Key Points
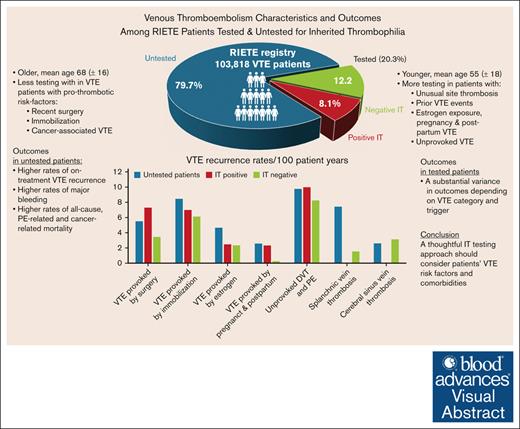
In a RIETE registry analysis of 103 818 patients with VTE, 20.3% were tested for IT, showing a substantial variance in outcomes.
A thoughtful IT testing approach should consider patients’ VTE risk factors and comorbidities.
Visual Abstract
Inherited thrombophilia (IT) workup is commonly pursued in patients with venous thromboembolism (VTE). Recent American Society of Hematology guidelines recommend a selective approach to IT testing, nevertheless, evidence on whether thrombophilia testing can actually improve patient-important outcomes through tailored management is limited. Data from the large, prospective Registro Informatizado de Enfermedad TromboEmbólica (RIETE) registry were analyzed to compare VTE risk factors, management, and outcomes between patients who were tested for IT and untested patients, during anticoagulant treatment and after its discontinuation. Among 103 818 patients enrolled in RIETE, 21 089 (20.3%) were tested for IT, 8422 (8.1%) tested positive, and 82 729 (79.7%) were not tested. IT testing was more frequent in patients with VTE provoked by minor risk factors and less common in those with major risk factors such as surgery or active cancer. Choices of anticoagulant treatment did not differ based on IT testing results. Untested patients exhibited inferior outcomes across all VTE categories, with higher rates of VTE recurrence, major bleeding, mortality, and notably, cancer-related mortality. After treatment discontinuation, IT-negative patients with surgically provoked VTE showed lower recurrence rates. For immobilization-related VTE as well as in estrogen-related VTE, no significant differences in recurrence rates were observed between IT-negative and IT-positive patients. However, IT-negative patients with pregnancy or postpartum-related VTE had significantly lower recurrence rates. Patients with unprovoked VTE, particularly those testing positive for IT, had high recurrence rates after treatment. These findings underscore the complex role of IT testing in managing VTE, supporting personalized treatment strategies that consider VTE risk factors and comorbidities. The trial was registered at www.clinicaltrials.gov as #NCT02832245.
Introduction
Inherited thrombophilia (IT) is a hereditary predisposition to venous thromboembolism (VTE), and genetic defects in this clinical context include deficiencies of the endogenous anticoagulants antithrombin, protein C, and protein S and gain-of-function polymorphisms in factor V (factor V Leiden [FVL]) and prothrombin (PT G20210A).1-5
Thrombophilia testing is commonly conducted in patients with VTE, especially in those who are young, experience recurrent episodes, have thrombosis at unusual sites, or have a family history of VTE. Although testing patients with VTE or their relatives may result in positive findings, the incremental value of identifying thrombophilia is potentially low. Despite the growing understanding of VTE etiology, testing for IT often does not aid in guiding clinical decisions and, therefore, should not be conducted routinely.1,2 Current guidelines on VTE treatment recommend indefinite anticoagulant treatment for most patients after a first episode of unprovoked VTE.6-8 In light of this, IT testing should be performed in a highly selective manner. Recent guidelines put forth by the American Society of Hematology (ASH) recommend restricting IT testing to patients who have experienced VTE associated with substantial transient or hormonal risk factors, those with thrombosis occurring at unusual sites, individuals who are considering thromboprophylaxis due to minor provoking risk factors associated with familial severe IT, pregnant women with a family history of severe IT, and certain patients with cancer with a family history of VTE. In most other cases, the practice of IT testing is discouraged.9 Nevertheless, evidence on whether thrombophilia testing can actually improve patient-important outcomes through tailored management is limited yet.
Recognizing the gaps in current knowledge, we used data from the prospective, international Registro Informatizado de Enfermedad TromboEmbólica (RIETE)10 to explore and compare clinical characteristics and VTE outcomes between patients who were tested for IT and patients who were not tested.
Methods
Study design and participants
RIETE is an ongoing registry of patients with acute VTE, spanning 194 centers across 26 countries in Europe, the Americas, and Asia (ClinicalTrials.gov identifier NCT02832245). The rationale, design, and methodology of RIETE have been previously described.10
The study included patients with objectively confirmed VTE consecutively enrolled in RIETE. All diagnostic and therapeutic decisions were determined solely by the attending physicians, because the study protocol did not mandate any specific medical interventions. Patients participating in ongoing blinded randomized controlled trials addressing VTE treatment are excluded from RIETE. Written informed consent was obtained from all participants in compliance with local ethical standards.
Data acquisition
The participating centers in RIETE attempt to enroll consecutive patients diagnosed with VTE. At each collaborating center, attending physicians gather and record data using a computer–based case report form, which is then securely transmitted to the registry's coordinating center via a dedicated website. To uphold confidentiality, a distinct identification number is assigned to each patient. The coordinating center of RIETE is responsible for data management and quality control, including thorough checks for inconsistencies or errors, resolved through direct communication with local coordinators.
The RIETE reporting system tracks the duration of anticoagulant treatment, noting the start and end dates, and systematically updates outcomes and their occurrence dates during follow-up visits. Patients are eligible for inclusion if they have a minimum follow-up period of 80 days. Data for this study were extracted from the registry database on 31 May 2023, covering patient enrollments since 2001.
Variables and outcomes definitions
The variables recorded in RIETE include patient baseline characteristics, coexisting medical conditions, risk factors for VTE, treatment after VTE diagnosis, and outcomes during anticoagulant treatment and after its discontinuation. Immobility is defined as at least 4 days of complete bed rest, including bathroom privileges, within the 2 months before VTE diagnosis. Surgical patients are defined as those undergoing surgery within 2 months before VTE. Active cancer refers to newly diagnosed or ongoing malignancy not in remission or when patients are receiving antineoplastic treatment. A history of cancer is noted when the disease is in remission, and no cancer treatment has been administered for at least 90 days before VTE diagnosis. Hormonal therapy includes treatments with estrogen, progesterone, combined oral contraceptives, and selective estrogen receptor modulators. VTE after in vitro fertilization procedures involving such hormonal therapies within 2 months is also categorized under hormonal therapy. Unprovoked VTE is identified in the absence of factors such as active cancer, recent immobility, surgery, central venous catheters, hormone use, pregnancy, postpartum status, or prolonged air travel. Patients are documented as having FVL and PT G20210A mutation, whether heterozygous or homozygous carriers, or low activated protein C resistance in the case of FVL. Combined thrombophilia refers to patients with >1 positive test for IT.
Primary clinical outcomes include all-cause mortality, confirmed VTE recurrence, and major bleeding as defined by the International Society on Thrombosis and Haemostasis.11 Nonmajor bleeding is any overt bleeding not meeting major bleeding criteria. Recurrent VTE is a new VTE event occurring after the initial diagnosis, regardless of completed previous treatment. Fatal pulmonary embolism (PE) is death within 10 days of PE diagnosis when no other cause of death is evident. Supplementary data on the cause of death and the nature of bleeding are also collected, although outcome adjudication is not centrally conducted.
Statistical analyses
Continuous variables are expressed as mean ± standard deviation or median (interquartile range or range, as appropriate). Categorical variables are presented as counts and percentages. The χ2 test (2-sided) and Fisher exact test (2-sided) were used for comparing categorical variables, considering a P value ≤.05 as statistically significant. Student t test and the Mann-Whitney U test were used for continuous variables, as applicable. The incidence rates of outcomes (VTE recurrences, major bleeding, and mortality) during anticoagulant treatment and after its discontinuation were calculated as cumulative incidence (events per 100 patient-years).
Patients tested for IT were compared with those not tested. Untested patients serve as the reference group for all comparisons. To further assess the impact of IT testing on clinical outcomes, subgroup analyses were conducted based on the categorization of VTE events into unprovoked VTE, VTE provoked by specific risk factors, and VTE at unusual sites, adhering to the framework suggested by the ASH guidelines on IT testing.9 All statistical analyses were conducted with the SPSS (Statistical Package for Social Sciences) program (SPSS for Windows version 25.0; SPSS Inc, Chicago, IL).
Role of the funding source
The sponsors of RIETE have not been involved in designing the registry, nor do they possess any rights to access the database, or to review or comment on studies from RIETE before publication.
