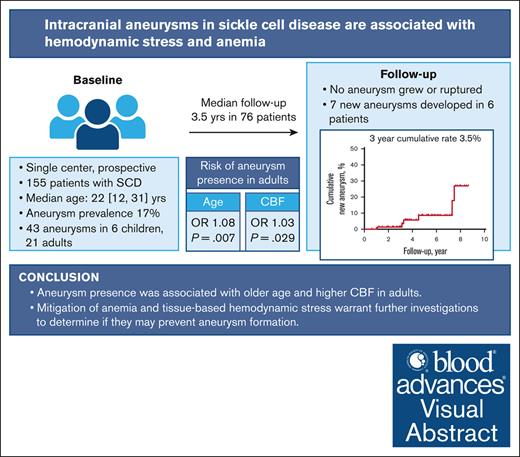
Key Points
Intracranial aneurysms are more prevalent than previously described, affecting 1 in 6 participants in our cohort.
Tissue-based cerebral blood flow may serve as a neuroimaging biomarker of hemodynamic stress underlying aneurysmal formation.
Visual Abstract
Although hemodynamic stress plays a key role in aneurysm formation outside of sickle cell disease (SCD), its role is understudied in patients with SCD. We hypothesized that tissue-based markers of hemodynamic stress are associated with aneurysm presence in a prospective SCD cohort. Children and adults with SCD, with and without aneurysms, underwent longitudinal brain magnetic resonance imaging/magnetic resonance angiography (MRA) to assess cerebral blood flow (CBF) and oxygen extraction fraction (OEF). Baseline characteristics were recorded. In the subgroup of adults, stepwise mixed-effect logistic regression examined clinical variables, CBF, and OEF as predictors of aneurysm presence. Cumulative rates of new aneurysm formation were estimated using Kaplan-Meier analyses. Forty-three aneurysms were found in 27 of 155 patients (17%). Most aneurysms were ≤3 mm and in the intracranial internal carotid artery. On univariate analysis, older age (P = .07), lower hemoglobin (P = .002), higher CBF (P = .03), and higher OEF (P = .02) were associated with aneurysm presence. On multivariable analysis, age and CBF remained independently associated with aneurysm presence. Seventy-six patients (49% of enrollment) received follow-up MRAs (median, 3.5 years). No aneurysm grew or ruptured, however, 7 new aneurysms developed in 6 patients. The 3-year cumulative rate of aneurysm formation was 3.5%. In 155 patients with SCD, 17% had intracranial aneurysms. Three-year aneurysm formation rate was 3.5%, although limited by small longitudinal sample size and short follow-up duration. Aneurysm presence was associated with elevated CBF in adults, as a tissue-based marker of cerebral hemodynamic stress. Future studies may examine the predictive role of CBF in aneurysm development in SCD.
Introduction
Patients with sickle cell disease (SCD) are more likely to develop intracranial aneurysms than the general population. The prevalence rate of aneurysms ranges from 3% in children to 11% in adults with SCD,1-5 in contrast to 0% in children and 3% in adults without SCD.6 Of patients with SCD and aneurysms, 40% to 80% will have multiple aneurysms.1,5,7 The annual risk of aneurysm rupture is unknown given the lack of observational, longitudinal data; however it is a major cause of intracranial hemorrhage in SCD,8 resulting in higher morbidity and mortality rates than ischemic stroke.9
Because of the lack of clear guidance from prospective, observational studies, the management of unruptured intracranial aneurysms in SCD varies widely across institutions.10 Although timely treatment of high-risk aneurysms, which are prone to rupture, is recommended,7 the aneurysmal features that define “high risk” in SCD are understudied and may differ from those of non-SCD aneurysms.11 For example, aneurysms in SCD tend to rupture at a smaller size and younger age than the general population.5,12,13 Additionally, the clinical risk factors and pathophysiology for aneurysm development in SCD are largely unknown. In patients without SCD, clinical risk factors such as age of >50 years, female sex, hypertension, diabetes mellitus, and tobacco use are associated with aneurysm development.6
Blood flow velocity on transcranial Doppler ultrasound and tissue-based cerebral blood flow (CBF) and oxygen extraction fraction (OEF) on advanced magnetic resonance imaging (MRI) are elevated in patients with SCD in response to reduced arterial oxygen content due to anemia. When these compensatory mechanisms are maximized and insufficient to meet oxygen metabolic demand, they are associated with white matter injury and silent cerebral infarcts (SCIs).14,15 Although yet to be examined in relation to intracranial aneurysms, risks such as sustained increase in CBF due to anemia and rigid sickled red blood cells may contribute to turbulent flow and endothelial injury.16 Studies of aneurysm pathogenesis in non-SCD populations suggest mechanical shear stress damages the endothelium, triggering an inflammatory cascade, ultimately leading to vessel wall thinning and aneurysmal formation.17-19 Past work investigating intracranial aneurysms in SCD has been limited to case series and descriptive cohort studies, without standardized imaging protocols, physiological studies, or longitudinal follow-up.5,10
In this prospective, longitudinal imaging study, we quantified aneurysm prevalence, growth, and new aneurysm formation in children and young adults with SCD. Additionally, we evaluated clinical and radiological variables associated with aneurysm presence. We hypothesized that individuals with aneurysms would demonstrate greater levels of hemodynamic stress, as measured by CBF, and tissue hypoxia, as measured by OEF, than those without aneurysms.
