Key Points
LMWH prevents NLRP3 inflammasome activation and placental thromboinflammation in a PE-like mouse model.
LMWH activates HBEGF-AKT pathway in trophoblasts and human placental explants, preventing inflammasome activation independent of platelets.
Visual Abstract
Low molecular weight heparins (LMWH) are used to prevent or treat thromboembolic events during pregnancy. Although studies suggest an overall protective effect of LMWH in preeclampsia (PE), their use in PE remains controversial. LMWH may convey beneficial effects in PE independent of their anticoagulant activity, possibly by inhibiting inflammation. Here, we evaluated whether LMWH inhibit placental thromboinflammation and trophoblast NLRP3 inflammasome activation. Using an established procoagulant extracellular vesicle–induced and platelet-dependent PE-like mouse model, we show that LMWH reduces pregnancy loss and trophoblast inflammasome activation, restores altered trophoblast differentiation, and improves trophoblast proliferation in vivo and in vitro. Moreover, LMWH inhibits platelet-independent trophoblast NLRP3 (NLR family pyrin domain containing 3) inflammasome activation. Mechanistically, LMWH activates via heparin-binding epidermal growth factor (HBEGF) signaling the PI3-kinase-AKT pathway in trophoblasts, thus preventing inflammasome activation. In human PE placental explants, inflammasome activation and PI3-kinase-AKT signaling events were reduced with LMWH treatment compared with those without LMWH treatment. Thus, LMWH inhibits sterile inflammation via the HBEGF signaling pathway in trophoblasts and ameliorates PE-associated complications. These findings suggest that drugs targeting the inflammasome may be evaluated in PE and identify a signaling mechanism through which LMWH ameliorates PE, thus providing a rationale for the use of LMWH in PE.
Introduction
Gestational vascular diseases (GVDs) such as preeclampsia (PE) are a major cause of feto-maternal morbidity and mortality.1,2 We have meager mechanistic knowledge and hence lack specific therapeutic approaches. Hemostasis regulators locally expressed within placenta control the hemostatic balance in the placenta, conveying both physiological and pathological effects during pregnancy.3 Inherited or acquired thrombophilia are a risk factor for placenta-mediated pregnancy complications such as PE, spontaneous abortions, recurrent miscarriages, and preterm birth, but the underlying mechanism remains poorly defined.
Platelets contribute to placenta-associated GVDs in humans and mice.4,5 Beyond their function in hemostasis, platelets modulate innate immune response, causing inflammation.5,6 In this regard, we have previously shown that procoagulant extracellular vesicles (EVs), known to be associated with GVDs, activate platelets, causing the release of ATP (Adenosine triphosphate) and DAMPs (danger-associated molecular pattern). Excess ATP causes inflammasome activation in trophoblast cells via purinergic signaling, resulting in a PE-like phenotype in mice.5,7,8 These studies identified a possible druggable pathway to restrict placental thromboinflammation in GVDs.
Low molecular weight heparins (LMWHs) are commonly used therapeutics for preventing thromboembolic events and associated placental dysfunction during pregnancy.9,10 They do not cross the placenta and have been frequently proposed to convey a therapeutic benefit in placental dysfunction. However, although studies suggest an overall protective effect,11,12 the role of anticoagulants such as heparins in PE remains controversial. Beyond their anticoagulant activity, LMWH-mediated effects include their angiogenic properties, improved endothelial functions, and immunomodulatory effects. Despite several proposed mechanisms,13-15 how LMWHs prevent PE-associated complications remain unknown. Therefore, understanding these mechanisms will provide new insights that may be used to evaluate the efficacy of LMWHs. Here, we use an established PE-like mouse model and human placental explants to evaluate whether LMWH targets placental thromboinflammation in PE.
Methods
Materials
The following antibodies were used in this study: rabbit cryoprin (NLRP3; Novus Biologicals, Wiesbaden, Germany), rabbit interleukin-1β (IL-1β; Boster Biologics, Pleasanton, CA, or Cell Signaling Technologies, Frankfurt am Main, Germany), rabbit cleaved caspase-1 (Cell Signaling, New England Biolabs, Frankfurt am Main, Germany), rabbit CD62P (P-selectin; Novus Biologicals, Wiesbaden, Germany), rabbit GAPDH (Glyceraldehyde 3-phosphate dehydrogenase; Sigma Aldrich, Taufkirchen, Germany), and rabbit Ki-67 (New England Biolabs, Frankfurt am Main, Germany). The following Horse raddish peroxidase (HRP)-conjugated secondary antibodies were used for immunoblotting: goat anti-rabbit IgG (Immunoglobulin, New England Biolabs, Frankfurt am Main, Germany) and rabbit anti-goat IgG (Abcam, Cambridge, UK).
Other reagents used in this study are described in supplemental Material.
Human placental explant culture
Term placental tissues from control and PE pregnancies were obtained after informed consent at the Department of Obstetrics and Gynecology, Medical University of Graz.16 Placental villous tissue of ∼1 to 2 mm was dissected, washed, and cultured in 6-well dishes in a hypoxic workstation under 8% oxygen and 5% CO2 in a humidified atmosphere at 37°C. In a subgroup of explants, LMWH (0.1 mg/mL) was added, and explants were cultured for 48 hours. Detailed procedure for explant isolation and culture are described in supplemental Material.
Mice
Wild-type C57BL/6 mice were obtained from The Jackson Laboratory. Plugged female mice were separated from males and injected at day 10.5 post-coitus (p.c.) and 11.5 p.c. with 600 nM/kg body weight (procoagulant activity) endothelial- or platelet-derived EVs intra-venously (IV), and the pregnancy outcome was analyzed at day 12.5 p.c.5,17,18 Control mice were injected with an equal volume of supernatant from the last PBS (phosphate buffer saline) wash of EVs during the isolation procedure. LMWH (enoxaparin-sodium) was injected subcutaneously (4 mg/kg) 30 minutes before each EV injection.5 All animal experiments were conducted following standards and procedures approved by the local Animal Care and Use Committee (Landesverwaltungsamt Halle and Leipzig, Germany).
Histology
Tissues were fixed in 4% buffered paraformaldehyde for 2 days, embedded in paraffin, and processed for sectioning. Placental morphology was analyzed on hematoxylin and eosin–stained sections. In each section, at least 10 randomly selected microscopic fields within the labyrinthine region from 3 nonconsecutive placental sections (magnification, 40×) were acquired. The vascular spaces were outlined using National Institutes of Health (NIH) ImageJ free hand tool and added using region of interest (ROI) manager. For maternal vascular area, blood vessels with enucleated erythrocytes were outlined, whereas for fetal vascular area, blood vessels with nucleated erythrocytes were outlined. The coverage percentage was calculated as the ratio between the number of pixels covered by the area defined by the selection and the overall number of pixels in the image. The average area for each placenta was used to calculate the significance. Analyses were performed by a blinded investigator.