The study was approved by the institutional review boards at Sheba Medical Center and at each center participating in RIETE. Written informed consent was obtained from all participants in compliance with local ethical standards.
Results
Patient characteristics and VTE risk factors
Of 103 818 patients with VTE enrolled in RIETE by 31 May 2023, a total of 21 089 (20.3%) were tested for IT. Baseline characteristics of these patients are presented in Table 1. Tested individuals were notably younger, with a higher prevalence of isolated deep venous thrombosis (DVT), and less PE than untested patients (P < .001). Unusual site thrombosis involving the splanchnic or cerebral veins was also more prevalent among patients who underwent IT testing (P < .001). IT testing varied with VTE risk factors, being more common in patients with prior VTE events, estrogen exposure, or pregnancy-related events and less frequent in those with surgery, immobilization, or cancer.
Baseline characteristics of patients with VTE in RIETE, according to IT testing and its results
| . | Not tested . | Tested . | Positive IT (all IT types) . | Protein C deficiency only . | Protein S deficiency only . | Antithrombin deficiency only . | FVL only . | Prothrombin mutation only . | Combined IT . | Negative IT . |
|---|---|---|---|---|---|---|---|---|---|---|
| Patients, N | 82 729 | 21 089 | 8422 | 294 | 726 | 240 | 2248 | 1434 | 3480 | 12 667 |
| Demographics | ||||||||||
| Male sex | 40 214 (49%) | 11 021 (52%)∗ | 4592 (55%)∗ | 167 (57%)† | 327 (45%) | 147 (61%)∗ | 1271 (57%)∗ | 755 (53%)† | 1925 (55%)∗ | 6429 (51%)∗ |
| Age (mean y ± SD) | 68 ± 16 | 55 ± 18∗ | 53 ± 18∗ | 52 ± 17∗ | 52 ± 19∗ | 50 ± 19∗ | 50 ± 17∗ | 52 ± 17∗ | 57 ± 18∗ | 56 ± 18∗ |
| Body weight (mean kg ± SD) | 76 ± 16 | 78 ± 16∗ | 78 ± 17∗ | 77 ± 14 | 75 ± 16 | 79 ± 18† | 80 ± 17∗ | 79 ± 16∗ | 78 ± 17∗ | 78 ± 16∗ |
| Initial VTE presentation | ||||||||||
| PE | de | 10 164 (48%)∗ | 3747 (44%)∗ | 136 (46%)‡ | 316 (44%)∗ | 117 (49%) | 779 (35%)∗ | 685 (48%)∗ | 1714 (49%)∗ | 6417 (51%)∗ |
| Isolated lower-limb DVT | 32 057 (39%) | 9116 (43%)∗ | 3968 (47%)∗ | 135 (46%)‡ | 353 (49%)∗ | 102 (43%) | 1264 (56%)∗ | 642 (45%)∗ | 1472 (42%)∗ | 5148 (41%)∗ |
| Isolated upper-limb DVT | 3494 (4.2%) | 891 (4.2%) | 306 (3.6%)† | 9 (3.1%) | 20 (2.8%) | 7 (2.9%) | 91 (4.0%) | 38 (2.6%)† | 141 (4.1%) | 585 (4.6%)‡ |
| Splanchnic vein thrombosis | 386 (0.47%) | 223 (1.1%)∗ | 93 (1.1%)∗ | 4 (1.4%) | 9 (1.2%)† | 8 (3.3%)∗ | 6 (0.27%) | 23 (1.6%)∗ | 43 (1.2%)∗ | 130 (1.0%)∗ |
| Superficial vein thrombosis | 1777 (2.1%) | 441 (2.1%) | 199 (2.4%) | 5 (1.7%) | 20 (2.8%) | 5 (2.1%) | 84 (3.7%)∗ | 26 (1.8%) | 59 (1.7%) | 242 (1.9%) |
| Cerebral sinus vein thrombosis | 95 (0.11%) | 105 (0.50%)∗ | 47 (0.56%)∗ | 0 | 4 (0.55%)‡ | 1 (0.42%) | 7 (0.31%)‡ | 14 (0.98%)∗ | 21 (0.60%)∗ | 58 (0.46%)∗ |
| Other locations | 695 (0.84%) | 149 (0.71%) | 62 (0.74%) | 5 (1.7%) | 4 (0.55%) | 0 | 17 (0.76%) | 6 (0.42%) | 30 (0.86%) | 87 (0.69%) |
| Risk factors for VTE | ||||||||||
| Recent surgery | 9018 (11%) | 2075 (9.8%)∗ | 761 (9.0%)∗ | 26 (8.8%) | 76 (10%) | 20 (8.3%) | 164 (7.3%)∗ | 155 (11%) | 320 (9.2%)† | 1314 (10%) |
| Immobilization ≥4 days | 19 199 (23%) | 3762 (18%)∗ | 1313 (16%)∗ | 45 (15%)† | 127 (17%)∗ | 30 (13%)∗ | 297 (13%)∗ | 229 (16%)∗ | 585 (17%)∗ | 2449 (19%)∗ |
| Active cancer | 17 124 (21%) | 1595 (7.6%)∗ | 619 (7.3%)∗ | 14 (4.8%)∗ | 42 (5.8%)∗ | 12 (5.0%)∗ | 106 (4.7%)∗ | 88 (6.1%)∗ | 357 (10%)∗ | 976 (7.7%)∗ |
| Estrogen use | 3466 (4.2%) | 2365 (11%)∗ | 917 (11%)∗ | 20 (6.8%)‡ | 79 (11%)∗ | 16 (6.7%) | 286 (13%)∗ | 200 (14%)∗ | 316 (9.1%)∗ | 1448 (11%)∗ |
| Pregnancy or postpartum | 676 (0.8%) | 608 (2.9%)∗ | 295 (3.5%)∗ | 11 (3.7%)∗ | 43 (5.9%)∗ | 15 (6.3%)∗ | 75 (3.3%)∗ | 58 (4.0%)∗ | 93 (2.7%)∗ | 313 (2.5%)∗ |
| None of the above (unprovoked) | 40 190 (49%) | 11 945 (57%)∗ | 4986 (59%)∗ | 186 (63%)∗ | 404 (56%)∗ | 156 (65%)∗ | 1426 (63%)∗ | 801 (56%)∗ | 2013 (58%)∗ | 6959 (55%)∗ |
| Prior VTE | 11 300 (14%) | 3769 (18%)∗ | 1976 (23%)∗ | 96 (33%)∗ | 175 (24%)∗ | 81 (34%)∗ | 580 (26%)∗ | 292 (20%)∗ | 752 (22%)∗ | 1793 (14%) |
| . | Not tested . | Tested . | Positive IT (all IT types) . | Protein C deficiency only . | Protein S deficiency only . | Antithrombin deficiency only . | FVL only . | Prothrombin mutation only . | Combined IT . | Negative IT . |
|---|---|---|---|---|---|---|---|---|---|---|
| Patients, N | 82 729 | 21 089 | 8422 | 294 | 726 | 240 | 2248 | 1434 | 3480 | 12 667 |
| Demographics | ||||||||||
| Male sex | 40 214 (49%) | 11 021 (52%)∗ | 4592 (55%)∗ | 167 (57%)† | 327 (45%) | 147 (61%)∗ | 1271 (57%)∗ | 755 (53%)† | 1925 (55%)∗ | 6429 (51%)∗ |
| Age (mean y ± SD) | 68 ± 16 | 55 ± 18∗ | 53 ± 18∗ | 52 ± 17∗ | 52 ± 19∗ | 50 ± 19∗ | 50 ± 17∗ | 52 ± 17∗ | 57 ± 18∗ | 56 ± 18∗ |
| Body weight (mean kg ± SD) | 76 ± 16 | 78 ± 16∗ | 78 ± 17∗ | 77 ± 14 | 75 ± 16 | 79 ± 18† | 80 ± 17∗ | 79 ± 16∗ | 78 ± 17∗ | 78 ± 16∗ |
| Initial VTE presentation | ||||||||||