Methods
Standard protocol approvals, registrations, and patient consents
The institutional review board at Washington University in St. Louis approved this study, and written informed consent was obtained from all participants aged ≥18 years old or parents/guardians for participants aged <18 years old.
Participants
Children and adults with SCD were prospectively enrolled in a longitudinal MRI study. Patients underwent a brain MRI and time-of-flight (TOF) magnetic resonance angiography (MRA) of the head at baseline and followed-up every 3 years. We also obtained the latest clinical MRAs, if any, as a part of medical care for each participant. Participants with hemoglobin SS (HbSS), HbS β0 thalassemia, HbSC, or HbS β+ thalassemia who were able to tolerate an unsedated MRI were included in the study. Participants may be on hydroxyurea or chronic transfusion therapy or have a history of cerebral vasculopathy. Patients with a history of stem cell transplantation or primary neurological illnesses other than stroke were excluded to specifically measure the neurological impact of SCD on the brain, rather than effects from other neurological injuries.
Laboratory parameters, such as venous Hb, venous cooximetry, capillary gel electrophoresis, and vital sign data were obtained before each scan visit. Clinical diagnoses of hypertension, diabetes mellitus, smoking history, and stroke were extracted from medical records. A diagnosis of hypertension or diabetes was based on charted diagnosis during an outpatient visit to a hematologist or family physician and/or the use of antihypertensive or diabetic medication. A diagnosis of stroke was classified as (1) overt strokes, defined as a clinical history of ischemic or hemorrhagic stroke associated with clinical symptoms; or (2) SCIs, defined as nonovert strokes with cerebral lesions of ≥3 mm in diameter on axial plane of fluid-attenuated inversion recovery (FLAIR) images.20
Imaging protocol and processing
MRI acquisition, segmentation, and coregistration
Participants underwent brain MRI and TOF MRA of the head on a Siemens 3 Tesla MR system (Erlangen, Germany). Standard 3-dimensional magnetization prepared rapid gradient echo T1-weighted echo time/repetition time (TE/TR) = 2.95/1800 ms, TI = 1000 ms, flip angle = 8°, resolution = 1.0 × 1.0 × 1.0 mm), and 2-dimensional T2-weighted FLAIR (TE/TR = 93/9000 ms, TI = 2500 ms, resolution = 1.0 × 0.9 × 5.0 mm for children, 1.0 × 0.9 × 3.0 mm for adults) was acquired. Research 3-dimensional TOF MRA parameters were TE/TR = 3.59/21.0 ms, TD = 0 ms, flip angle = 18°, resolution = 0.6 × 0.6 × 0.7 mm.
Three-dimensional magnetization prepared rapid gradient echo T1 images were skull-stripped and segmented into gray and white matter with Statistical Parametric Mapping version 12 (Wellcome Institute of Neurology, London, United Kingdom).21 Coregistration aligned all images within a scan using the FMRIB Linear Image Registration Tool, with accuracy confirmed by visual inspection.
A board-certified neuroradiologist (M.N.R.), blinded to participant cohort and baseline characteristics, reviewed all MR images and identified SCIs. For each participant with SCIs, a board-certified vascular neurologist (A.L.F.), who was blinded to the participant cohort and baseline characteristics, manually delineated lesions on FLAIR images using the Medical Image Processing Analysis and Visualization (https://mipav.cit.nih.gov/) application. The program automatically thresholds differences in T2 signal intensity regionally to allow the user to accurately capture the area of infarct. Voxels positive for lesions were excluded from whole brain. High-error voxels involving skull-air interface and cerebral spinal fluid were also excluded. This allowed for measurements of whole brain CBF and OEF unaffected by infarcts and artifacts.
