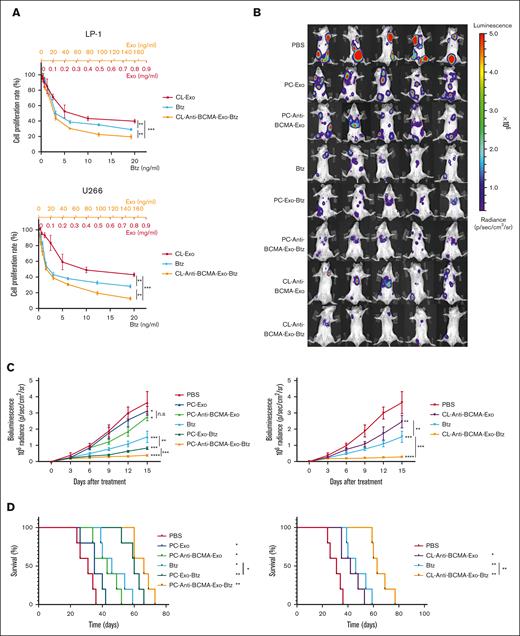
The in vitro and in vivo antitumor effect of anti-BCMA-Exo-Btz in MM. (A) Cell viability of MM cells in response to treatment with free Btz, CL-Exo, and CL-anti-BCMA-Exo-Btz. (B-C) In situ myeloma models were constructed by injecting LP-1 cells in the tail vein of NSG mice and measured once every 3 days after treatment. The fluorescence intensity (B) and tumor growth curve (C) of the mice are shown. (D) Kaplan-Meier analysis revealed describe the survival rate of the mice in experiment. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
The in vitro and in vivo antitumor effect of anti-BCMA-Exo-Btz in MM. (A) Cell viability of MM cells in response to treatment with free Btz, CL-Exo, and CL-anti-BCMA-Exo-Btz. (B-C) In situ myeloma models were constructed by injecting LP-1 cells in the tail vein of NSG mice and measured once every 3 days after treatment. The fluorescence intensity (B) and tumor growth curve (C) of the mice are shown. (D) Kaplan-Meier analysis revealed describe the survival rate of the mice in experiment. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
To evaluate the efficacy of anti-BCMA-Exo-Btz in vivo, orthotopic MM xenograft models were established. Luciferase-labeled myeloma cell line LP-1 was injected via the lateral tail vein in NSG mice. Mice bearing established myelomas were randomly sorted into 8 groups, and the groups were treated as follows: PBS, PC-Exo, PC-Exo-Btz, PC-Anti-BCMA-Exo, PC-Anti-BCMA-Exo-Btz, CL-Anti-BCMA-Exo-Btz, CL-Anti-BCMA-Exo, and an equivalent dose of free Btz. Tumor burden was assessed by serial bioluminescence imaging (Figure 5B).
The fluorescence intensity of tumor burden demonstrated that monocyte exosomes had a certain antitumor activity in MM mice. Loading Btz into exosomes enhanced the antimyeloma effect compared with free Btz treatment. Importantly, anti-BCMA-Exo-Btz exhibited the most robust antimyeloma effect, surpassing the efficacy of free Btz, Exo, Exo-Btz, and anti-BCMA-Exo (Figure 5C), leading to extended overall survival (Figure 5D). This pattern was observed in drug-loaded anti-BCMA-Exo derived from both a human monocyte cell line and human primary monocytes. Furthermore, this pattern was consistent with the in vitro antimyeloma effect. Moreover, the pharmacokinetic studies indicated that anti-BCMA-Exo-Btz exhibited higher concentrations and extended blood circulation time than free Btz (supplemental Figure 1G). These results suggested that anti-BCMA-Exo-Btz had a significant antitumor effect in MM mice.