| PE | de | 10 164 (48%)∗ | 3747 (44%)∗ | 136 (46%)‡ | 316 (44%)∗ | 117 (49%) | 779 (35%)∗ | 685 (48%)∗ | 1714 (49%)∗ | 6417 (51%)∗ |
| Isolated lower-limb DVT | 32 057 (39%) | 9116 (43%)∗ | 3968 (47%)∗ | 135 (46%)‡ | 353 (49%)∗ | 102 (43%) | 1264 (56%)∗ | 642 (45%)∗ | 1472 (42%)∗ | 5148 (41%)∗ |
| Isolated upper-limb DVT | 3494 (4.2%) | 891 (4.2%) | 306 (3.6%)† | 9 (3.1%) | 20 (2.8%) | 7 (2.9%) | 91 (4.0%) | 38 (2.6%)† | 141 (4.1%) | 585 (4.6%)‡ |
| Splanchnic vein thrombosis | 386 (0.47%) | 223 (1.1%)∗ | 93 (1.1%)∗ | 4 (1.4%) | 9 (1.2%)† | 8 (3.3%)∗ | 6 (0.27%) | 23 (1.6%)∗ | 43 (1.2%)∗ | 130 (1.0%)∗ |
| Superficial vein thrombosis | 1777 (2.1%) | 441 (2.1%) | 199 (2.4%) | 5 (1.7%) | 20 (2.8%) | 5 (2.1%) | 84 (3.7%)∗ | 26 (1.8%) | 59 (1.7%) | 242 (1.9%) |
| Cerebral sinus vein thrombosis | 95 (0.11%) | 105 (0.50%)∗ | 47 (0.56%)∗ | 0 | 4 (0.55%)‡ | 1 (0.42%) | 7 (0.31%)‡ | 14 (0.98%)∗ | 21 (0.60%)∗ | 58 (0.46%)∗ |
| Other locations | 695 (0.84%) | 149 (0.71%) | 62 (0.74%) | 5 (1.7%) | 4 (0.55%) | 0 | 17 (0.76%) | 6 (0.42%) | 30 (0.86%) | 87 (0.69%) |
| Risk factors for VTE | ||||||||||
| Recent surgery | 9018 (11%) | 2075 (9.8%)∗ | 761 (9.0%)∗ | 26 (8.8%) | 76 (10%) | 20 (8.3%) | 164 (7.3%)∗ | 155 (11%) | 320 (9.2%)† | 1314 (10%) |
| Immobilization ≥4 days | 19 199 (23%) | 3762 (18%)∗ | 1313 (16%)∗ | 45 (15%)† | 127 (17%)∗ | 30 (13%)∗ | 297 (13%)∗ | 229 (16%)∗ | 585 (17%)∗ | 2449 (19%)∗ |
| Active cancer | 17 124 (21%) | 1595 (7.6%)∗ | 619 (7.3%)∗ | 14 (4.8%)∗ | 42 (5.8%)∗ | 12 (5.0%)∗ | 106 (4.7%)∗ | 88 (6.1%)∗ | 357 (10%)∗ | 976 (7.7%)∗ |
| Estrogen use | 3466 (4.2%) | 2365 (11%)∗ | 917 (11%)∗ | 20 (6.8%)‡ | 79 (11%)∗ | 16 (6.7%) | 286 (13%)∗ | 200 (14%)∗ | 316 (9.1%)∗ | 1448 (11%)∗ |
| Pregnancy or postpartum | 676 (0.8%) | 608 (2.9%)∗ | 295 (3.5%)∗ | 11 (3.7%)∗ | 43 (5.9%)∗ | 15 (6.3%)∗ | 75 (3.3%)∗ | 58 (4.0%)∗ | 93 (2.7%)∗ | 313 (2.5%)∗ |
| None of the above (unprovoked) | 40 190 (49%) | 11 945 (57%)∗ | 4986 (59%)∗ | 186 (63%)∗ | 404 (56%)∗ | 156 (65%)∗ | 1426 (63%)∗ | 801 (56%)∗ | 2013 (58%)∗ | 6959 (55%)∗ |
| Prior VTE | 11 300 (14%) | 3769 (18%)∗ | 1976 (23%)∗ | 96 (33%)∗ | 175 (24%)∗ | 81 (34%)∗ | 580 (26%)∗ | 292 (20%)∗ | 752 (22%)∗ | 1793 (14%) |
Patients not tested for IT were used as reference for all comparisons with the remaining subgroups.
DVT, Deep venous thrombosis; SD, Standard deviation.
P < .001.
P < .01.
P < .05.
VTE treatment strategies
Most patients (86%) initially received parenteral anticoagulants (low molecular-weight heparin, unfractionated heparin, and fondaparinux), with no significant differences in rates between those tested for IT and untested patients. Similar patterns were observed in the rates of thrombolysis and the insertion of inferior vena cava filters across these groups. Most also transitioned to oral anticoagulants for long-term treatment. Among IT-tested patients, vitamin K antagonists were preferred (65%), with direct oral anticoagulants used less frequently compared with untested patients (P < .001). Percentage rates of patients treated with low molecular-weight heparin, vitamin K antagonists, and direct oral anticoagulants were comparable between those who tested positive for IT and those who tested negative (Table 2).
VTE treatment strategies according to IT testing, its results, and by thrombophilia subgroups
| . | Not tested . | Tested . | Positive IT (ALL IT types) . | Protein C deficiency only . | Protein S deficiency only . | Antithrombin deficiency only . | Factor V Leiden only . | Prothrombin mutation only . | Combined IT . | Negative IT . |
|---|---|---|---|---|---|---|---|---|---|---|
| Patients, n | 82 729 | 21 089 | 8422 | 294 | 726 | 240 | 2248 | 1434 | 3480 | 12 667 |
| Initial therapy | ||||||||||
| LMWH | 71 327 (86%) | 18 097 (86%) | 7387 (88%)∗ | 258 (88%) | 649 (89%)† | 206 (86%) | 1946 (87%) | 1276 (89%)‡ | 3052 (88%)† | 10 710 (85%)∗ |
| UFH | 4402 (5.3%) | 1201 (5.7%)† | 411 (4.9%) | 19 (6.5%) | 31 (4.3%) | 21 (8.8%)† | 77 (3.4%)∗ | 69 (4.8%) | 194 (5.6%) | 790 (6.2%)∗ |
| Fondaparinux | 1883 (2.3%) | 417 (2.0%)‡ | 192 (2.3%) | 3 (1.0%) | 7 (1.0%)† | 7 (2.9%) | 77 (3.4%)∗ | 28 (2.0%) | 70 (2.0%) | 225 (1.8%)∗ |
| DOACs | 3193 (3.9%) | 759 (3.6%) | 225 (2.7%)∗ | 7 (2.4%) | 17 (2.4%)† | 2 (0.8%)† | 99 (4.4%) | 25 (1.7%)∗ | 75 (2.2%)∗ | 534 (4.2%) |
| Rivaroxaban | 2122 (2.6%) | 637 (3.0%)∗ | 175 (2.1%)‡ | 5 (1.7%) | 12 (1.7%) | 2 (0.8%) | 82 (3.6%)‡ | 20 (1.4%)‡ | 54 (1.6%)∗ | 462 (3.6%)∗ |
| Apixaban | 969 (1.2%) | 112 (0.5%)∗ | 45 (0.53%)∗ | 2 (0.7%) | 4 (0.5%) | 0 | 15 (0.7%)† | 5 (0.3%)‡ | 19 (0.5%)∗ | 67 (0.5%)∗ |
| Systemic thrombolysis | 472 (0.6%) | 92 (0.5%)‡ | 38 (0.49%) | 1 (0.4%) | 3 (0.4%) | 4 (1.8%) | 7 (0.3%) | 6 (0.4%) | 17 (0.5%) | 54 (0.5%)† |
| Mechanical thrombolysis | 343 (1.1%) | 94 (1.3%) | 43 (1.6%)† | 0 | 7 (3.1%)† | 1 (1.4%) | 9 (1.2%) | 9 (1.9%) | 17 (1.7%) | 51 (1.1%) |