CBF and OEF imaging parameters
CBF was measured using a pseudocontinuous arterial spin labeling (ASL; TE/TR = 12/3780 ms, resolution = 3.0 × 3.0 × 5.0 mm, labeling duration = 1500 ms, postlabeling delay [PLD] = 1500 ms for adults) sequence with 2-dimensional echo planar imaging readouts.22 For children, the PLD was changed from 1000 to 1500 ms, with a large subset of participants having obtained both 1000 and 1500 ms. We performed a linear regression analysis using 50 scans that used both PLDs (β = 0.79, standard error = 0.028, P < .001, R2 = 0.94) and imputed CBF with 1500 ms PLD for the 49 scans with a PLD of 1000 ms using the equation CBF1500ms = CBF1000ms × 0.79 + 7.88. Blood T1 was individually measured per participant to improve CBF reproducibility because T1 varies with age and hematocrit.23 Blood T1 was measured in the superior sagittal sinus and estimated using a 4-parameter model as previously described.24
OEF was quantified using a duo-echo asymmetric spin echo (ASE) sequence, measuring tissue deoxyhemoglobin (TE1/TE2/TR = 64.0/99.2/4400 ms, the time intervals (t) between the center of pulse and TE/2 are 0 to 22 ms with an increment of 0.5 ms, repetition = 2, resolution = 1.72 × 1.72 × 3 mm3, matrix size = 128 × 128, 26 slices).25 Partial volume correction was performed for both pseudocontinuous ASL and ASE.26
Aneurysm definition
Two board-certified neuroradiologists (M.N.R. and M.S.P.), blinded to baseline characteristics and participant cohort, independently reviewed the TOF MR angiograms. Of the participants with multiple MR angiograms, the MR angiograms were reviewed sequentially to classify aneurysms as unchanged, grown, or de novo compared with previous imaging. Aneurysm presence, diameter, and location were recorded posterior circulation location included vertebral artery, basilar artery, cerebellar arteries, and posterior cerebral artery.6 Disagreement on aneurysm presence occurred in 2 of 155 patients. A consensus was reached between the neuroradiologists after jointly reviewing the studies. Inconclusive cases of aneurysm vs infundibulum occurred in 2 children and were included in the “without aneurysm” cohort. Any stenosis or occlusion of a major cerebral artery was also measured and documented.27
Statistical analysis
Data are presented as median (interquartile range [IQR]). Baseline clinical characteristics, laboratory parameters, and imaging parameters were compared between participants with and without aneurysms. Mann-Whitney U and Fisher exact test were used for continuous and categorical variables, respectively. Bivariate correlations were performed with Spearman correlation.
Given the known relationship between age and CBF28 (Rho = −0.42, P < .001, in this cohort) and our overall small sample size of children with aneurysms (6 children vs 21 adults), we restricted the CBF and OEF analysis to adults (aged ≥18 years, supplemental Table 1 for baseline characteristics). To evaluate the effects of CBF and OEF on aneurysm presence, whole brain CBF and OEF from baseline and follow-up MRIs were included in a stepwise mixed-effects logistic regression model via generalized estimating equations. Repeated measurements within individuals, from the contribution of repeated scans over time, were included as a random effect. We used stepwise entry for variables with P value <.15, and P value <.05 to be retained in the final model. Collinearity between variables was assessed using a variance inflation factor of ≤2. Because of collinearity between OEF and Hb, we used 2 multivariable models to determine predictors of aneurysm presence. Model 1 included Hb as an independent variable, whereas model 2 included OEF as an independent variable. Odds ratio (OR) with 95% confidence intervals (CIs) were reported. The fit of the multivariable models was confirmed by assessing the distribution of the regression residuals.
On longitudinal analysis of aneurysm progression, we examined the subgroup of participants with ≥2 MRAs. In addition to research MRAs, the latest clinical MRAs, if any existed, were also included. Follow-up periods were calculated from enrollment until the time of a new aneurysm detected on MRA. The Kaplan-Meier product-limit method was used to estimate cumulative rates of new aneurysm formation. All statistical analyses were conducted using SAS software, version 9.4 of the SAS System for Windows (SAS Institute Inc, Cary, NC).
Results
Characteristics of participants with MRA-defined aneurysms
A total of 155 patients with SCD were enrolled, with a median age of 22 years (IQR, 12-31). Baseline characteristics of patients with and without aneurysms are shown in Table 1. At baseline, 27 patients (17%) had ≥1 aneurysms. Compared with patients without aneurysms, those with aneurysms were older (30 vs 21 years old, P < .003). There was no sex difference between patients with and without aneurysms. The prevalence of patients taking hydroxyurea or on chronic exchange transfusion at the time of their baseline scan was not different between the 2 groups. Likewise, the diagnoses of hypertension, type 2 diabetes, or active smoking were infrequent and similar between the 2 groups.