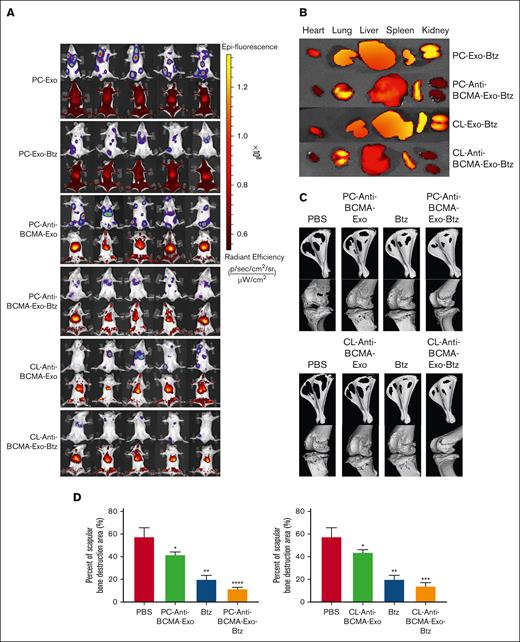
To assess the biodistribution and targeting capability of anti-BCMA-Exo-Btz, exosomes were labeled with VivoTrack DiR (Fluorescent). Fluorescence signals at the tumor site were observed for anti-BCMA-Exo-Btz, whereas Exo-Btz exhibited systemic distribution (Figure 6A). Six hours after injection, organ removal revealed weaker fluorescence signals in the heart and kidney for anti-BCMA-Exo-Btz, confirming its specific targeting of Btz to myeloma in vivo (Figure 6B). These data suggested that anti-BCMA-Exo-Btz specifically targeted Btz to myeloma in vivo.
The distribution of DiR-labeled exosomes in vivo and its effect on bone lesion repair. (A) Biodistribution of DiR-labeled exosomes in LP-1 tumor bearing NSG mice. (B) Biodistribution of DiR-labeled exosomes in the heart, kidney, lung, liver and spleen of the mice. (C) Micro-CT image shown the bone destruction of the scapular. (D) The percentage of scapular bone destruction area in different treatment groups. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
The distribution of DiR-labeled exosomes in vivo and its effect on bone lesion repair. (A) Biodistribution of DiR-labeled exosomes in LP-1 tumor bearing NSG mice. (B) Biodistribution of DiR-labeled exosomes in the heart, kidney, lung, liver and spleen of the mice. (C) Micro-CT image shown the bone destruction of the scapular. (D) The percentage of scapular bone destruction area in different treatment groups. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Because the above results suggested that monocyte exosomes enhanced the osteogenic potential of MSCs, we proceeded to investigate the impact of anti-BCMA-Exo-Btz on myeloma-related bone lesions in mouse models. Micro-CT scanning and 3-dimensional reconstruction were used to analyze bone destruction in the scapula and knee joints. In the PBS treatment group, there was evident and extensive bone destruction, with significant cavities appearing inside the scapula and pathological fractures occurring at the scapular spine. Severe bone damage was also observed around the knee joint, with tibial plateau collapse and pathological fractures around the femoral condyles. The anti-BCMA-Exo group showed a tendency to alleviate bone destruction compared with the PBS control group. The Btz group showed superior treatment outcomes compared with the previous 2 groups. Furthermore, in comparison with the PBS group, anti-BCMA-Exo group, and Btz group, mice in the anti-BCMA-Exo-Btz group displayed the mildest bone destruction. This consistent pattern was observed in 2 groups in which anti-BCMA-Exo-Btz was used; 1 derived from a human monocyte cell line and the other from human primary monocytes (Figure 6C-D).
Safety assessment of anti-BCMA-Exo-Btz
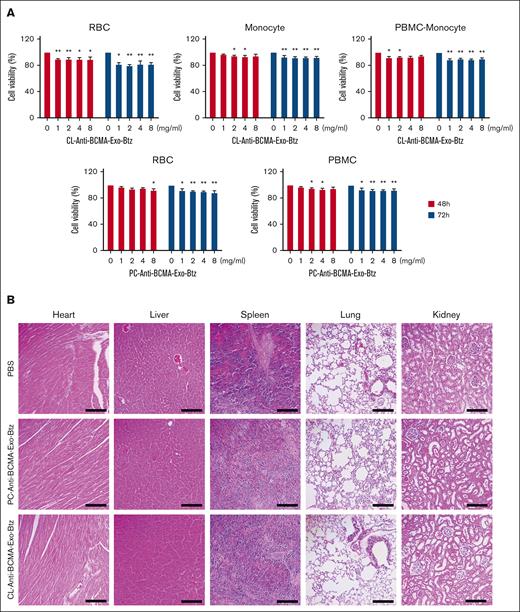
As with any therapeutic, safety assessments are necessary before exosome-based drug delivery systems can be widely used. In our research, PC-anti-BCMA-Exo-Btz and CL-anti-BCMA-Exo-Btz had a slight effect on the cell viability of red blood cells, PBMCs, monocytes, and PBMCs except monocytes (PBMC-monocyte) (Figure 7A). However, the concentrations used in the experiment were significantly higher than those used in treating MM cells. To assess potential toxicity, we conducted histological examinations of vital organs, including the heart, liver, spleen, lung, and kidney tissues. Gratifyingly, no toxicity or significant histological damage was observed in any of these organs in the anti-BCMA-Exo-Btz–treated group (Figure 7B). These results demonstrated that anti-BCMA-Exo-Btz caused no adverse effects on blood cells or vital organs, suggesting its safety for potential clinical applications.