| Inferior vena cava filter | 2167 (2.6%) | 421 (2.0%)∗ | 170 (2.0%)∗ | 7 (2.4%) | 19 (2.6%) | 10 (4.2%) | 38 (1.7%)‡ | 24 (1.7%)† | 72 (2.1%)† | 251 (2.0%)∗ |
| No anticoagulant therapy | 319 (0.4%) | 44 (0.2%)∗ | 18 (0.21%)† | 0 | 4 (0.5%) | 0 | 5 (0.2%) | 4 (0.3%) | 5 (0.1%)† | 26 (0.2%)‡ |
| Long-term therapy | ||||||||||
| LMWH | 25 872 (31%) | 4634 (22%)∗ | 1773 (21%)∗ | 35 (12%)∗ | 176 (24%)∗ | 51 (21%)∗ | 443 (20%)∗ | 303 (21%)∗ | 765 (22%)∗ | 2861 (23%)∗ |
| VKAs | 40 548 (49%) | 13 629 (65%)∗ | 5588 (66%)∗ | 224 (76%)∗ | 467 (64%)∗ | 165 (69%)∗ | 1434 (64%)∗ | 962 (67%)∗ | 2336 (67%)∗ | 8041 (63%)∗ |
| DOACs | 11 790 (15%) | 2399 (11%)∗ | 884 (11%)∗ | 28 (9.7%)† | 69 (9.6%)∗ | 14 (5.9%)∗ | 323 (14%) | 154 (11%)∗ | 296 (8.6%)∗ | 1515 (12%)∗ |
| Rivaroxaban | 5946 (7.2%) | 1508 (7.2%) | 523 (6.2%)∗ | 19 (6.5%) | 41 (5.6%) | 12 (5.0%) | 205 (9.1%)∗ | 76 (5.3%)‡ | 170 (4.9%)∗ | 985 (7.8%)† |
| Apixaban | 4049 (4.9%) | 514 (2.4%)∗ | 203 (2.4%)∗ | 8 (2.7%) | 13 (1.8%)∗ | 0 | 65 (2.9%)∗ | 37 (2.6%)∗ | 80 (2.3%)∗ | 311 (2.5%)∗ |
| Edoxaban | 1442 (1.7%) | 279 (1.3%)∗ | 113 (1.3%)‡ | 1 (0.3%) | 11 (1.5%) | 1 (0.4%) | 35 (1.6%) | 30 (2.1%) | 35 (1.0%)‡ | 166 (1.3%)∗ |
| Dabigatran | 353 (0.4%) | 98 (0.5%) | 45 (0.53%) | 0 | 4 (0.5%) | 1 (0.4%) | 18 (0.8%)† | 11 (0.8%) | 11 (0.3%) | 53 (0.4%) |
| Fondaparinux | 830 (1.0%) | 148 (0.7%)∗ | 80 (0.95%) | 2 (0.7%) | 3 (0.4%) | 5 (2.1%) | 25 (1.1%) | 7 (0.5%) | 38 (1.1%) | 68 (0.5%)∗ |
| No anticoagulant therapy | 634 (0.8%) | 64 (0.3%)∗ | 24 (0.28%)∗ | 2 (0.7%) | 1 (0.14%) | 2 (0.8%) | 7 (0.3%)† | 3 (0.2%)† | 9 (0.3%)∗ | 40 (0.3%)∗ |
| . | Not tested . | Tested . | Positive IT (ALL IT types) . | Protein C deficiency only . | Protein S deficiency only . | Antithrombin deficiency only . | Factor V Leiden only . | Prothrombin mutation only . | Combined IT . | Negative IT . |
|---|---|---|---|---|---|---|---|---|---|---|
| Patients, n | 82 729 | 21 089 | 8422 | 294 | 726 | 240 | 2248 | 1434 | 3480 | 12 667 |
| Initial therapy | ||||||||||
| LMWH | 71 327 (86%) | 18 097 (86%) | 7387 (88%)∗ | 258 (88%) | 649 (89%)† | 206 (86%) | 1946 (87%) | 1276 (89%)‡ | 3052 (88%)† | 10 710 (85%)∗ |
| UFH | 4402 (5.3%) | 1201 (5.7%)† | 411 (4.9%) | 19 (6.5%) | 31 (4.3%) | 21 (8.8%)† | 77 (3.4%)∗ | 69 (4.8%) | 194 (5.6%) | 790 (6.2%)∗ |
| Fondaparinux | 1883 (2.3%) | 417 (2.0%)‡ | 192 (2.3%) | 3 (1.0%) | 7 (1.0%)† | 7 (2.9%) | 77 (3.4%)∗ | 28 (2.0%) | 70 (2.0%) | 225 (1.8%)∗ |
| DOACs | 3193 (3.9%) | 759 (3.6%) | 225 (2.7%)∗ | 7 (2.4%) | 17 (2.4%)† | 2 (0.8%)† | 99 (4.4%) | 25 (1.7%)∗ | 75 (2.2%)∗ | 534 (4.2%) |
| Rivaroxaban | 2122 (2.6%) | 637 (3.0%)∗ | 175 (2.1%)‡ | 5 (1.7%) | 12 (1.7%) | 2 (0.8%) | 82 (3.6%)‡ | 20 (1.4%)‡ | 54 (1.6%)∗ | 462 (3.6%)∗ |
| Apixaban | 969 (1.2%) | 112 (0.5%)∗ | 45 (0.53%)∗ | 2 (0.7%) | 4 (0.5%) | 0 | 15 (0.7%)† | 5 (0.3%)‡ | 19 (0.5%)∗ | 67 (0.5%)∗ |
| Systemic thrombolysis | 472 (0.6%) | 92 (0.5%)‡ | 38 (0.49%) | 1 (0.4%) | 3 (0.4%) | 4 (1.8%) | 7 (0.3%) | 6 (0.4%) | 17 (0.5%) | 54 (0.5%)† |
| Mechanical thrombolysis | 343 (1.1%) | 94 (1.3%) | 43 (1.6%)† | 0 | 7 (3.1%)† | 1 (1.4%) | 9 (1.2%) | 9 (1.9%) | 17 (1.7%) | 51 (1.1%) |
| Inferior vena cava filter | 2167 (2.6%) | 421 (2.0%)∗ | 170 (2.0%)∗ | 7 (2.4%) | 19 (2.6%) | 10 (4.2%) | 38 (1.7%)‡ | 24 (1.7%)† | 72 (2.1%)† | 251 (2.0%)∗ |
| No anticoagulant therapy | 319 (0.4%) | 44 (0.2%)∗ | 18 (0.21%)† | 0 | 4 (0.5%) | 0 | 5 (0.2%) | 4 (0.3%) | 5 (0.1%)† | 26 (0.2%)‡ |
| Long-term therapy | ||||||||||
| LMWH | 25 872 (31%) | 4634 (22%)∗ | 1773 (21%)∗ | 35 (12%)∗ | 176 (24%)∗ | 51 (21%)∗ | 443 (20%)∗ | 303 (21%)∗ | 765 (22%)∗ | 2861 (23%)∗ |
| VKAs | 40 548 (49%) | 13 629 (65%)∗ | 5588 (66%)∗ | 224 (76%)∗ | 467 (64%)∗ | 165 (69%)∗ | 1434 (64%)∗ | 962 (67%)∗ | 2336 (67%)∗ | 8041 (63%)∗ |
| DOACs | 11 790 (15%) | 2399 (11%)∗ | 884 (11%)∗ | 28 (9.7%)† | 69 (9.6%)∗ | 14 (5.9%)∗ | 323 (14%) | 154 (11%)∗ | 296 (8.6%)∗ | 1515 (12%)∗ |
| Rivaroxaban | 5946 (7.2%) | 1508 (7.2%) | 523 (6.2%)∗ | 19 (6.5%) | 41 (5.6%) | 12 (5.0%) | 205 (9.1%)∗ | 76 (5.3%)‡ | 170 (4.9%)∗ | 985 (7.8%)† |
| Apixaban | 4049 (4.9%) | 514 (2.4%)∗ | 203 (2.4%)∗ | 8 (2.7%) | 13 (1.8%)∗ | 0 | 65 (2.9%)∗ | 37 (2.6%)∗ | 80 (2.3%)∗ | 311 (2.5%)∗ |
| Edoxaban | 1442 (1.7%) | 279 (1.3%)∗ | 113 (1.3%)‡ | 1 (0.3%) | 11 (1.5%) | 1 (0.4%) | 35 (1.6%) | 30 (2.1%) | 35 (1.0%)‡ | 166 (1.3%)∗ |
| Dabigatran | 353 (0.4%) | 98 (0.5%) | 45 (0.53%) | 0 | 4 (0.5%) | 1 (0.4%) | 18 (0.8%)† | 11 (0.8%) | 11 (0.3%) | 53 (0.4%) |
| Fondaparinux | 830 (1.0%) | 148 (0.7%)∗ | 80 (0.95%) | 2 (0.7%) | 3 (0.4%) | 5 (2.1%) | 25 (1.1%) | 7 (0.5%) | 38 (1.1%) | 68 (0.5%)∗ |
| No anticoagulant therapy | 634 (0.8%) | 64 (0.3%)∗ | 24 (0.28%)∗ | 2 (0.7%) | 1 (0.14%) | 2 (0.8%) | 7 (0.3%)† | 3 (0.2%)† | 9 (0.3%)∗ | 40 (0.3%)∗ |
Patients not tested for IT were used as reference for all comparisons with the remaining subgroups.
DVT, deep venous thrombosis; DOACs, direct oral anticoagulants; LMWH, low molecular-weight heparin; UFH, unfractionated heparin; VKAs, vitamin K antagonists.
P < .001.
P < .05.
P < .01.