Generation of procoagulant EVs
Mouse-derived SVEC4-10 cells (mouse endothelial cells; American Type Culture Collection, ATCC, LGC Standards GmbH, Wesel, Germany) or human-derived EA.hy926 (human endothelial cells; American Type Culture Collection, ATCC) cells were serum starved for 72 hours to generate EVs. Cell culture supernatant was collected, centrifuged at 200g for 10 minutes, followed by high-speed centrifugation at 20 000g for 45 minutes to pellet endothelial cell–derived EVs. The EV pellet was finally resuspended in PBS, aliquoted, and stored at –80°C until further use. Supernatant from the last wash was used as control for all experiments. Procoagulant activity (thrombin generation potential in nM) of EVs (thawed once) was assessed using Zymuphen MP-Activity ELISA (Enzyme-linked Immunosorbent Assay). EV used for experiments were likewise only thawed once. EV concentration was adjusted to 600 nM/kg body-weight procoagulant activity before injection. Human cell–derived EVs were only used for studies with human trophoblast cells, whereas mouse cell–derived EVs were used for studies with mouse trophoblast cells and in mice.
Cell culture
Platelet-rich plasma (PRP) was prepared as previously described.5 Mouse trophoblast stem cells were treated with EVs (final procoagulant activity, 7.5-nM thrombin equivalent) with PRP and in some experiments pretreated with LMWH (0.1 mg/mL), followed by EVs and PRP for 24 hours in differentiation media. Human trophoblast-like cells (JEG-3) were treated with EVs (final procoagulant activity, 7.5-nM thrombin equivalent) with PRP, and in some experiments, LMWHs (0.1 mg/mL) were used along with EVs and PRP for 24 hours. For experiments with lipopolysaccharide (LPS) with ATP treatment, cells were either treated with 200 ng/mL (24 hours) and then 1-mM ATP (1 hour) or ATP after 30-minute pretreatment with LMWH (0.1 mg/mL). In experiments in which AKTi (Protein kinase B, AKT inhibitor) and HBEGFi (HBEGF inhibitor) were used, cells were pretreated for additional 30 minutes with either 10-μM LY294002 (AKTi) or 10 μg/mL CRM197 (HBEGFi).
Immunoblotting
Immunoblotting was conducted as previously described.5 The antibodies used are described within “Materials” section. Detailed protocol is provided in the supplemental Material.
Immunostaining
Immunostaining for CD62P was conducted as previously described. A detailed protocol is available in the supplemental Material.
Measurement of ATP release
ATP release from PRP was studied using Cell-titer Glo reagent (Promega Corporation, Walldorf, Germany) according to manufacturer’s protocol. EVs were coincubated with PRP for 10 minutes, followed by addition of Cell-titer Glo reagent. Luminescence was measured using a plate reader.
Measurement of cell proliferation
Trophoblast proliferation was studied using bromodeoxyuridine proliferation assay (in vitro) or Ki-67 immunohistochemistry (ex vivo). Detailed protocols are provided in the supplemental Material.
Statistical analysis
Data are summarized as the means ± standard errors of the mean. Statistical analyses were performed with Student t test or analysis of variance, as appropriate. Post hoc comparisons of analysis of variance were corrected with Šídák multiple comparisons test. The Kolmogorov-Smirnov test or D’Agostino-Pearson normality-test was used to determine whether the data are consistent with a Gaussian distribution. Statistical analyses performed are delineated in each figure legend using Graphpad Prism. Statistical significance was accepted at P values of <.05.
Results
LMWH improves embryonic survival and placental function
To evaluate the effect of LMWH on placental thromboinflammation and embryonic survival, we used an established mouse model of EV-induced placental thromboinflammation, which mimics PE-like phenotype in humans.5,18 Procoagulant EVs were injected in pregnant C57Bl6 mice at days 10.5 and 11.5 p.c. LMWH injections before EV injections improved embryonic survival, growth restriction (evaluated by embryonic height), and placental vascularization, compared with EV-injected mice (Figure 1A-E). Maternal vascularized area, as seen by blood vessels with enucleated erythrocytes, was increased in EV-injected mice, suggesting large lacunae, and was reduced upon LMWH injections. On the contrary, fetal vasculature area, as identified by blood vessels containing nucleated erythrocytes, was reduced in EV-injected mice but restored upon LMWH injections (Figure 1D-E). Trophoblast proliferation, as measured by Ki-67+ nuclei within the placenta, which was reduced upon EV-induced thromboinflammation, was normalized by LMWH injections (Figure 1F-G; supplemental Figure 1A). Activated (P-selectin–positive) platelets were detected within the placenta of EV-injected mice without LMWH injections but not in those with LMWH injections (Figure 1H). LMWH prevented procoagulant EV-induced platelet activation, as studied using ATP measurement assay (supplemental Figure 2). In parallel, expression of trophoblast differentiation markers that were altered upon EV-induced thromboinflammation, were normalized by LMWH injections (Figure 1I). These results suggest that LMWH conveys protection in a PE-like mouse model of placental thromboinflammation.
LMWH prevents EV-mediated placental dysfunction. (A-C) Representative images (A; top, embryos; bottom, placenta) and bar graphs (B-C) showing reduced fetal death (B) and improved embryonic height (C) after LMWH treatment in EV-injected mice. Pregnancy outcome assessed in C57BL/6 mice at day 12.5 p.c. after IV injection of mouse endothelial cell–derived procoagulant EVs at day 10.5 p.c. and 11.5 p.c. (D-E) Hematoxylin and eosin (H&E) staining of murine placenta (representative images [D]; bar graph summarizing results [E]) showing LMWH prevents EV-induced enhanced maternal vascularization (blood lacunae; enucleated erythrocytes, arrows) and reduced fetal vascularization (nucleated erythrocytes, arrow heads) after EV injections. Vascularized areas were calculated using ImageJ. Analyses performed at day 12.5 p.c. Size bar represent 20 μm. (F-G) Ki-67 staining on murine placenta (representative images [F]; bar graph summarizing results [G]) showing LMWH restores EV-induced impaired placental proliferation. Size bar represent 20 μm. (H) Representative Immunostaining images from mice placenta showing increased CD62P (P-selectin, red; DAPI [4′,6-diamidino-2-phenylindole]–stained nucleus, blue) signals in placentas from EV-injected dams, suggesting increased activated platelets within the placenta. LMWH-treated mice (EV+LMWH) showed reduced CD62P signaling, suggesting LMWH prevents platelet activation. Size bar represent 20 μm. (I) Bar graphs summarizing results from qRT-PCR for mouse trophoblast differentiation gene (PL-II, Tpbpa, Esx1, and Gcm-1) showing that LMWH prevents EV-induced abnormal placental differentiation. Control mice, C, were injected with the supernatant obtained after the last PBS wash during EV isolation. For panels B-C,E,G,I, data shown represent mean ± standard error of the mean (SEM); n = 5 to 8 placentas from 3 dams (∗P < .05, nonsignificant [ns]; analysis of variance [ANOVA]; Šídák multiple comparisons test). C, control; EV, extracellular vesicle (EV)-injected mice; EV+LMWH, EV-injected mice with low-molecular weight heaprin (LMWH) injections; qRT-PCR, quantitative reverse transcriptase-polymerase chain reaction; PL-II, placental lactogen-II; Tpbpa, trophoblast specific protein alpha; Esx1, Extraembryonic, Spermatogenesis, Homeobox 1 and Gcm-1, Glial Cells Missing Transcription Factor 1.