Safety assessment of anti-BCMA-Exo-Btz. (A) Red blood cells (RBCs), PBMCs, monocytes, and PBMCs except monocytes (PBMC-monocyte) were exposed to varying concentrations of 1, 2, 4, and 8 mg/mL anti-BCMA-Exo loaded with Btz for 48 and 72 hours, and the cell viability were detected. (B) H&E staining of the tissue of heart, kidney, lung, liver, and spleen. Scale bar, 200 μm. H&E, hematoxylin and eosin.
Safety assessment of anti-BCMA-Exo-Btz. (A) Red blood cells (RBCs), PBMCs, monocytes, and PBMCs except monocytes (PBMC-monocyte) were exposed to varying concentrations of 1, 2, 4, and 8 mg/mL anti-BCMA-Exo loaded with Btz for 48 and 72 hours, and the cell viability were detected. (B) H&E staining of the tissue of heart, kidney, lung, liver, and spleen. Scale bar, 200 μm. H&E, hematoxylin and eosin.
Discussion
MM, the second most common hematologic malignancy, is characterized by the uncontrolled proliferation of monoclonal plasma cells within the bone marrow, leading to the excessive synthesis of nonfunctional intact immunoglobulins or immunoglobulin chains.16,17 Recent advancements in the treatment of MM have expanded therapeutic options, incorporating both single-agent and combination regimens involving chemotherapy drugs and immunomodulatory agents, significantly improving the prognosis for patients with MM.1,17 Despite these strides, it is important to note that MM remains an incurable condition.
Btz is the first US Food and Drug Administration–approved proteasome inhibitor, holding significance as a pivotal anticancer drug widely used in the treatment of various cancer types, including MM, non–small cell lung cancer, and breast cancer.18-20 The clinical efficacy of Btz is, however, limited by its poor stability, quick clearance, and low selectivity.21,22 In addition, Btz induces dose-limiting side effects such as peripheral neuropathy, gastrointestinal toxicity, and viral infections, reducing the quality of life of patients with MM and potentially leading to dose reduction or treatment interruption.23,24 Therefore, an effective drug delivery method is needed to overcome these obstacles associated with chemotherapy based on Btz.
In this context, a biomimetic nanomaterial-based drug delivery system, such as exosomes, emerges as a highly promising strategy.25 Exosomes, with a diameter ranging from 30 to 150 nm, are small vesicles released by cells, consisting of a bilayer of phospholipid membranes and internal biomolecules.26 The internal cavity of exosomes facilitates the encapsulation of drugs.25 In addition, the membrane provides a protective shield for the drugs they carry, slowing enzymatic hydrolysis or degradation in the circulation. This protective effect enhances drug stability and prolongs its half-life in the blood, extending the duration that the drug remains effective in circulation.27,28 Furthermore, exosomes can be engineered with specific ligands to facilitate selective receptor binding, enabling targeted therapeutic interventions.6 Owing to their circulating stability, low immunogenicity, and modifiable properties, exosomes have emerged as promising candidates for drug delivery vectors.29 Based on the above, we selected monocytes as the maternal cells for the source of exosomes, which exhibit low immunogenicity, making them relatively resistant to active clearance by the immune system. In our study, monocyte-derived exosomes demonstrated remarkable efficacy in reshaping the tumor microenvironment and displaying antitumor effects. Monocyte-derived exosomes enhanced the cytotoxic effect of NK cells on MM cells, induced the polarization of macrophages to the antitumor M1 subtype, and promoted the osteogenic differentiation of MSCs. Moreover, monocyte-derived exosomes induced apoptosis and cell cycle arrest in MM cells. These results collectively indicate that monocyte-derived exosomes are an ideal drug delivery vehicle for MM treatment.