Clinical outcomes during anticoagulant treatment and after its discontinuation
VTE outcomes during anticoagulant treatment and after its discontinuation are presented in Table 3. During anticoagulant therapy, untested patients showed higher VTE recurrence rates (3.46 [95% confidence interval, 3.31-3.62]) than those tested for IT (2.58 [2.38-2.80]), regardless of IT status (P < .001). They also exhibited higher rates of major bleeding (4.44 [4.27-4.61]), all-cause mortality (16.2 [15.9-16.5]), PE-related mortality (1.32 [1.23-1.41]), and bleeding-related mortality (0.65 [0.58-0.71]) than tested patients (P < .001). Major bleeding and all-cause mortality rates were the lowest in patients with FVL and PT G20210A mutations.
VTE-related outcomes during anticoagulation and after its discontinuation, by thrombophilia subgroups
| . | Patients not tested for IT . | Tested patients (all) . | Negative IT . | All positive IT . | Protein C deficiency . | Protein S deficiency . | AT deficiency . | FVL . | PT G20210A . | Combined IT . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | N . | Events per 100 patient-y . | N . | Events per 100 patient-y . | N . | Events per 100 patient-y . | N . | Events per 100 patient-y . | N . | Events per 100 patient-y . | N . | Events per 100 patient-y . | N . | Events per 100 patient-y . | N . | Events per 100 patient-y . | N . | Events per 100 patient-y . | N . | Events per 100 patient-y . |
| During anticoagulation | ||||||||||||||||||||
| Patients, N | 82 729 | 21 089 | 12 667 | 8422 | 294 | 726 | 240 | 2248 | 1434 | 3480 | ||||||||||
| Duration of therapy | ||||||||||||||||||||
| Mean days (±SD) | 264 ± 371 | 407 ± 594∗ | 362 ± 533∗ | 475 ± 670∗ | 520 ± 702∗ | 554 ± 712∗ | 507 ± 695∗ | 444 ± 644∗ | 477 ± 667∗ | 471 ± 672∗ | ||||||||||
| Median days (IQR) | 164 (97-282) | 216 (133-398)∗ | 204 (125-373)∗ | 244 (146-467)∗ | 270 (154-545)∗ | 275 (151-609)∗ | 277 (129-572)∗ | 230 (133-434)∗ | 254 (159-472)∗ | 241 (148-462)∗ | ||||||||||
| Outcomes | ||||||||||||||||||||
| VTE recurrences | 2016 | 3.46 (3.31-3.62) | 581 | 2.58 (2.38-2.80)∗ | 310 | 2.57 (2.29-2.87)∗ | 271 | 2.59 (2.30-2.92)∗ | 8 | 1.95 (0.91-3.70) | 25 | 2.38 (1.57-3.46) | 16 | 5.31 (3.15-8.45)∗ | 70 | 2.69 (2.11-3.38)† | 35 | 1.95 (1.38-2.68)∗ | 117 | 2.73 (2.27-3.26)‡ |
| Recurrent DVT | 1054 | 1.79 (1.68-1.90) | 360 | 1.57 (1.42-1.74)† | 191 | 1.56 (1.35-1.79) | 169 | 1.59 (1.36-1.84) | 6 | 1.45 (0.59-3.02) | 17 | 1.60 (0.96-2.51) | 11 | 3.45 (1.81-5.99)† | 45 | 1.71 (1.26-2.26) | 22 | 1.20 (0.77-1.79) | 68 | 1.56 (1.22-1.96) |
| Recurrent PE | 1006 | 1.70 (1.60-1.81) | 235 | 1.02 (0.89-1.15)∗ | 126 | 1.02 (0.85-1.21)∗ | 109 | 1.01 (0.84-1.22)∗ | 2 | 0.48 (0.08-1.59)† | 9 | 0.83 (0.40-1.52)† | 5 | 1.58 (0.58-3.51) | 25 | 0.93 (0.62-1.35)‡ | 14 | 0.76 (0.43-1.25)∗ | 54 | 1.23 (0.93-1.59)† |
| Major bleeding | 2614 | 4.44 (4.27-4.61) | 364 | 1.57 (1.41-1.73)∗ | 234 | 1.89 (1.66-2.14)∗ | 130 | 1.20 (1.00-1.42)∗ | 5 | 1.20 (0.44-2.67)∗ | 16 | 1.47 (0.87-2.34)∗ | 4 | 1.20 (0.38-2.90)‡ | 19 | 0.70 (0.43-1.07)∗ | 17 | 0.91 (0.55-1.43)∗ | 69 | 1.55 (1.22-1.95)∗ |
| All-cause mortality | 9692 | 16.2 (15.9-16.5) | 449 | 1.91 (1.74-2.09)∗ | 248 | 1.97 (1.74-2.23)∗ | 201 | 1.84 (1.60-2.11)∗ | 6 | 1.43 (0.58-2.98)∗ | 14 | 1.27 (0.72-2.08)∗ | 12 | 3.60 (1.95-6.12)∗ | 28 | 1.03 (0.70-1.46)∗ | 15 | 0.80 (0.47-1.29)∗ | 126 | 2.81 (2.35-3.33)∗ |
| Causes of death | ||||||||||||||||||||
| PE | 788 | 1.32 (1.23-1.41) | 19 | 0.08 (0.05-0.12)∗ | 11 | 0.09 (0.05-0.15)∗ | 8 | 0.07 (0.03-0.14)∗ | 0 | - | 0 | - | 0 | - | 1 | 0.04 (0.00-0.18)∗ | 3 | 0.16 (0.04-0.44)∗ | 4 | 0.09 (0.03-0.22)∗ |
| Bleeding | 386 | 0.65 (0.58-0.71) | 22 | 0.09 (0.06-0.14)∗ | 11 | 0.09 (0.05-0.15)∗ | 11 | 0.10 (0.05-0.17)∗ | 0 | - | 1 | 0.09 (0.00-0.45)‡ | 1 | 0.30 (0.02-1.48) | 1 | 0.04 (0.00-0.18)∗ | 0 | - | 8 | 0.18 (0.08-0.34)∗ |
| Cancer | 3676 | 6.15 (5.96-6.36) | 192 | 0.82 (0.71-0.94)∗ | 100 | 0.80 (0.65-0.96)∗ | 92 | 0.84 (0.68-1.03)∗ | 2 | 0.48 (0.08-1.58)∗ | 4 | 0.36 (0.12-0.88)∗ | 4 | 1.20 (0.38-2.89)∗ | 11 | 0.40 (0.21-0.70)∗ | 4 | 0.21 (0.07-0.52)∗ | 67 | 1.49 (1.17-1.89)∗ |
| After anticoagulation discontinuation | ||||||||||||||||||||
| Patients, n | 26 049 | 10 048 | 6560 | 3488 | 92 | 266 | 68 | 958 | 632 | 1472 | ||||||||||
| Duration of follow-up | ||||||||||||||||||||
| Mean days (±SD) | 423±623 | 688±891∗ | 673±869∗ | 717±930∗ | 790±1175∗ | 661±811∗ | 695±887† | 722±897∗ | 773±996∗ | 696±928∗ | ||||||||||
| Median days (IQR) | 193 (64-516) | 370 (127-903)∗ | 373 (130-891)∗ | 368 (123-938)∗ | 254 (88-984) | 419 (130-927)∗ | 382 (111-997)† | 393 (147-955)∗ | 430 (144-949)∗ | 331 (112-903)∗ | ||||||||||
| Outcomes | ||||||||||||||||||||