LMWH prevents EV-mediated placental dysfunction. (A-C) Representative images (A; top, embryos; bottom, placenta) and bar graphs (B-C) showing reduced fetal death (B) and improved embryonic height (C) after LMWH treatment in EV-injected mice. Pregnancy outcome assessed in C57BL/6 mice at day 12.5 p.c. after IV injection of mouse endothelial cell–derived procoagulant EVs at day 10.5 p.c. and 11.5 p.c. (D-E) Hematoxylin and eosin (H&E) staining of murine placenta (representative images [D]; bar graph summarizing results [E]) showing LMWH prevents EV-induced enhanced maternal vascularization (blood lacunae; enucleated erythrocytes, arrows) and reduced fetal vascularization (nucleated erythrocytes, arrow heads) after EV injections. Vascularized areas were calculated using ImageJ. Analyses performed at day 12.5 p.c. Size bar represent 20 μm. (F-G) Ki-67 staining on murine placenta (representative images [F]; bar graph summarizing results [G]) showing LMWH restores EV-induced impaired placental proliferation. Size bar represent 20 μm. (H) Representative Immunostaining images from mice placenta showing increased CD62P (P-selectin, red; DAPI [4′,6-diamidino-2-phenylindole]–stained nucleus, blue) signals in placentas from EV-injected dams, suggesting increased activated platelets within the placenta. LMWH-treated mice (EV+LMWH) showed reduced CD62P signaling, suggesting LMWH prevents platelet activation. Size bar represent 20 μm. (I) Bar graphs summarizing results from qRT-PCR for mouse trophoblast differentiation gene (PL-II, Tpbpa, Esx1, and Gcm-1) showing that LMWH prevents EV-induced abnormal placental differentiation. Control mice, C, were injected with the supernatant obtained after the last PBS wash during EV isolation. For panels B-C,E,G,I, data shown represent mean ± standard error of the mean (SEM); n = 5 to 8 placentas from 3 dams (∗P < .05, nonsignificant [ns]; analysis of variance [ANOVA]; Šídák multiple comparisons test). C, control; EV, extracellular vesicle (EV)-injected mice; EV+LMWH, EV-injected mice with low-molecular weight heaprin (LMWH) injections; qRT-PCR, quantitative reverse transcriptase-polymerase chain reaction; PL-II, placental lactogen-II; Tpbpa, trophoblast specific protein alpha; Esx1, Extraembryonic, Spermatogenesis, Homeobox 1 and Gcm-1, Glial Cells Missing Transcription Factor 1.
LMWH prevents NLRP3 inflammasome activation in placenta
In addition to platelet activation, EV- and platelet-induced thromboinflammatory phenotype is associated with placental NLRP3 inflammasome activation.5,17,18 Therefore, we evaluated the effect of LMWH on NLRP3 inflammasome activation. LMWH treatment reduced the EV-induced expression of NLRP3, cleaved IL-1β, and cleaved caspase-1 (Figure 2A-B) in mouse placenta, suggesting that LMWH prevents placental inflammasome activation. Similarly, LMWH reduced inflammasome activation in murine differentiated trophoblast stem cells and human trophoblast-like (JEG-3) cells stimulated with platelets and EVs in vitro (Figure 2C-E). To determine whether LMWH inhibits trophoblast NLRP3 inflammasome activation independent of platelets, murine differentiated trophoblast stem cells, and JEG-3 cells were stimulated directly with LPS+ATP, a known inducer of inflammasome.19-21 LMWH prevented LPS+ATP–induced inflammasome activation in trophoblasts (Figure 2F-H), suggesting that not platelet-specific but rather DAMP-mediated inflammasome activation is inhibited by LMWH. In addition, LMWH normalized the LPS+ATP–mediated reduced proliferation in trophoblasts (supplemental Figure 1B). These findings suggest that LMWH not only prevents platelet-mediated EV-induced thromboinflammation but also trophoblast-dependent inflammation and restores trophoblast differentiation, placental function, and embryonic growth.
LMWH prevents NLRP3 inflammasome activation. (A-B) Inflammasome activation in murine placentas after LMWH and EV injections. Representative immunoblots (A; each lane represents individual mice placentae) and bar graphs (B) showing reduced EV and platelet-induced expression of NLRP3, cleaved IL-1β, and cleaved caspase-1 in murine placenta analyzed at day 12.5 p.c. Control mice, C, were injected with the supernatant obtained after the last PBS wash during EV isolation. (C-E) Inflammasome activation in murine and human trophoblast cells. Representative immunoblots (C) and bar graphs (D, E) showing reduced EV and platelet (PRP) induced expression of NLRP3, cleaved IL-1β, and cleaved caspase-1 due to LMWH in mouse (trophoblast cells [TS] cells C-D) and human (JEG-3, C,E) trophoblasts. Control cells, C, were exposed to supernatant obtained after the last PBS wash during EV isolation. EV represents cells exposed to EV and platelets (PRP); EV+LMWH, cells pretreated with LMWH and exposed to EVs and platelets. (F-H) Representative immunoblots (F) and bar graphs (G-H) showing that LMWH prevented LPS+ATP–induced expression of NLRP3, cleaved IL-1β, and cleaved caspase-1 in mouse (TS cells [F-G]) and human (JEG-3 [F,H]) trophoblasts. Control cells, C, were exposed to PBS. LPS+ATP cells were pretreated with 200 ng/mL LPS (24 hours) and then exposed to 1-mM ATP (1 hour). ATP+LMWH cells pretreated with LMWH (30 minutes), followed by treatment with LPS (24 hour) and then exposed to ATP (1 hour). Inactive (proform) and active (cleaved form) forms of IL-1β or caspase-1 are indicated in panels A,C,F. Only the active form (cleaved form) was quantified in panels B,D-E,G-H. For panels B,D-E,G-H, data shown represent mean ± SEM; n = 5 to 8 placentas from 3 dams for panel B or 3 independent repeat experiments for panels D-E,G-H. ∗P < .05; ns; ANOVA, Šídák multiple comparisons test. EV, EV-injected mice; EV+LMWH, EV-injected mice with LMWH injections.