Baseline characteristics by patients with and without aneurysms
| . | Patients without aneurysm (n = 128) . | Patients with aneurysm (n = 27) . | P value . |
|---|---|---|---|
| Age, y | 21 (11, 28) | 30 (20, 37) | .003 |
| Female, n (%) | 70 (55%) | 18 (67%) | .25 |
| SCD type | .37 | ||
| HbS β+ thalassemia | 3 (2%) | 1 (4%) | |
| HbS β0 thalassemia | 9 (7%) | 4 (15%) | |
| HbSC | 15 (12%) | 1 (4%) | |
| HbSS | 101 (79%) | 22 (78%) | |
| Hb, g/dL | 8.9 (7.9, 10) | 8.3 (7.5, 9.3) | .07 |
| HbA, % | 0 (0, 29.3) | 0 (0, 28.3) | .96 |
| HbS, % | 71 (51, 83) | 77 (51, 83) | .32 |
| Hydroxyurea, n (%) | 49 (37%) | 11 (41%) | .83 |
| Exchange transfusion, n (%) | 31 (24%) | 4 (15%) | .29 |
| Hypertension | 14 (11%) | 3 (11%) | 1.00 |
| Diabetes mellitus | 4 (3%) | 0 (0%) | .35 |
| Current smoker∗ | 11 (9%) | 3 (16%) | .40 |
| SCI, n (%) | 77 (60%) | 17 (63%) | .79 |
| Overt stroke, n (%)† | 20 (16%) | 4 (15%) | .91 |
| Concurrent cerebral arterial stenosis of ≥50% or occlusion | 16 (13%) | 6 (22%) | .24 |
| Number of aneurysms per patient | - | ||
| 1 | 20 (74%) | ||
| 2 | 4 (15%) | ||
| ≥3 | 3 (11%) | ||
| By aneurysm (n = 43) | - | ||
| Size (mm), n (%) | |||
| <2 | 3 (7%) | ||
| 2-3 | 32 (74%) | ||
| 4-5 | 5 (12%) | ||
| ≥6 | 3 (7%) | ||
| Location, n (%) | |||
| Internal carotid artery | 17 (40%) | ||
| Ophthalmic artery | 6 (14%) | ||
| Anterior communicating artery | 5 (12%) | ||
| Posterior communicating artery | 4 (9%) | ||
| Posterior cerebral artery | 3 (7%) | ||
| Basilar artery | 3 (7%) | ||
| Middle cerebral artery | 2 (5%) | ||
| Vertebral artery | 2 (5%) | ||
| Anterior cerebral artery | 1 (2%) | ||
| Laterality, n (%) | |||
| Left hemisphere | 21 (50%) | ||
| Right hemisphere | 14 (32%) | ||
| Midline | 8 (18%) |
| . | Patients without aneurysm (n = 128) . | Patients with aneurysm (n = 27) . | P value . |
|---|---|---|---|
| Age, y | 21 (11, 28) | 30 (20, 37) | .003 |
| Female, n (%) | 70 (55%) | 18 (67%) | .25 |
| SCD type | .37 | ||
| HbS β+ thalassemia | 3 (2%) | 1 (4%) | |
| HbS β0 thalassemia | 9 (7%) | 4 (15%) | |
| HbSC | 15 (12%) | 1 (4%) | |
| HbSS | 101 (79%) | 22 (78%) | |
| Hb, g/dL | 8.9 (7.9, 10) | 8.3 (7.5, 9.3) | .07 |
| HbA, % | 0 (0, 29.3) | 0 (0, 28.3) | .96 |
| HbS, % | 71 (51, 83) | 77 (51, 83) | .32 |
| Hydroxyurea, n (%) | 49 (37%) | 11 (41%) | .83 |
| Exchange transfusion, n (%) | 31 (24%) | 4 (15%) | .29 |
| Hypertension | 14 (11%) | 3 (11%) | 1.00 |
| Diabetes mellitus | 4 (3%) | 0 (0%) | .35 |
| Current smoker∗ | 11 (9%) | 3 (16%) | .40 |
| SCI, n (%) | 77 (60%) | 17 (63%) | .79 |
| Overt stroke, n (%)† | 20 (16%) | 4 (15%) | .91 |
| Concurrent cerebral arterial stenosis of ≥50% or occlusion | 16 (13%) | 6 (22%) | .24 |
| Number of aneurysms per patient | - | ||
| 1 | 20 (74%) | ||
| 2 | 4 (15%) | ||
| ≥3 | 3 (11%) | ||
| By aneurysm (n = 43) | - | ||
| Size (mm), n (%) | |||
| <2 | 3 (7%) | ||
| 2-3 | 32 (74%) | ||
| 4-5 | 5 (12%) | ||
| ≥6 | 3 (7%) | ||
| Location, n (%) | |||
| Internal carotid artery | 17 (40%) | ||
| Ophthalmic artery | 6 (14%) | ||
| Anterior communicating artery | 5 (12%) | ||
| Posterior communicating artery | 4 (9%) | ||
| Posterior cerebral artery | 3 (7%) | ||
| Basilar artery | 3 (7%) | ||
| Middle cerebral artery | 2 (5%) | ||
| Vertebral artery | 2 (5%) | ||
| Anterior cerebral artery | 1 (2%) | ||
| Laterality, n (%) | |||
| Left hemisphere | 21 (50%) | ||
| Right hemisphere | 14 (32%) | ||
| Midline | 8 (18%) |
Smoking history missing in 11 and 8 patients without and with aneurysms, respectively.
Before study enrollment, 2 patients without aneurysms had a history of intracranial hemorrhage due to arteriovenous malformation and traumatic brain injury. All other patients with overt strokes experienced ischemic strokes.
Characteristics of intracranial aneurysms in SCD
A total of 43 aneurysms were found in 27 patients at baseline MRA. Multiple aneurysms per patient were found in 25% of the cases. Aneurysmal size ranged from 1 to 9 mm, however the majority were ≤3 mm (n = 35, 81%). Aneurysms were predominately found in the anterior (n = 35, 81%) vs posterior (n = 8, 19%) circulation (binomial test, P < .001), with the intracranial portion of the internal carotid artery as the most common location (40%), followed by the ophthalmic artery (14%), and anterior communicating artery (12%). There was no left vs right hemispheric preference. The size of the largest aneurysm of each patient positively correlated with age (Rho = 0.39, P = .04), suggesting the growth of aneurysms with age. The comparison between children and adults with aneurysms is included in supplemental Table 2.