| VTE recurrences | 2390 | 8.69 (8.35-9.05) | 1172 | 6.99 (6.59-7.40) | 711 | 6.58 (6.11-7.08) | 461 | 7.72 (7.04-8.45) | 18 | 11.8 (7.20-18.3) | 27 | 6.12 (4.11-8.77) | 9 | 7.86 (3.83-14.4) | 135 | 8.09 (6.81-9.54) | 92 | 8.06 (6.54-9.84) | 180 | 7.34 (6.33-8.48)† |
| Recurrent DVT | 1283 | 4.46 (4.22-4.71) | 692 | 3.89 (3.61-4.19)‡ | 400 | 3.50 (3.17-3.85)∗ | 292 | 4.61 (4.10-5.16) | 10 | 5.77 (2.93-10.3) | 21 | 4.67 (2.97-7.02) | 4 | 3.18 (1.01-7.68) | 99 | 5.65 (4.62-6.85)† | 57 | 4.62 (3.53-5.94) | 101 | 3.88 (3.18-4.70) |
| Recurrent PE | 1141 | 3.96 (3.73-4.19) | 501 | 2.80 (2.56-3.05)∗ | 324 | 2.83 (2.54-3.15)∗ | 177 | 2.74 (2.36-3.16)∗ | 9 | 5.11 (2.49-9.37) | 7 | 1.48 (0.65-2.93)‡ | 5 | 4.23 (1.55-9.36) | 39 | 2.16 (1.56-2.92)∗ | 35 | 2.82 (1.99-3.87)† | 82 | 3.09 (2.48-3.82)† |
| Major bleeding | 342 | 1.14 (1.03-1.27) | 98 | 0.52 (0.43-0.63)∗ | 60 | 0.50 (0.38-0.64)∗ | 38 | 0.56 (0.40-0.76)∗ | 1 | 0.50 (0.03-2.49) | 2 | 0.42 (0.07-1.38) | 0 | - | 6 | 0.32 (0.13-0.66)∗ | 7 | 0.53 (0.23-1.04)† | 22 | 0.79 (0.51-1.18) |
| All-cause mortality | 3949 | 13.1 (12.7-13.5) | 460 | 2.43 (2.22-2.66)∗ | 288 | 2.38 (2.12-2.67)∗ | 172 | 2.51 (2.16-2.91)∗ | 1 | 0.50 (0.03-2.48)∗ | 19 | 3.95 (2.45-6.05)∗ | 6 | 4.64 (1.88-9.64)‡ | 25 | 1.32 (0.87-1.92)∗ | 24 | 1.80 (1.18-2.63)∗ | 97 | 3.46 (2.82-4.20)∗ |
| Causes of death | ||||||||||||||||||||
| PE | 51 | 0.17 (0.13-0.22) | 13 | 0.07 (0.04-0.11)‡ | 6 | 0.05 (0.02-0.10)‡ | 7 | 0.10 (0.04-0.20) | 0 | - | 0 | - | 0 | - | 1 | 0.05 (0.00-0.26) | 0 | - | 6 | 0.21 (0.09-0.45) |
| Bleeding | 188 | 0.62 (0.54-0.72) | 27 | 0.14 (0.10-0.20)∗ | 15 | 0.12 (0.07-0.20)∗ | 12 | 0.18 (0.09-0.30)∗ | 0 | - | 1 | 0.21 (0.01-1.02 | 0 | - | 2 | 0.11 (0.02-0.35)∗ | 2 | 0.15 (0.03-0.49)† | 7 | 0.25 (0.11-0.49)‡ |
| Cancer | 1560 | 5.18 (4.92-5.44) | 184 | 0.97 (0.84-1.12)∗ | 124 | 1.03 (0.86-1.22)∗ | 60 | 0.88 (0.67-1.12)∗ | 0 | - | 5 | 1.04 (0.38-2.30)∗ | 1 | 0.77 (0.04-3.81)† | 12 | 0.63 (0.34-1.08)∗ | 5 | 0.37 (0.14-0.83)∗ | 37 | 1.32 (0.94-1.80)∗ |
| . | Patients not tested for IT . | Tested patients (all) . | Negative IT . | All positive IT . | Protein C deficiency . | Protein S deficiency . | AT deficiency . | FVL . | PT G20210A . | Combined IT . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | N . | Events per 100 patient-y . | N . | Events per 100 patient-y . | N . | Events per 100 patient-y . | N . | Events per 100 patient-y . | N . | Events per 100 patient-y . | N . | Events per 100 patient-y . | N . | Events per 100 patient-y . | N . | Events per 100 patient-y . | N . | Events per 100 patient-y . | N . | Events per 100 patient-y . |
| During anticoagulation | ||||||||||||||||||||
| Patients, N | 82 729 | 21 089 | 12 667 | 8422 | 294 | 726 | 240 | 2248 | 1434 | 3480 | ||||||||||
| Duration of therapy | ||||||||||||||||||||
| Mean days (±SD) | 264 ± 371 | 407 ± 594∗ | 362 ± 533∗ | 475 ± 670∗ | 520 ± 702∗ | 554 ± 712∗ | 507 ± 695∗ | 444 ± 644∗ | 477 ± 667∗ | 471 ± 672∗ | ||||||||||
| Median days (IQR) | 164 (97-282) | 216 (133-398)∗ | 204 (125-373)∗ | 244 (146-467)∗ | 270 (154-545)∗ | 275 (151-609)∗ | 277 (129-572)∗ | 230 (133-434)∗ | 254 (159-472)∗ | 241 (148-462)∗ | ||||||||||
| Outcomes | ||||||||||||||||||||
| VTE recurrences | 2016 | 3.46 (3.31-3.62) | 581 | 2.58 (2.38-2.80)∗ | 310 | 2.57 (2.29-2.87)∗ | 271 | 2.59 (2.30-2.92)∗ | 8 | 1.95 (0.91-3.70) | 25 | 2.38 (1.57-3.46) | 16 | 5.31 (3.15-8.45)∗ | 70 | 2.69 (2.11-3.38)† | 35 | 1.95 (1.38-2.68)∗ | 117 | 2.73 (2.27-3.26)‡ |
| Recurrent DVT | 1054 | 1.79 (1.68-1.90) | 360 | 1.57 (1.42-1.74)† | 191 | 1.56 (1.35-1.79) | 169 | 1.59 (1.36-1.84) | 6 | 1.45 (0.59-3.02) | 17 | 1.60 (0.96-2.51) | 11 | 3.45 (1.81-5.99)† | 45 | 1.71 (1.26-2.26) | 22 | 1.20 (0.77-1.79) | 68 | 1.56 (1.22-1.96) |
| Recurrent PE | 1006 | 1.70 (1.60-1.81) | 235 | 1.02 (0.89-1.15)∗ | 126 | 1.02 (0.85-1.21)∗ | 109 | 1.01 (0.84-1.22)∗ | 2 | 0.48 (0.08-1.59)† | 9 | 0.83 (0.40-1.52)† | 5 | 1.58 (0.58-3.51) | 25 | 0.93 (0.62-1.35)‡ | 14 | 0.76 (0.43-1.25)∗ | 54 | 1.23 (0.93-1.59)† |
| Major bleeding | 2614 | 4.44 (4.27-4.61) | 364 | 1.57 (1.41-1.73)∗ | 234 | 1.89 (1.66-2.14)∗ | 130 | 1.20 (1.00-1.42)∗ | 5 | 1.20 (0.44-2.67)∗ | 16 | 1.47 (0.87-2.34)∗ | 4 | 1.20 (0.38-2.90)‡ | 19 | 0.70 (0.43-1.07)∗ | 17 | 0.91 (0.55-1.43)∗ | 69 | 1.55 (1.22-1.95)∗ |
| All-cause mortality | 9692 | 16.2 (15.9-16.5) | 449 | 1.91 (1.74-2.09)∗ | 248 | 1.97 (1.74-2.23)∗ | 201 | 1.84 (1.60-2.11)∗ | 6 | 1.43 (0.58-2.98)∗ | 14 | 1.27 (0.72-2.08)∗ | 12 | 3.60 (1.95-6.12)∗ | 28 | 1.03 (0.70-1.46)∗ | 15 | 0.80 (0.47-1.29)∗ | 126 | 2.81 (2.35-3.33)∗ |
| Causes of death | ||||||||||||||||||||
| PE | 788 | 1.32 (1.23-1.41) | 19 | 0.08 (0.05-0.12)∗ | 11 | 0.09 (0.05-0.15)∗ | 8 | 0.07 (0.03-0.14)∗ | 0 | - | 0 | - | 0 | - | 1 | 0.04 (0.00-0.18)∗ | 3 | 0.16 (0.04-0.44)∗ | 4 | 0.09 (0.03-0.22)∗ |