LMWH prevents NLRP3 inflammasome activation. (A-B) Inflammasome activation in murine placentas after LMWH and EV injections. Representative immunoblots (A; each lane represents individual mice placentae) and bar graphs (B) showing reduced EV and platelet-induced expression of NLRP3, cleaved IL-1β, and cleaved caspase-1 in murine placenta analyzed at day 12.5 p.c. Control mice, C, were injected with the supernatant obtained after the last PBS wash during EV isolation. (C-E) Inflammasome activation in murine and human trophoblast cells. Representative immunoblots (C) and bar graphs (D, E) showing reduced EV and platelet (PRP) induced expression of NLRP3, cleaved IL-1β, and cleaved caspase-1 due to LMWH in mouse (trophoblast cells [TS] cells C-D) and human (JEG-3, C,E) trophoblasts. Control cells, C, were exposed to supernatant obtained after the last PBS wash during EV isolation. EV represents cells exposed to EV and platelets (PRP); EV+LMWH, cells pretreated with LMWH and exposed to EVs and platelets. (F-H) Representative immunoblots (F) and bar graphs (G-H) showing that LMWH prevented LPS+ATP–induced expression of NLRP3, cleaved IL-1β, and cleaved caspase-1 in mouse (TS cells [F-G]) and human (JEG-3 [F,H]) trophoblasts. Control cells, C, were exposed to PBS. LPS+ATP cells were pretreated with 200 ng/mL LPS (24 hours) and then exposed to 1-mM ATP (1 hour). ATP+LMWH cells pretreated with LMWH (30 minutes), followed by treatment with LPS (24 hour) and then exposed to ATP (1 hour). Inactive (proform) and active (cleaved form) forms of IL-1β or caspase-1 are indicated in panels A,C,F. Only the active form (cleaved form) was quantified in panels B,D-E,G-H. For panels B,D-E,G-H, data shown represent mean ± SEM; n = 5 to 8 placentas from 3 dams for panel B or 3 independent repeat experiments for panels D-E,G-H. ∗P < .05; ns; ANOVA, Šídák multiple comparisons test. EV, EV-injected mice; EV+LMWH, EV-injected mice with LMWH injections.
LMWH activates HBEGF signaling to prevent NLRP3 inflammasome
LMWH targets heparin-binding epidermal growth factor (HBEGF) expression on trophoblasts,22,23 and HBEGF regulates MAPK (ERK1/2, Extracellular signal-regulated kinases 1/2) and AKT (Protein kinase B) signaling,24 which in turn regulate trophoblast cell function. In addition, increased AKT signaling is known to inhibit NLRP3 activation.25,26 Therefore, we evaluated whether LMWH prevents inflammasome activation in trophoblasts and restores trophoblast differentiation by modulating HBEGF-EGFR-AKT signaling. Levels of pAKT (phosphorylated AKT) and pERK1/2 (phosphorylated ERK1/2) were reduced in the placenta of EV-injected mice. LMWH induced HBEGF and pEGFR (phosphorylated epidermal growth factor receptor) and restored pAKT and pERK1/2 expression (Figure 3A-B). This indicates that LMWH activates HBEGF signaling, thereby increasing the expression of pAKT and pERK1/2. Similarly, platelets and EVs suppressed HBEGF-PI3K-AKT signaling and associated inflammasome activation in murine trophoblasts in vitro, which was reversed by LMWH (Figure 3C-D). To establish causality, we used CRM197 (HBEGFi), which binds to and specifically inhibits HBEGF. Pretreatment with CRM197 before LMWH exposure abolished the protective effect of LMWH on EV-induced inflammasome activation in trophoblasts (Figure 3C-D). To prove a causal relationship between phosphorylation of AKT and inflammasome inhibition, we used LY294002 (AKTi), an inhibitor blocking PI3 kinase-dependent AKT phosphorylation and kinase activity (Figure 3C-D). The protective effect of LMWH on EV-induced inflammasome activation in trophoblasts was lost upon pretreatment with LY294002 (AKTi) before LMWH exposure. Therefore, when phosphorylation of AKT is inhibited, NLRP3 remains activated, and eventually, caspase-1 remains activated. The experiments with LY294002 and CRM197 support the conclusion that LMWH conveys its protective effect via HBEGF-PI3K-AKT signaling (Figure 3C-D).
LMWH prevents platelet and EV-induced inflammasome activation via HBEGF-AKT signaling. (A-B) LMWH activates HBEGF-AKT signaling in placenta. Immunoblots of placenta from EV-injected pregnant mice (representative immunoblots [A]; bar graphs summarizing results [B]) showing restored HBEGF, pEGFR, pAKT, and pERK1/2 levels upon LMWH treatment. Placentas were assessed from C57BL/6 mice at day 12.5 p.c. after IV injection of mouse endothelial cell–derived procoagulant EVs at days 10.5 p.c. and 11.5 p.c. Control mice (C) were injected with the supernatant obtained after the last PBS wash during EV isolation. (C-D) Inhibition of HBEGF (HBEGFi, CRM197) or AKT (AKTi, LY294002) in vitro inhibits (representative immunoblots [C]; bar graphs summarizing results [D]) LMWH-mediated protective effects on platelet and EV-induced expression of NLRP3, cleaved IL-1β, and cleaved caspase-1. Cells were pretreated respective inhibitors before LMWH treatment. Control cells, C, were exposed to supernatant obtained after the last PBS wash during EV isolation. EV represents cells exposed to EV and platelets (PRP); EV+LMWH, cells pretreated with LMWH and exposed to EVs and platelets; EV+LMWH+HBEGFi, cells pretreated with HBEGFi and LMWH and exposed to EVs and platelets; EV+LMWH+AKTi, cells pretreated with AKTi and LMWH and exposed to EVs and platelets. Inactive (proform) and active (cleaved form) of IL-1β or caspase-1 are indicated in panels A,C. Only the active form (cleaved form) was quantified in panels B,D. Data shown represent mean ± SEM from 6 to 8 placentas from 3 dams for panels A-B or 3 independent repeat experiments for panels C-D. ∗P < .05, ns; ANOVA; Šídák multiple comparisons test. EV, EV-injected mice; EV+LMWH, EV-injected mice with LMWH injections.