Hemodynamic stress, tissue hypoxia, and anemia in association with aneurysms in adults with SCD
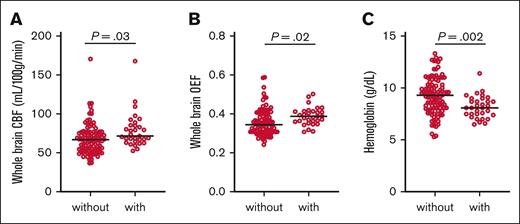
Elevated CBF and OEF may allow for tissue-based markers of elevated flow velocity and tissue hypoxia, respectively, which may potentiate endothelial injury, vessel wall remodeling, and aneurysm formation. On univariate mixed-effect regression (Table 2; Figure 1), we used MRIs from all time points (N = 129 MRIs with CBF and OEF). Adults with aneurysms were nonsignificantly older (P = .07), more anemic (P = .002), with higher CBF (P = .03) and higher OEF (P = .02) than patients without aneurysms. On stepwise multivariable regression, CBF and age were independently associated with aneurysm presence (CBF: OR, 1.03; 95% CI, 1.00-1.06; P = .029; age: OR, 1.08; 95% CI, 0.02-1.13; P = .007), whereas OEF and Hb were not retained in association with aneurysm presence.
Univariate and stepwise multivariable mixed-effect logistic regression of clinical and radiological predictors of aneurysm presence in adults, adjusting for repeated measurements
| Predictors . | Univariate . | Multivariable, model 1∗ . | Multivariable, model 2∗ . | |||
|---|---|---|---|---|---|---|
| OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | |
| CBF | 1.02 (1.00-1.05) | .033 | 1.03 (1.00-1.06) | .029 | 1.03 (1.00-1.06) | .029 |
| OEF | 2.27 (1.14-4.51) | .019 | - | - | NS | NS |
| Hb | 0.65 (0.50-0.86) | .002 | NS | NS | - | - |
| Age | 1.05 (0.99-1.10) | .071 | 1.08 (1.02-1.13) | .007 | 1.08 (1.02-1.13) | .007 |
| Predictors . | Univariate . | Multivariable, model 1∗ . | Multivariable, model 2∗ . | |||
|---|---|---|---|---|---|---|
| OR (95% CI) . | P . | OR (95% CI) . | P . | OR (95% CI) . | P . | |
| CBF | 1.02 (1.00-1.05) | .033 | 1.03 (1.00-1.06) | .029 | 1.03 (1.00-1.06) | .029 |
| OEF | 2.27 (1.14-4.51) | .019 | - | - | NS | NS |
| Hb | 0.65 (0.50-0.86) | .002 | NS | NS | - | - |
| Age | 1.05 (0.99-1.10) | .071 | 1.08 (1.02-1.13) | .007 | 1.08 (1.02-1.13) | .007 |
NS, variable not significant with P > .15 and removed from the final model.
Model 1: independent variables included CBF, Hb, and age. Model 2: independent variables included CBF, OEF, and age.
Hemodynamic stress, tissue hypoxia, and anemia in adult participants with and without aneurysms. We used MRIs from all time points (N = 129 MRIs with CBF and OEF). Adults with aneurysms exhibited higher CBF (A), higher OEF (B), and lower Hb (C) than patients without aneurysms. Black lines denote group median. Raw P values from univariate mixed-effect regression are shown.
Hemodynamic stress, tissue hypoxia, and anemia in adult participants with and without aneurysms. We used MRIs from all time points (N = 129 MRIs with CBF and OEF). Adults with aneurysms exhibited higher CBF (A), higher OEF (B), and lower Hb (C) than patients without aneurysms. Black lines denote group median. Raw P values from univariate mixed-effect regression are shown.
Longitudinal follow-up: prevalence of de novo aneurysm formation
Of 27 children and adults with aneurysms at baseline, 2 patients were referred to neurosurgery for assessment of aneurysms. Two additional patients underwent revascularization surgery for concurrent moyamoya syndrome. All other patients were managed conservatively.