| Bleeding | 386 | 0.65 (0.58-0.71) | 22 | 0.09 (0.06-0.14)∗ | 11 | 0.09 (0.05-0.15)∗ | 11 | 0.10 (0.05-0.17)∗ | 0 | - | 1 | 0.09 (0.00-0.45)‡ | 1 | 0.30 (0.02-1.48) | 1 | 0.04 (0.00-0.18)∗ | 0 | - | 8 | 0.18 (0.08-0.34)∗ |
| Cancer | 3676 | 6.15 (5.96-6.36) | 192 | 0.82 (0.71-0.94)∗ | 100 | 0.80 (0.65-0.96)∗ | 92 | 0.84 (0.68-1.03)∗ | 2 | 0.48 (0.08-1.58)∗ | 4 | 0.36 (0.12-0.88)∗ | 4 | 1.20 (0.38-2.89)∗ | 11 | 0.40 (0.21-0.70)∗ | 4 | 0.21 (0.07-0.52)∗ | 67 | 1.49 (1.17-1.89)∗ |
| After anticoagulation discontinuation | ||||||||||||||||||||
| Patients, n | 26 049 | 10 048 | 6560 | 3488 | 92 | 266 | 68 | 958 | 632 | 1472 | ||||||||||
| Duration of follow-up | ||||||||||||||||||||
| Mean days (±SD) | 423±623 | 688±891∗ | 673±869∗ | 717±930∗ | 790±1175∗ | 661±811∗ | 695±887† | 722±897∗ | 773±996∗ | 696±928∗ | ||||||||||
| Median days (IQR) | 193 (64-516) | 370 (127-903)∗ | 373 (130-891)∗ | 368 (123-938)∗ | 254 (88-984) | 419 (130-927)∗ | 382 (111-997)† | 393 (147-955)∗ | 430 (144-949)∗ | 331 (112-903)∗ | ||||||||||
| Outcomes | ||||||||||||||||||||
| VTE recurrences | 2390 | 8.69 (8.35-9.05) | 1172 | 6.99 (6.59-7.40) | 711 | 6.58 (6.11-7.08) | 461 | 7.72 (7.04-8.45) | 18 | 11.8 (7.20-18.3) | 27 | 6.12 (4.11-8.77) | 9 | 7.86 (3.83-14.4) | 135 | 8.09 (6.81-9.54) | 92 | 8.06 (6.54-9.84) | 180 | 7.34 (6.33-8.48)† |
| Recurrent DVT | 1283 | 4.46 (4.22-4.71) | 692 | 3.89 (3.61-4.19)‡ | 400 | 3.50 (3.17-3.85)∗ | 292 | 4.61 (4.10-5.16) | 10 | 5.77 (2.93-10.3) | 21 | 4.67 (2.97-7.02) | 4 | 3.18 (1.01-7.68) | 99 | 5.65 (4.62-6.85)† | 57 | 4.62 (3.53-5.94) | 101 | 3.88 (3.18-4.70) |
| Recurrent PE | 1141 | 3.96 (3.73-4.19) | 501 | 2.80 (2.56-3.05)∗ | 324 | 2.83 (2.54-3.15)∗ | 177 | 2.74 (2.36-3.16)∗ | 9 | 5.11 (2.49-9.37) | 7 | 1.48 (0.65-2.93)‡ | 5 | 4.23 (1.55-9.36) | 39 | 2.16 (1.56-2.92)∗ | 35 | 2.82 (1.99-3.87)† | 82 | 3.09 (2.48-3.82)† |
| Major bleeding | 342 | 1.14 (1.03-1.27) | 98 | 0.52 (0.43-0.63)∗ | 60 | 0.50 (0.38-0.64)∗ | 38 | 0.56 (0.40-0.76)∗ | 1 | 0.50 (0.03-2.49) | 2 | 0.42 (0.07-1.38) | 0 | - | 6 | 0.32 (0.13-0.66)∗ | 7 | 0.53 (0.23-1.04)† | 22 | 0.79 (0.51-1.18) |
| All-cause mortality | 3949 | 13.1 (12.7-13.5) | 460 | 2.43 (2.22-2.66)∗ | 288 | 2.38 (2.12-2.67)∗ | 172 | 2.51 (2.16-2.91)∗ | 1 | 0.50 (0.03-2.48)∗ | 19 | 3.95 (2.45-6.05)∗ | 6 | 4.64 (1.88-9.64)‡ | 25 | 1.32 (0.87-1.92)∗ | 24 | 1.80 (1.18-2.63)∗ | 97 | 3.46 (2.82-4.20)∗ |
| Causes of death | ||||||||||||||||||||
| PE | 51 | 0.17 (0.13-0.22) | 13 | 0.07 (0.04-0.11)‡ | 6 | 0.05 (0.02-0.10)‡ | 7 | 0.10 (0.04-0.20) | 0 | - | 0 | - | 0 | - | 1 | 0.05 (0.00-0.26) | 0 | - | 6 | 0.21 (0.09-0.45) |
| Bleeding | 188 | 0.62 (0.54-0.72) | 27 | 0.14 (0.10-0.20)∗ | 15 | 0.12 (0.07-0.20)∗ | 12 | 0.18 (0.09-0.30)∗ | 0 | - | 1 | 0.21 (0.01-1.02 | 0 | - | 2 | 0.11 (0.02-0.35)∗ | 2 | 0.15 (0.03-0.49)† | 7 | 0.25 (0.11-0.49)‡ |
| Cancer | 1560 | 5.18 (4.92-5.44) | 184 | 0.97 (0.84-1.12)∗ | 124 | 1.03 (0.86-1.22)∗ | 60 | 0.88 (0.67-1.12)∗ | 0 | - | 5 | 1.04 (0.38-2.30)∗ | 1 | 0.77 (0.04-3.81)† | 12 | 0.63 (0.34-1.08)∗ | 5 | 0.37 (0.14-0.83)∗ | 37 | 1.32 (0.94-1.80)∗ |
Patients not tested for IT were used as reference for all comparisons with the remaining subgroups.
AT, antithrombin; DVT, deep venous thrombosis; IQR, interquartile range; SD, standard deviation.
P < .001.
P < .05.
P < .01.
Anticoagulant treatment was discontinued in 36 097 patients, of whom 10 048 (27.8%) were tested for IT, and 3488 were positive. Untested patients continued to exhibit higher incidences of VTE recurrence (8.69 [8.35-9.05]), major bleeding (1.14 [1.03-1.27]), and all-cause mortality (13.1 [12.7-13.5]), PE-related mortality (0.17 [0.13-0.22]), and bleeding-related mortality (0.62 [0.54-0.72]) than tested patients (P < .001).
These higher rates of adverse outcomes remained evident even after excluding patients with cancer from the analysis, albeit to a lesser extent. Nonetheless, untested patients uniformly exhibited higher cancer-related mortality both during anticoagulation treatment (6.15 [5.96-6.36] vs 0.82 [0.71-0.94]; P < .001) and after its discontinuation (5.18 [4.92-5.44] vs 0.97 [0.84-1.12]; P < .001) than tested patients, indicating a substantial impact of cancer on outcomes.
Outcomes according to IT testing and patient groups of interest
VTE-related outcomes according to IT testing across various patient groups of interest are presented in Table 4. Across all VTE patient groups, the duration of anticoagulant treatment was consistently longer for those tested for IT (P < .001). Across all categories, untested patients experienced higher rates of major bleeding, all-cause and VTE-related mortality, and notably, cancer-related mortality. Excluding patients with splanchnic vein thrombosis, IT-positive individuals typically exhibited the lowest rates of major bleeding.