LMWH prevents platelet and EV-induced inflammasome activation via HBEGF-AKT signaling. (A-B) LMWH activates HBEGF-AKT signaling in placenta. Immunoblots of placenta from EV-injected pregnant mice (representative immunoblots [A]; bar graphs summarizing results [B]) showing restored HBEGF, pEGFR, pAKT, and pERK1/2 levels upon LMWH treatment. Placentas were assessed from C57BL/6 mice at day 12.5 p.c. after IV injection of mouse endothelial cell–derived procoagulant EVs at days 10.5 p.c. and 11.5 p.c. Control mice (C) were injected with the supernatant obtained after the last PBS wash during EV isolation. (C-D) Inhibition of HBEGF (HBEGFi, CRM197) or AKT (AKTi, LY294002) in vitro inhibits (representative immunoblots [C]; bar graphs summarizing results [D]) LMWH-mediated protective effects on platelet and EV-induced expression of NLRP3, cleaved IL-1β, and cleaved caspase-1. Cells were pretreated respective inhibitors before LMWH treatment. Control cells, C, were exposed to supernatant obtained after the last PBS wash during EV isolation. EV represents cells exposed to EV and platelets (PRP); EV+LMWH, cells pretreated with LMWH and exposed to EVs and platelets; EV+LMWH+HBEGFi, cells pretreated with HBEGFi and LMWH and exposed to EVs and platelets; EV+LMWH+AKTi, cells pretreated with AKTi and LMWH and exposed to EVs and platelets. Inactive (proform) and active (cleaved form) of IL-1β or caspase-1 are indicated in panels A,C. Only the active form (cleaved form) was quantified in panels B,D. Data shown represent mean ± SEM from 6 to 8 placentas from 3 dams for panels A-B or 3 independent repeat experiments for panels C-D. ∗P < .05, ns; ANOVA; Šídák multiple comparisons test. EV, EV-injected mice; EV+LMWH, EV-injected mice with LMWH injections.
LMWH activates HBEGF signaling and prevents NLRP3 activation in human placental explants
To establish translational relevance, we conducted ex vivo human term placenta explant experiments from control and patients with PE. HBEGF expression and pAKT and pERK1/2 levels were increased, whereas the expression of NLRP3, cleaved caspase-1, and cleaved IL-1β was reduced in explants obtained from PE cases treated with LMWH compared with those without LMWH treatment. (Figure 4). These human data corroborate our findings obtained in the in vivo and in vitro models, suggesting that LMWH prevents placental thromboinflammation, allowing for protection in PE.
LMWH prevents inflammasome activation in human PE placental explants. (A-B) Human explants from term placentas obtained from patients with PE exposed to LMWH ex vivo (PE+LMWH) and healthy controls (Cs; non-PE) showed an inhibition of NLRP3, cleaved IL-1β and cleaved caspase-1 and an increase in HBEGF-AKT signaling (representative immunoblots [A]; bar graphs summarizing results [B]). Control explants, C, were obtained from healthy term placenta and exposed to PBS. Data shown represent mean ± SEM from 3 different patients. ∗P < .05, ns, ANOVA, Šídák multiple comparisons test.
LMWH prevents inflammasome activation in human PE placental explants. (A-B) Human explants from term placentas obtained from patients with PE exposed to LMWH ex vivo (PE+LMWH) and healthy controls (Cs; non-PE) showed an inhibition of NLRP3, cleaved IL-1β and cleaved caspase-1 and an increase in HBEGF-AKT signaling (representative immunoblots [A]; bar graphs summarizing results [B]). Control explants, C, were obtained from healthy term placenta and exposed to PBS. Data shown represent mean ± SEM from 3 different patients. ∗P < .05, ns, ANOVA, Šídák multiple comparisons test.
Discussion
Lack of mechanistic insights into the pathophysiology of PE has resulted in limited efficient therapeutic options to manage PE. Delivery of the placenta remains the only “remedy,” which is a matter of concern in preterm patients with PE. This ultimately results in preterm birth and predispose the offspring to an increased disease risk in later life. LMWH use was described to be associated with a significant reduced risk for PE when therapy was started before 16 weeks.27 However, its use remains largely controversial. The current results support a model in which LMWH prevents placental thromboinflammation, promotes trophoblast proliferation, and inhibits NLRP3 inflammasome by enhancing HBEGF-PI3K-AKT signaling in PE (Figure 5). Together with previous reports,27-30 these results suggest that LMWH conveys a dual beneficial (anticoagulant and anti-inflammatory) effect in PE.
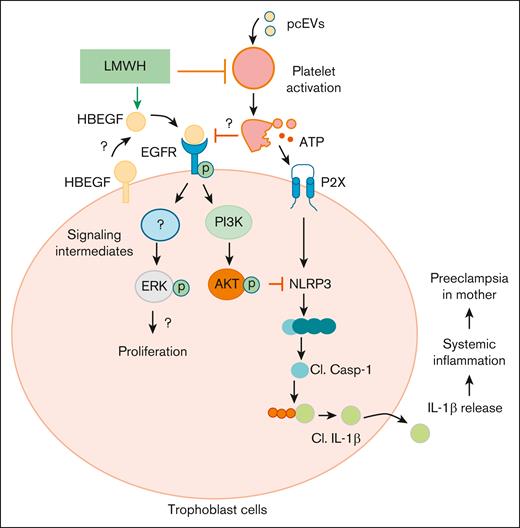
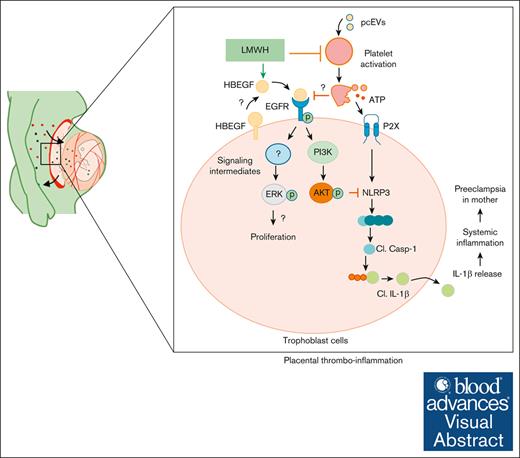
Proposed mechanism by which LMWH promotes HBEGF-AKT signaling and prevents ensuing placental thromboinflammation, proliferation, and differentiation. On 1 hand, LMWH prevents platelet activation. On the other, LMWH activates HBEGF signaling, thereby activating AKT signaling and preventing inflammasome activation. Cl., cleaved.