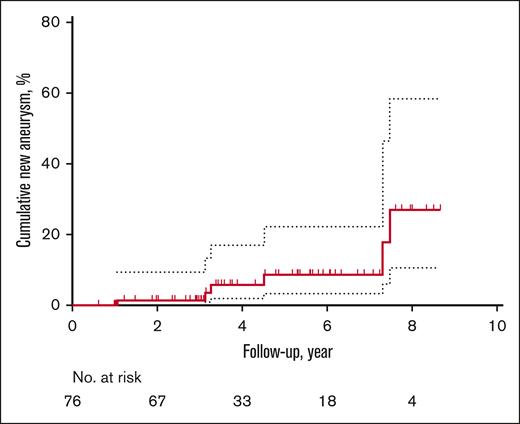
Of the entire cohort including those without aneurysms, 76 patients (49%) received follow-up MRAs, including 35 clinical MRAs. Given that less than half of the study population received follow-up and a high proportion of the follow-up was guided by the clinicians, the results have inherent biases. Fifty-one and 25 patients had 1 and 2 follow-up MRAs, respectively. On sensitivity analysis, compared with patients without follow-up imaging, patients with serial MRAs were younger (aged 12 vs 26 years, P < .001); however, there was no difference in the proportion of patients with aneurysm at baseline (without serial MRA, 14 [18%] vs with serial MRA, 13 [17%], P = .92) or in sex (female without serial MRA, 46 [58%] vs with serial MRA 42 [55%], P = .71). The median duration of follow-up was 3.5 years (IQR, 3.0-5.8), with a total of 328.2 person-years of follow-up. Although neither preexisting aneurysm growth nor rupture occurred, surveillance scans demonstrated the development of de novo aneurysms in 6 (8%) patients (Table 3). The 1- and 3-year cumulative rates of new aneurysm formation were 1.4% (95% CI, 0-4) and 3.5% (95% CI, 0-8), respectively (Figure 2). Four patients who developed de novo aneurysms had no aneurysms at baseline; notably, they were the youngest 4 of 6 patients. The oldest 2 patients with prior aneurysms developed new aneurysms in a location nonadjacent to the original aneurysms.
Demographics, aneurysm description, and management of patients with de novo aneurysms
| Age (y), sex, genotype . | Age (y) on follow-up . | Location, size of aneurysm on baseline MRA . | Location, size of aneurysm on follow-up MRA . | Time between MRAs, y . | Management . |
|---|---|---|---|---|---|
| 10, F, HbSS | 18 | None | Left M2, 2 mm | 7.3 | Conservative |
| 12, F, HbSS | 17 | None | R cavernous ICA tiny saccular aneurysm | 4.5 | Conservative |
| 15, M, HbSS | 18 | None | Left Pcom, 1 mm | 3.3 | Conservative |
| 18, F, HbSS | 20 | None | Right P1, 1.5 mm | 1.1 | Conservative |
| 26, F, HbSS | 33 | Right cavernous ICA 2 mm | Right cavernous ICA, 2 mm; Right A2, 2 mm | 7.5 | Conservative |
| 37, F, HbSβ0thal | 40 | Right cavernous ICA, 9 mm; | Right cavernous ICA 9 mm; basilar tip, 4 mm; left P1, 2 mm | 3.1 | Digital subtraction angiography was recommended but declined by patient and family |
| Age (y), sex, genotype . | Age (y) on follow-up . | Location, size of aneurysm on baseline MRA . | Location, size of aneurysm on follow-up MRA . | Time between MRAs, y . | Management . |
|---|---|---|---|---|---|
| 10, F, HbSS | 18 | None | Left M2, 2 mm | 7.3 | Conservative |
| 12, F, HbSS | 17 | None | R cavernous ICA tiny saccular aneurysm | 4.5 | Conservative |
| 15, M, HbSS | 18 | None | Left Pcom, 1 mm | 3.3 | Conservative |
| 18, F, HbSS | 20 | None | Right P1, 1.5 mm | 1.1 | Conservative |
| 26, F, HbSS | 33 | Right cavernous ICA 2 mm | Right cavernous ICA, 2 mm; Right A2, 2 mm | 7.5 | Conservative |
| 37, F, HbSβ0thal | 40 | Right cavernous ICA, 9 mm; | Right cavernous ICA 9 mm; basilar tip, 4 mm; left P1, 2 mm | 3.1 | Digital subtraction angiography was recommended but declined by patient and family |
A2, second segment of anterior cerebral artery; ICA, internal carotid artery; M2, second segment of middle cerebral artery; P1, first segment of posterior cerebral artery; Pcom, posterior communicating artery.
Unadjusted Kaplan-Meier event curve for de novo aneurysm formation from the time of enrollment to follow-up. Dotted lines indicate 95% CI.
Unadjusted Kaplan-Meier event curve for de novo aneurysm formation from the time of enrollment to follow-up. Dotted lines indicate 95% CI.
Discussion
Intracranial aneurysms affected 1 in 6 participants in our cohort of children and young adults with SCD. In univariate analysis, we found older age, lower Hb, elevated CBF, and elevated OEF in adults with aneurysms. In multivariable analysis, only older age and elevated CBF were retained. These findings suggest that aging, anemia, hemodynamic stress, and tissue hypoxia may be associated with progressive vessel wall injury, remodeling, and ultimately aneurysm formation. In longitudinal analysis over a short follow-up period of 3.5 years, we observed new aneurysms in 8% of those with follow-up MRA: 4 children and 2 adults.