BCMA is reported to be overexpressed in MM cells, making it a prominent target for MM-targeted therapy and immunotherapy.12,30 Thus, anti-BCMA was selected to increase the selectivity of exosomes to MM cells. We developed a targeted drug delivery system for Btz using monocyte-derived exosomes and evaluated its antitumor effect in MM. To specifically deliver Btz-loaded exosomal carriers to MM cells, the monocyte-derived exosome was first modified with anti-BCMA before being incubated with Btz to achieve anti-BCMA-Exo-Btz. Subsequently, the morphology, particle size, encapsulation efficiency, and stability of anti-BCMA-Exo-Btz were characterized. The zeta potential, considered to be a characteristic property of the exosomes, reflects the surface charge of the exosome and its stability in solution.31 The results suggested that the surface morphology, particle size, and the zeta potential of exosomes did not significantly change after modification and Btz encapsulation, indicating that the processes of modification and loading did not have adverse effects on the morphological properties and stability of natural exosomes. In addition, the encapsulation efficiency of Btz achieved 12.05 ± 0.50%, indicating that the exosomes provided an efficient capacity for Btz loading. Assessing drug release from exosomes and drug accumulation within cells provides valuable information about drug delivery kinetics and the potential for sustained drug release. Anti-BCMA-Exo-Btz exhibited enhanced accessibility to MM cells and sustained intracellular accumulation over an extended duration, compared with free Btz. Besides, the release pattern of Btz from anti-BCMA-Exo exhibited a time-dependent pattern, with 50% of Btz released at 6 hours and nearly 100% released after 17 hours.
The in vitro antitumor results demonstrated that anti-BCMA-Exo-Btz effectively inhibited the proliferation of MM cells, exhibiting a superior inhibitory effect compared with free Btz. This enhanced efficacy could be attributed to the effective fusion of exosomes with cells, promoting drug absorption and accumulation and subsequently exerting their therapeutic effects. In cytotoxicity experiments involving normal cells, anti-BCMA-Exo-Btz had a slight effect on the cell viability of red blood cells, PBMCs, monocytes, and PBMCs except monocytes (PBMC-monocyte) derived from healthy donor. However, it is important to note that the concentrations used in the experiment were significantly higher than those used in treating MM cells. This finding suggests that anti-BCMA-Exo-Btz is safe for potential clinical applications.
In view of the significant efficacy of the above in vitro cell efficacy, we proceeded to investigate the in vivo targeted properties and treatment efficacy of anti-BCMA-Exo-Btz. The administration of anti-BCMA-Exo-Btz significantly inhibited tumor growth and prolonged the overall survival of the animals. Importantly, treatment with anti-BCMA-Exo-Btz did not induce significant histological damage to vital organs, including the heart, liver, spleen, lung, and kidney tissues, indicating that anti-BCMA-Exo-Btz exhibits good safety for injection and does not induce toxic or side effects in animals. In vivo imaging results showed that fluorescence signals of VivoTrack DiR–labeled exosomes at the tumor site were observed for anti-BCMA-Exo-Btz, whereas Exo-Btz exhibited systemic distribution. This suggests that anti-BCMA-Exo-Btz reaches the tumor site by actively targeting BCMA in MM cells, facilitating better targeting of Btz to the tumor site and exerting a therapeutic effect.
In brief, our findings demonstrate that anti-BCMA-Exo-Btz actively and selectively targets MM cells, efficiently inhibiting tumor proliferation without inducing toxicity or side effects. We strongly believe that our research provides a reference for the further development of targeted delivery drugs for the treatment of MM.
Acknowledgments
The research leading to these results has received funding from the National Natural Science Foundation of China (grants 82270197 and 82270211); Suzhou City Basic Research Program-Key Clinical Technology Research (grant SKY2023010); the project of State Key Laboratory of Radiation Medicine and Protection, Soochow University (grant GZK12023020); the special project of “Technological innovation” of China National Nuclear Corporation (CNNC) Medical Industry Co Ltd (grant ZHYLYB2021002); and Nantong University Research Fund Clinical Medicine Special Funding Project (grant 2023JY016).
Authorship
Contribution: B.L. and W.Z. designed the research and contributed reagents and other essential materials; S.Y. and Q.L. performed research and wrote the manuscript; C.H., M.B., H.X., X.Z., Y.Z., and Z.W. analyzed data; and M.Z. and Y.C. modified the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bingzong Li, Department of Hematology, The Second Affiliated Hospital of Soochow University, San Xiang Rd 1055, Suzhou, 215006, China; email: lbzwz0907@hotmail.com; and Wenzhuo Zhuang, Department of Cell Biology, School of Biology & Basic Medical Sciences, Suzhou Medical College of Soochow University, Ren Ai Rd 199, Suzhou, 215123, China; email: zhuangwenzhuo@suda.edu.cn.
References
Author notes
S.Y. and Q.L. contributed equally to this study.
The data supporting the findings of this study can be found in the article or are available upon reasonable request from the corresponding authors, Bingzong Li (lbzwz0907@hotmail.com) and Wenzhuo Zhuang (zhuangwenzhuo@suda.edu.cn).
The full-text version of this article contains a data supplement.