Proposed mechanism by which LMWH promotes HBEGF-AKT signaling and prevents ensuing placental thromboinflammation, proliferation, and differentiation. On 1 hand, LMWH prevents platelet activation. On the other, LMWH activates HBEGF signaling, thereby activating AKT signaling and preventing inflammasome activation. Cl., cleaved.
We and others have previously shown that platelet inhibition by aspirin can prevent placental thromboinflammation,5,31,32 but whether LMWH, also frequently used in pregnancy, targets placental thromboinflammation remained unknown. Clinical studies (eg, ASPRE, [Aspirin for Evidence-Based Preeclampsia Prevention trial]) investigating the role of aspirin in PE in a larger cohort with a high adherence rate reported a lower incidence of PE.33 Studies evaluating LMWHs in pregnancy focus on thromboprophylaxis for the prevention of venous thromboembolism in pregnant women (eg, ALIFE2, Anticoagulants for Living Fetuses-2 study and HighLow study).34-36 Our data suggest that LMWH directly regulates HBEGF–PI3K-AKT signaling and associated inflammation in trophoblasts, in addition to its anticoagulant effects on plasmatic coagulation factors and platelets. Accordingly, we propose that a combination treatment of aspirin and LMWH may be superior to aspirin monotherapy. Because both drugs, aspirin and LMWH, have been used frequently in pregnant women alone or in combination,32 such approach seems feasible.
Systematic meta-analysis studies conducted on data from 10 clinical studies suggested that addition of LMWH to low-dose aspirin reduced the risk of PE.37 Moreover, additional benefits on live births, placental abruption, and gestational hypertension were reported in these studies. Further subgroup analysis also suggested a positive effect of LMWH in combination with aspirin on women with a history of miscarriages. Taken together, LMWH combined with aspirin may be beneficial in high-risk women, suggesting the need for risk-assessment studies and better stratification of pregnant women. Given these and our current insights (this report), studies not only focusing on venous thromboembolism but evaluating the effect of LMWH (plus aspirin) on placental thromboinflammation and/or PE are warranted.
NLRP3 inflammasome has been widely shown to be involved in pathophysiology of pregnancy complications including PE and preterm birth.17,38-41 Although these studies highlight a pathological role of inflammasomes in placenta, a physiological role of NLRP3 inflammasome cannot be excluded. Activation of NLRP3 inflammasome has been shown to be required for endometrial receptivity and embryo implantation.42,43 Similarly, its presence provides essential protection against infectious diseases.39,44 Expression of cleaved caspase-1 and cleaved IL-1β in healthy placenta tissues in mice and humans may indicate a physiological activation of NLRP3 inflammasome. Future studies exploring a potential physiological role of NLRP3 and caspase-1 in the placenta are required.
Mechanisms of thromboinflammation in pregnancy are largely understudied. We have previously shown that EVs and platelets promote NLRP3 inflammasome activation in the placenta, thereby promoting thromboinflammation.5,17,18 Using mice lacking either NLRP3 or caspase-1, we demonstrated in these studies that NLRP3 is required for platelet and EV-mediated placental thromboinflammation and impaired pregnancy outcome.5,17,18 However, we do not exclude that other mechanisms, for example, activation of neutrophil extracellular traps or activation of other inflammasomes, may contribute to placental thromboinflammation. Although the possible role of such pathways and a possible impact of LMWH on these pathways in PE remains to be established, this study together with the previously published data support a model in which the EV- and platelet-mediated NLRP3 inflammasome activation in the placenta is ameliorated by LMWH.
AKT and ERK signaling are known to promote trophoblast proliferation, migration, and growth, processes that are essential for placental development.45-47 Our results suggest an activation of these signaling mediators via HBEGF signaling. Increased/excess AKT signaling, in turn, prevents NLRP3 activation.25,26 Accordingly, these signaling pathways need to be well balanced, possibly in a cell and/or stimulus dependent manner. Hence, detailed mechanistic studies are required to completely understand the regulation of these signaling pathways and NLRP3 in trophoblast cells.
Effects of LMWH-independent coagulation inhibition include their angiogenic properties, improved endothelial functions, and immunomodulatory effects.13-15 This study suggests a beneficial effect of LMWH on NLRP3 inflammasome inhibition via HBEGF signaling. LMWHs are a heterogenous group of pharmacological substances, which differ in their specific profile. LMWHs optimized for HBEGF inhibition or HBEGF inhibitors may be superior to other LMWHs. The preclinical characterization of LMWHs’ effect on HBEGF inhibition may provide the rational to evaluate specific LMWH preparations in preclinical models and eventually in clinical studies. Such “optimized” LMWHs may be used at a lower dose, with less impact on hemostasis, and may thus reduce the risk of hemorrhage, in particular if combined with aspirin. Alternatively, specific HBEGF inhibitors may reduce side effects associated with (long-term) heparin treatment in pregnant women, such as an increased risk of hemorrhage, heparin-induced immune reactions, or possibly osteoporosis.48
A role of HBEGF pathway in pregnancy and PE is not well understood. Although we observe effects on HBEGF-EGFR-AKT pathway in the absence of LMWHs, the mechanisms by which EVs or platelets regulate this pathway remains unknown. On 1 hand, EVs and platelets via P2X signaling activate the NLRP3 signaling5; on the other hand, LMWHs via HBEGF-EGFR-AKT signaling prevents NLRP3 signaling (this study). These are 2 independent pathways, which counteract each other. Although we observe some effects on the HBEGF-EGFR-AKT pathway due to platelets and EVs, the mechanisms by which EVs and platelet activation can modulate this pathway remains unknown.
It is becoming increasingly evident that disease mechanisms may be primed already in utero. Similarly, recent studies demonstrated long-term effects of coagulation protease-dependent signaling. Therefore, it is important to evaluate the long-term effects of placental thromboinflammation on offspring health. The safety and efficacy of therapies preventing thromboinflammation in pregnancy will also depend upon these long-term effects. Whether LMWH (alone or in combination with aspirin) has an impact on long-term disease priming mechanisms remains to be investigated.
As any study, this study has limitations. LMWH is suggested as a second-line therapy in patients with PE, often prescribed on top of aspirin. Therefore, clinical samples in which only LMWH was used were not available. However, data from human placental explants corroborated findings observed in vivo and in vitro. In addition, the current results only suggest a preventive effect of LMWH because it was given 30 minutes before the in vivo injections of EV or pretreated in the case of in vitro experiments. Given the protective effects, further studies focusing a therapeutic effect and potential long-term effects are required. Although our data strongly suggest that LMWHs inhibit NLRP3 inflammasome activation, experimental evidence to prove causality for the LMWH-dependent suppression of the NLRP3 inflammasome, for example, using a gain of function approach, is missing. Such experiments are, however, not easy, because a gain of function of the NLRP3 inflammasome is expected to impair placental development and pregnancy outcome irrespective of the presence or absence of a pathogenetic stimulus.