Our study highlights several potential mechanisms underlying aneurysm formation in SCD. First, elevated CBF in patients with aneurysms aligns with the known risk of hemodynamic stress in non-SCD–related aneurysm development.17,18 Although we measured tissue-level CBF of the whole brain, non-SCD studies have measured wall shear stress of the parent vessel, historically modeled from computational fluid dynamics (CFD) by incorporating participant-specific artery and aneurysm morphology,19 and, more recently, calculated from phase-contrast MRI by measuring artery-specific flow velocity.29 In SCD, although we did not find studies on shear stress and aneurysm, elevated shear stress on CFD was reported in the distal internal carotid artery and basilar artery in patients with SCD and without aneurysms.30 Interestingly, these vessel segments are more frequently affected by aneurysms in SCD than in the general population.31 Second, our findings of anemia and elevated OEF in patients with aneurysms on univariate analysis suggest hypoxemia may represent an additional risk factor. In a non-SCD animal model of intracranial aneurysms, a hypoxic microenvironment was found within the intracranial arterial wall, and hypoxia-induced inflammation was associated with aneurysm rupture.32 Alternatively, anemia may reflect a hemolytic state; however, we did not measure markers of hemolysis. We hypothesize that the release of proinflammatory cell content through hemolysis triggers thrombotic and inflammatory cascades, further injuring endothelial cells and weakening the arterial wall.33 Indeed, treatments with anti-inflammatory therapies such as matrix metalloproteinase-9 and tumor necrosis factor-α inhibitors have shown promise in preclinical models of non-SCD–related aneurysms.34 Overall, persistent hyperemia and hypoxemia combined with systemic inflammation may explain why aneurysms form in SCD at an earlier age and more frequently compared with the general population. SCD therapeutics, such as voxelotor and crizanlizumab, aiming to target hemolytic anemia and leukocyte adhesion, may be explored in the medical prevention of aneurysms.35,36
In our study, aneurysm presence was more associated with CBF than Hb or OEF. This suggests that hemodynamic stress may play a stronger pathogenic role in aneurysm development in SCD than anemia or tissue hypoxia. In SCD, CBF elevation is the result of anemia through increased cardiac output37 and vasodilation through cerebral autoregulation.38 Although not examined in our study, there may be additional regional elevation in flow velocity from mild ipsilateral stenosis39 and increase in flow rate from contralateral flow-limiting stenosis and occlusion40 to cause aneurysm formation. Supporting this theory, we observed a nonsignificantly higher proportion of patients with concurrent cerebral arterial stenosis and occlusion among those with aneurysms.
Our metric of “hemodynamic stress” or tissue-based CBF differs from the more commonly used metrics of hemodynamic stress, such as wall shear stress and flow velocity, in non-SCD aneurysm literature.17,18,41 In non-SCD research, although elevated blood flow of the parent artery was associated with aneurysm presence18 and size,42 none studied tissue-based CBF. In SCD aneurysm literature, we did not find neuroimaging studies on shear stress. Only 1 study reported no association between transcranial Doppler flow velocity and aneurysm in 5 children with SCD.1 The aforementioned study, however, did not restrict flow velocity comparison to parent vessels. In our study, we used CBF as a surrogate marker for flow velocity. Although tissue-based CBF does not represent true velocity, the 2 terms are proportional to each other.43 Although CFD analysis provides localized hemodynamic metrics to the parent artery and the aneurysm dome, its computational complexity makes it unsuitable for large-scale studies on aneurysm pathogenesis and treatment.19 The advantage of using tissue-based CBF is that voxel-based measures may better reflect blood flow within brain tissue, potentially influencing vascular remodeling through thrombo-inflammatory pathways within capillary-tissue beds. If validated against more commonly used metrics of hemodynamic stress, CBF may serve as a noninvasive neuroimaging biomarker of hemodynamic stress underlying aneurysmal formation and risk of rupture in future studies.
The prevalence of aneurysms in our cohort (17%) was higher than previously reported. Prevalence rates estimated from retrospective SCD clinical databases ranged from 3% in children to 11% in adults.1-5 The variation in clinical practice on ordering screening MRAs in asymptomatic individuals may have led to an underestimation of aneurysm prevalence in the prior reports. Additionally, we reported 1.4% and 3.5% cumulative rates of new aneurysm formation at 1- and 3-years, respectively, similar to an incidence rate of 1290.3 per 100 000 patient-years reported from a pooled analysis of retrospective cohorts.4 Our reported cumulative rate may also be an underestimation because adults were more likely to have aneurysms but were less likely to have follow-up imaging in our study. Currently, the guideline recommends screening MRIs for children with SCD aged >6 years and MRA only for children on chronic transfusions who wish to discontinue therapy.44 Given aneurysms may affect up to 1 of 6 patients with SCD and that its rupture carries a high mortality rate,8 there may be a need for routine screening MRAs, in addition to MRIs, in all individuals with SCD.