Taken together, this study identifies a new mechanism through which LMWH may reduce the risk of PE and associated thromboinflammation and placental dysfunction. Future studies comparing the anti-inflammatory and trophoblast-protective effects of aspirin, LMWHs, and the combination thereof are required to determine the most efficient therapeutic approach to prevent thromboinflammation in PE.
Acknowledgments
The authors thank Kathrin Deneser, Rumiya Makarova, Silke Borchert, Ihsan Gadi, and Estela Mena Plaza for excellent technical support. The authors thank research nurse Bettina Amtmann for collection of placental tissue at the Department of Obstetrics and Gynaecology, Medical University Graz, Austria.
This work was funded by grants of the “Deutsche Forschungsgemeinschaft” (KO-5736/1-1 and KO-5736/5-1 [S.K.]; IS 67/16-1, IS 67/22-1, IS-67/25-1, and IS-67/26-1 [B.I.]), by the Federal Ministry of Education and Research under the funding code 01GR2304A and by the Open Access Publishing Fund of Leipzig University, supported by the German Research Foundation within the program Open Access Publication Funding. The project is a member of Centers for Reproductive Sciences.
Authorship
Contribution: K.K.S and A.G. designed, performed, and interpreted in vivo, in vitro and ex vivo experiments and prepared the manuscript; D. F., J. G., N. H., M.L., M.S.A., A.P.S., S.F., and S.S. supported in vitro experiments and ex vivo analysis; D. F., J. G., M.L., A.D.-S., H. S., and M.G. acquired placental tissues and conducted explant experiments; K.S., H. S., and B. I. interpreted the experimental work and supported experimental design; B.I. revised the manuscript; and S.K. performed in vivo experiments, designed and interpreted the experimental work, and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shrey Kohli, Institute of Laboratory Medicine, Clinical Chemistry and Molecular Diagnostics, University Hospital Leipzig, Leipzig University, Paul-List Str 13/15, 04103 Leipzig, Germany; email: shrey.kohli@medizin.uni-leipzig.de.
References
Author notes
K.K.S. and A.G. contributed equally to this study.
Data are available upon reasonable request from the corresponding author, Shrey Kohli (shrey.kohli@medizin.uni-leipzig.de).
The full-text version of this article contains a data supplement.

![LMWH prevents EV-mediated placental dysfunction. (A-C) Representative images (A; top, embryos; bottom, placenta) and bar graphs (B-C) showing reduced fetal death (B) and improved embryonic height (C) after LMWH treatment in EV-injected mice. Pregnancy outcome assessed in C57BL/6 mice at day 12.5 p.c. after IV injection of mouse endothelial cell–derived procoagulant EVs at day 10.5 p.c. and 11.5 p.c. (D-E) Hematoxylin and eosin (H&E) staining of murine placenta (representative images [D]; bar graph summarizing results [E]) showing LMWH prevents EV-induced enhanced maternal vascularization (blood lacunae; enucleated erythrocytes, arrows) and reduced fetal vascularization (nucleated erythrocytes, arrow heads) after EV injections. Vascularized areas were calculated using ImageJ. Analyses performed at day 12.5 p.c. Size bar represent 20 μm. (F-G) Ki-67 staining on murine placenta (representative images [F]; bar graph summarizing results [G]) showing LMWH restores EV-induced impaired placental proliferation. Size bar represent 20 μm. (H) Representative Immunostaining images from mice placenta showing increased CD62P (P-selectin, red; DAPI [4′,6-diamidino-2-phenylindole]–stained nucleus, blue) signals in placentas from EV-injected dams, suggesting increased activated platelets within the placenta. LMWH-treated mice (EV+LMWH) showed reduced CD62P signaling, suggesting LMWH prevents platelet activation. Size bar represent 20 μm. (I) Bar graphs summarizing results from qRT-PCR for mouse trophoblast differentiation gene (PL-II, Tpbpa, Esx1, and Gcm-1) showing that LMWH prevents EV-induced abnormal placental differentiation. Control mice, C, were injected with the supernatant obtained after the last PBS wash during EV isolation. For panels B-C,E,G,I, data shown represent mean ± standard error of the mean (SEM); n = 5 to 8 placentas from 3 dams (∗P < .05, nonsignificant [ns]; analysis of variance [ANOVA]; Šídák multiple comparisons test). C, control; EV, extracellular vesicle (EV)-injected mice; EV+LMWH, EV-injected mice with low-molecular weight heaprin (LMWH) injections; qRT-PCR, quantitative reverse transcriptase-polymerase chain reaction; PL-II, placental lactogen-II; Tpbpa, trophoblast specific protein alpha; Esx1, Extraembryonic, Spermatogenesis, Homeobox 1 and Gcm-1, Glial Cells Missing Transcription Factor 1.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/18/10.1182_bloodadvances.2023011895/2/m_blooda_adv-2023-011895-gr1.jpeg?Expires=1769087550&Signature=QfkDi4VhLfgV2qU8Z4EeRFBA4B-BIt210~aZJ775hBUTN1N0n8mL95CoICE0A73BjQGZRgBlHkAZBbbloDX6O9zWGyd2Ehw63dfC5LrIcE3-VmwxyezozRXM40YQC-SZI9DR7X4fzksluEl4aHnM-lejPzOqGNIXF6k7izzPAtLHNFJ1w-Gf7IzV-Vg6f--pWnaS4oQ9e2lIweR7SpsuIrxvq9GdOs3pJoJ2BK8dJsdiz-GcBMmNzL~yB6t111PY69BWgshfalPccdXAe4j5DUK5MMOacIa4Y1AH3bBcibDjcJZVnuRnwlKjo9bnWLy4HsYkSq6EJWGJGjqTME2uQg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![LMWH prevents NLRP3 inflammasome activation. (A-B) Inflammasome activation in murine placentas after LMWH and EV injections. Representative immunoblots (A; each lane represents individual mice placentae) and bar graphs (B) showing reduced EV and platelet-induced expression of NLRP3, cleaved IL-1β, and cleaved caspase-1 in murine placenta analyzed at day 12.5 p.c. Control mice, C, were injected with the supernatant obtained after the last PBS wash during EV isolation. (C-E) Inflammasome activation in murine and human trophoblast cells. Representative immunoblots (C) and bar graphs (D, E) showing reduced EV and platelet (PRP) induced expression of NLRP3, cleaved IL-1β, and cleaved caspase-1 due to LMWH in mouse (trophoblast cells [TS] cells C-D) and human (JEG-3, C,E) trophoblasts. Control cells, C, were exposed to supernatant obtained after the last PBS wash during EV isolation. EV represents cells exposed to EV and platelets (PRP); EV+LMWH, cells pretreated with LMWH and exposed to EVs and platelets. (F-H) Representative immunoblots (F) and bar graphs (G-H) showing that LMWH prevented LPS+ATP–induced expression of NLRP3, cleaved IL-1β, and cleaved caspase-1 in mouse (TS cells [F-G]) and human (JEG-3 [F,H]) trophoblasts. Control cells, C, were exposed to PBS. LPS+ATP cells were pretreated with 200 ng/mL LPS (24 hours) and then exposed to 1-mM ATP (1 hour). ATP+LMWH cells pretreated with LMWH (30 minutes), followed by treatment with LPS (24 hour) and then exposed to ATP (1 hour). Inactive (proform) and active (cleaved form) forms of IL-1β or caspase-1 are indicated in panels A,C,F. Only the active form (cleaved form) was quantified in panels B,D-E,G-H. For panels B,D-E,G-H, data shown represent mean ± SEM; n = 5 to 8 placentas from 3 dams for panel B or 3 independent repeat experiments for panels D-E,G-H. ∗P < .05; ns; ANOVA, Šídák multiple comparisons test. EV, EV-injected mice; EV+LMWH, EV-injected mice with LMWH injections.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/18/10.1182_bloodadvances.2023011895/2/m_blooda_adv-2023-011895-gr2.jpeg?Expires=1769087550&Signature=OA9EAjTn1DSV2V3D5H11jCq1n~cdnhT6A83CsGANWhcD9mfPJEBxUOXj-rF9HDJ~C1AAMZAcPZ0AjrAump4W3Rlj-DfvfRDcRZz6z~10aki2mqj9DuCNG6~Ld31wejoehiGC9YPMChrmENtXWYp~s7OIDNoCBUtWU5kr1r4UsEG0OBAiZZXHOcgg5SDnppbPcIClSiAQf6iJH6c1cFO~833GpYiRNg0VroA7tHPjk16HaEGTEy6fT1YooxKc8oSM00p9XjS0NEgEGV8MHOZYGQ~G1jWnqDbav~pDC4Rw8YrrlsEniRvKqnNL3T03CWmHWBfM7XDkcKVEMMZYTRyKsg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![LMWH prevents platelet and EV-induced inflammasome activation via HBEGF-AKT signaling. (A-B) LMWH activates HBEGF-AKT signaling in placenta. Immunoblots of placenta from EV-injected pregnant mice (representative immunoblots [A]; bar graphs summarizing results [B]) showing restored HBEGF, pEGFR, pAKT, and pERK1/2 levels upon LMWH treatment. Placentas were assessed from C57BL/6 mice at day 12.5 p.c. after IV injection of mouse endothelial cell–derived procoagulant EVs at days 10.5 p.c. and 11.5 p.c. Control mice (C) were injected with the supernatant obtained after the last PBS wash during EV isolation. (C-D) Inhibition of HBEGF (HBEGFi, CRM197) or AKT (AKTi, LY294002) in vitro inhibits (representative immunoblots [C]; bar graphs summarizing results [D]) LMWH-mediated protective effects on platelet and EV-induced expression of NLRP3, cleaved IL-1β, and cleaved caspase-1. Cells were pretreated respective inhibitors before LMWH treatment. Control cells, C, were exposed to supernatant obtained after the last PBS wash during EV isolation. EV represents cells exposed to EV and platelets (PRP); EV+LMWH, cells pretreated with LMWH and exposed to EVs and platelets; EV+LMWH+HBEGFi, cells pretreated with HBEGFi and LMWH and exposed to EVs and platelets; EV+LMWH+AKTi, cells pretreated with AKTi and LMWH and exposed to EVs and platelets. Inactive (proform) and active (cleaved form) of IL-1β or caspase-1 are indicated in panels A,C. Only the active form (cleaved form) was quantified in panels B,D. Data shown represent mean ± SEM from 6 to 8 placentas from 3 dams for panels A-B or 3 independent repeat experiments for panels C-D. ∗P < .05, ns; ANOVA; Šídák multiple comparisons test. EV, EV-injected mice; EV+LMWH, EV-injected mice with LMWH injections.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/18/10.1182_bloodadvances.2023011895/2/m_blooda_adv-2023-011895-gr3.jpeg?Expires=1769087550&Signature=KdknyV9WS3sLmeXPwKqT41x0PlEWkI9wEWvWBsNi2vNINMl~bDKLtfodGRxhQZ4yhtYUw-AF2XKQGtzTLYrMcjPHjdybX6xvYySjKQi6tDvP8l6yV1VWs387vbh9OtzThdmgr0jwVq~qe~~PrKckxXVMpTLGyPE8xGVz87xiLa6QZU~SLee5QqnYx0hRyKs2JZaaMXFHnVXiYcskkEmx3juo5e5DJ5uDGGjEGrlqawkvi7Ks6pSVddeyr1~jbVSbd204e1uCrNoRJzls1zwqBSW6vSFBzyZHnDAIK~iKff6l8bst8~nSFbMiVNoNUvt9KsXE1XjAH6W3tOt71UxyaA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![LMWH prevents inflammasome activation in human PE placental explants. (A-B) Human explants from term placentas obtained from patients with PE exposed to LMWH ex vivo (PE+LMWH) and healthy controls (Cs; non-PE) showed an inhibition of NLRP3, cleaved IL-1β and cleaved caspase-1 and an increase in HBEGF-AKT signaling (representative immunoblots [A]; bar graphs summarizing results [B]). Control explants, C, were obtained from healthy term placenta and exposed to PBS. Data shown represent mean ± SEM from 3 different patients. ∗P < .05, ns, ANOVA, Šídák multiple comparisons test.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/18/10.1182_bloodadvances.2023011895/2/m_blooda_adv-2023-011895-gr4.jpeg?Expires=1769087550&Signature=Dok1GAJnRq2KlCW1tBWrVsWW0GqSscVF78EMeE-f7PFS4FHzROeNWdkVBH656kghROzjgzUt0wiiWlFzLJ-ft0XTTxTjJWLoaZ-BasyIJoFcMVqQ7enXtfvIl6LFsDmRoQvIIm4EKaOVLE1xx9DxBfOD-ZceI-rZ4umhR2c0eikL~QTipk532yZHSHWtNQOo6m0QxmhjXLnT7j74ocu9fCU37AP3Mrhk~ewKKQudyXcbvzlIC~2H56HsEAv0vRN1tG~8noDQIS1ZXb1czpVbhG5BA40XG~MkYmws5V8Vf4FBuJIbP~ldb-YjgnSC0-MC63qS417SjASd6rG-EnVosQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)