Optimal management of unruptured intracranial aneurysms in patients with SCD has yet to be established. In terms of surgical or endovascular treatment, based on the observation made through retrospective case series that ruptured aneurysms in SCD tend to be smaller than those in the non-SCD population,5,12,13 some have proposed setting a smaller size threshold for treatment than the 7-mm cutoff for the general population5,11; however, no definitive guideline on treatment size threshold has been made. The smaller aneurysms (<3 mm) are often monitored via imaging1,7; however, the modality (MRA, computed tomography angiogram, or digital subtraction angiography) and the frequency of follow-up imaging are undefined. Additionally, 20% to 30% of aneurysms in SCD occur in the posterior circulation, compared with 5% to 15% in the non-SCD population.6,10,45 Because the posterior circulation aneurysms carry a higher risk of rupture, the optimal management strategy for these aneurysms in patients with SCD needs further study.46 Regarding medical management, unlike other studies in non-SCD populations,6 we found no association between hypertension, diabetes, cigarette use, and aneurysm presence. Because individuals with SCD may develop these vascular risk factors over time,47,48 primary prevention and timely treatment of hypertension and diabetes, along with smoking abstinences remains crucial. Lastly, the effect of disease-modifying therapy on aneurysm is understudied. Our study did not demonstrate any association between disease-modifying therapy and aneurysm presence and was underpowered to assess longitudinal treatment effects. Preliminary data from a separate study of 87 adults who underwent bone marrow transplant reported no aneurysm growth or new formations over 3.3 years.49 Large prospective studies over decades are needed to understand aneurysm development and management in SCD.
In contrast to the non-SCD population,6 we did not find female sex as a risk factor of aneurysm occurrence in patients with SCD. In the general population, the increased risk in females has been attributed to potential factors such as higher vessel wall stress because of smaller vessel diameter and the diminishing protective effect of estrogen after menopause.50 In SCD, however, in which aneurysms tend to affect younger patients10 and male sex is associated with more severe disease complications,51 there is a clear need to examine sex differences in cerebral aneurysms in SCD.
Study limitations include, first, the cross-sectional associations from this study do not allow interpretation of causation. Second, by using whole-brain CBF and OEF, we did not restrict CBF and OEF measurements to voxels near individual aneurysms. Because ASL and ASE are tissue-based measurements, CBF and OEF elevation likely indicate global hyperperfusion and tissue hypoxia, whereas direct measures of local shear stress and flow velocity adjacent to the parent vessel wall in which the aneurysm arises may better reflect aneurysmal physiology. Alternative novel approaches such as time-resolved phase-contrast MRI may be used in future studies to assess shear stress magnitude and turbulence flow pattern within individual aneurysms and nearby inlet/outlet arterial segments.29 Lastly, our cohort had a median follow-up of 3.5 years. Despite the high rate of aneurysms, none of the existing aneurysms enlarged or ruptured on follow-up imaging. Similarly, because of variability in enrollment age, follow-up periods, clinician-guided follow-up in a subset, and the limited number of new aneurysms on follow-up, the longitudinal results have inherent biases and we were unable to assess clinical or imaging predictors of de novo aneurysm formation. We will continue to follow-up our cohort to better understand aneurysm development and risk of rupture over time.
Conclusion
The prevalence of intracranial aneurysms in SCD is higher than previously reported. Aneurysm presence was associated with higher CBF and older age in adults. Mitigation of anemia and tissue-based hemodynamic stress warrant further investigations to determine whether they may prevent aneurysm formation, growth, and rupture.
Acknowledgments
This research was supported by the National Center for Advancing Translational Sciences (5KL2TR002346 [Y.W.]), the National Institute of Neurological Disorders and Stroke (K23NS099472 [K.P.G.], RF1NS116565 [H.A. and A.L.F.], and R21NS127425 [H.A.]), and the National Institutes of Health, National Heart, Lung, and Blood Institute (K23HL136904 and R01HL157188 [M.E.F.], and R01HL129241 [H.A. and A.L.F.]).
Authorship
Contribution: Y.W., J.G., and A.L.F. designed the research, wrote the manuscript, and analyzed data; S.F., A.M., J.B.L., C.Y., and Y.C. performed research and analyzed data; M.N.R., M.S.P., and R.C. performed research; K.P.G., A.A.K., and J.-M.L. designed the research and reviewed the manuscript; M.E.F. and M.L.H. performed research and reviewed the manuscript; and H.A. designed research, analyzed data, and reviewed the manuscript.
Conflict-of-interest disclosure: M.E.F. reports equity ownership in Proclara Biosciences; received compensation from Global Blood Therapeutics and Pfizer Inc for consultant services; and received research funding from Pfizer Inc. M.L.H. reports compensation from Pfizer Inc, bluebird bio, and Novo Nordisk for consulting services, and spouse employment at Pfizer. H.A. reports compensation from Pfizer Inc for consultant services. The remaining authors declare no competing financial interests.
Correspondence: Yan Wang, Department of Neurology, Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8111, St. Louis, MO 63110; email: ywange@wustl.edu.
References
Author notes
Anonymized data not published within this article will be made available by request, from corresponding author Yan Wang (ywange@wustl.edu).
The full-text version of this article contains a data supplement.