VTE-related outcomes during anticoagulation and after its discontinuation, according to IT testing, and patient groups of interest
| Patient Population . | Thrombophilia Status . | Number of Patients . | Duration of AC Therapy (Months) Median, IQR . | Recurrent VTE on AC (Events/100 PY) . | Recurrent VTE after AC Discontinuation (Events/100 PY) . | Major Bleeding Events (Events/100 PY) . | Mortality (Events/100 PY) . | VTE-related mortality (Events/100 PY) . | Bleeding-related mortality (Events/100 PY) . | Cancer-related mortality (Events/100 PY) . | Notes . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients with VTE provoked by recent surgery | Patients not tested for IT | 9018 | 4.70 (3.15-7.69) | 3.18 (2.73-3.67) | 5.46 (4.78-6.20) | 3.58 (3.22-3.97) | 10.8 (10.2-11.5) | 0.62 (0.48-0.79) | 0.46 (0.34-0.61) | 4.22 (3.83-4.63) | |
| Tested patients (all) | 2075 | 6.41 (4.01-11.0)∗ | 3.72 (2.92-4.69) | 4.63 (3.78-5.62) | 1.21 (0.91-1.58)∗ | 1.79 (1.42-2.22)∗ | 0.07 (0.02-0.19)∗ | 0.07 (0.02-0.19)∗ | 0.93 (0.67-1.25)∗ | ||
| Negative thrombophilia | 1314 | 6.23 (3.91-9.63)∗ | 3.91 (2.82-5.29) | 3.42 (2.57-4.48)† | 1.28 (0.90-1.78)∗ | 1.64 (1.20-2.18)∗ | 0.04 (0.00-0.19)∗ | 0.08 (0.01-0.25)† | 0.84 (0.54-1.25)∗ | ||
| All positive thrombophilia | 761 | 7.00 (4.21-12.6)∗ | 3.51 (2.41-4.95) | 7.25 (5.42-9.50) | 1.10 (0.67-1.70)∗ | 2.03 (1.43-2.80)∗ | 0.12 (0.02-0.39)† | 0.06 (0.00-0.29)† | 1.07 (0.66-1.66)∗ | ||
| Patients with VTE provoked by immobilization ≥4 d | Patients not tested for IT | 19 199 | 4.47 (3.02-7.59) | 3.57 (3.24-3.94) | 8.38 (7.65-9.16) | 5.31 (4.98-5.67) | 25.3 (24.5-26.0) | 1.98 (1.78-2.19) | 1.15 (1.00-1.32) | 5.95 (5.60-6.32) | |
| Tested patients (all) | 3762 | 6.14 (3.61-11.1)∗ | 2.67 (2.15-3.29)‡ | 6.36 (5.48-7.35)† | 1.52 (1.24-1.85)∗ | 3.65 (3.21-4.14)∗ | 0.16 (0.08-0.28)∗ | 0.19 (0.10-0.32)∗ | 0.90 (0.69-1.16)∗ | ||
| Negative thrombophilia | 2449 | 5.82 (3.52-9.03)∗ | 2.26 (1.64-3.03)† | 6.09 (5.06-7.27)† | 1.54 (1.19-1.97)∗ | 3.53 (2.98-4.15)∗ | 0.13 (0.05-0.28)∗ | 0.15 (0.06-0.31)∗ | 0.85 (0.60-1.17)∗ | ||
| All positive thrombophilia | 1313 | 7.03 (4.09-13.4)∗ | 3.25 (2.38-4.33) | 6.95 (5.38-8.83) | 1.48 (1.05-2.04)∗ | 3.86 (3.13-4.70)∗ | 0.21 (0.08-0.46)∗ | 0.25 (0.10-0.52)∗ | 1.00 (0.65-1.46)∗ | ||
| Patients with VTE provoked by estrogen use | Patients not tested for IT | 3466 | 5.22 (3.22-8.54) | 2.55 (1.96-3.26) | 4.61 (3.60-5.83) | 2.12 (1.70-2.62) | 8.89 (7.99-9.86) | 0.46 (0.28-0.71) | 0.31 (0.17-0.52) | 4.25 (3.65-4.94) | |
| Tested patients (all) | 2365 | 6.54 (4.17-11.3)∗ | 1.56 (1.08-2.17)‡ | 2.35 (1.80-3.03)∗ | 0.59 (0.40-0.84)∗ | 0.58 (0.39-0.84)∗ | 0.04 (0.01-0.14)∗ | - | 0.32 (0.19-0.52)∗ | ||
| Negative thrombophilia | 1448 | 6.37 (4.01-9.76)∗ | 1.36 (0.79-2.20)‡ | 2.31 (1.63-3.17)∗ | 0.83 (0.53-1.23)∗ | 0.59 (0.35-0.94)∗ | 0.04 (0.00-0.18)∗ | - | 0.30 (0.14-0.56)∗ | ||
| All positive thrombophilia | 917 | 7.03 (4.57-12.4)∗ | 1.78 (1.07-2.79) | 2.43 (1.56-3.62)† | 0.26 (0.10-0.58)∗ | 0.57 (0.30-0.99)∗ | 0.05 (0.00-0.26)† | - | 0.36 (0.16-0.72)∗ | ||
| Patients with VTE provoked by pregnancy or postpartum | Patients not tested for IT | 676 | 4.67 (3.22-7.20) | 3.28 (1.72-5.70) | 2.53 (1.23-4.63) | 1.29 (0.63-2.38) | 0.99 (0.43-1.97) | 0.14 (0.01-0.70) | 0.14 (0.01-0.70) | 0.14 (0.01-0.70) | |
| Tested patients (all) | 608 | 6.47 (4.17-9.84)∗ | 3.02 (1.75-4.87) | 1.13 (0.55-2.07) | 0.53 (0.23-1.05) | 0.08 (0.00-0.37)† | - | - | 0.08 (0.00-0.37) | ||
| Negative thrombophilia | 313 | 6.34 (4.01-8.77)∗ | 2.95 (1.20-6.14) | 0.23 (0.01-1.11)† | 0.46 (0.12-1.26) | - | - | - | - | ||
| All positive thrombophilia | 295 | 6.64 (4.21-11.1)∗ | 3.07 (1.50-5.63) | 2.27 (1.05-4.31) | 0.60 (0.19-1.45) | 0.15 (0.01-0.73)‡ | - | - | 0.15 (0.01-0.73) | ||
| Unprovoked VTE | Patients not tested for IT | 40 190 | 6.11 (3.42-11.4) | 2.34 (2.18-2.51) | 9.73 (9.22-10.3) | 2.10 (1.97-2.23) | 5.82 (5.61-6.04) | 0.51 (0.45-0.57) | 0.36 (0.31-0.41) | 0.47 (0.41-0.54) | |
| Tested patients (all) | 11 945 | 8.10 (5.22-15.1)∗ | 2.24 (2.00-2.49) | 8.83 (8.22-9.48)‡ | 0.93 (0.81-1.06)∗ | 1.01 (0.90-1.15)∗ | 0.06 (0.03-0.09)∗ | 0.06 (0.04-0.10)∗ | 0.11 (0.08-0.16)∗ | ||
| Negative thrombophilia | 6959 | 7.59 (5.16-13.6)∗ | 2.19 (1.88-2.54) | 8.23 (7.49-9.02)† | 1.02 (0.86-1.20)∗ | 0.99 (0.83-1.16)∗ | 0.06 (0.03-0.11)∗ | 0.06 (0.03-0.11)∗ | 0.13 (0.08-0.20)∗ | ||
| All positive thrombophilia | 4986 | 9.05 (5.39-18.2)∗ | 2.29 (1.95-2.67) | 9.90 (8.83-11.07) | 0.81 (0.65-1.00)∗ | 1.05 (0.87-1.26)∗ | 0.06 (0.02-0.12)∗ | 0.07 (0.03-0.14)∗ | 0.09 (0.05-0.17)∗ | ||
| Patients with splanchnic vein thrombosis | Patients not tested for IT | 611 | 4.67 (2.92-9.33) | 3.26 (1.81-5.43) | 7.39 (4.11-12.32) | 7.85 (5.78-10.5) | 26.6 (22.7-31.0) | 0.17 (0.01-0.84) | 1.52 (0.74-2.80) | 14.7 (11.9-18.1) | |
| Tested patients (all) | 287 | 8.41 (3.75-20.4)∗ | 2.25 (1.10-4.12) | 1.00 (0.17-3.31)† | 1.48 (0.72-2.72)∗ | 1.13 (0.50-2.24)∗ | - | 0.16 (0.01-0.80)† | 0.32 (0.05-1.07)∗ | ||
| Negative thrombophilia | 171 | 6.18 (3.52-16.4)‡ | 2.66 (0.97-5.89) | 1.56 (0.26-5.16)‡ | 0.93 (0.24-2.52)∗ | 0.61 (0.10-2.01)∗ | - | - | 0.61 (0.10-2.01)∗ | ||
| All positive thrombophilia | 116 | 12.0 (4.11-28.8)∗ | 1.88 (0.60-4.54) | - | 2.12 (0.86-4.41)∗ | 1.73 (0.64-3.85)∗ | - | 0.35 (0.02-1.71) | - | ||
| Patients with cerebral sinus vein thrombosis | Patients not tested for IT | 162 | 5.22 (3.24-10.1) | 1.55 (0.26-5.11) | 2.56 (0.43-8.47) | 3.91 (1.82-7.42) | 8.62 (5.27-13.4) | 0.48 (0.02-2.36) | 0.96 (0.16-3.16) | 2.87 (1.16-5.97) | |
| Tested patients (all) | 130 | 9.00 (5.32-15.8)∗ | 1.12 (0.19-3.70) | 1.76 (0.30-5.82) | 1.37 (0.44-3.31) | 2.35 (1.03-4.65)† | - | 0.67 (0.11-2.22) | 1.68 (0.62-3.72) | ||
| Negative thrombophilia | 70 | 10.2 (4.58-15.9)† | 1.20 (0.06-5.94) | 3.06 (0.51-10.10) | 2.03 (0.52-5.51) | 2.60 (0.83-6.26)‡ | - | 0.65 (0.03-3.20) | 1.95 (0.50-5.30) | ||
| All positive thrombophilia | 60 | 8.18 (5.82-14.9)† | 1.05 (0.05-5.17) | - | 0.70 (0.03-3.43) | 2.09 (0.53-5.68)‡ | - | 0.70 (0.03-3.43) | 1.39 (0.23-4.59) |
| Patient Population . | Thrombophilia Status . | Number of Patients . | Duration of AC Therapy (Months) Median, IQR . | Recurrent VTE on AC (Events/100 PY) . | Recurrent VTE after AC Discontinuation (Events/100 PY) . | Major Bleeding Events (Events/100 PY) . | Mortality (Events/100 PY) . | VTE-related mortality (Events/100 PY) . | Bleeding-related mortality (Events/100 PY) . | Cancer-related mortality (Events/100 PY) . | Notes . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients with VTE provoked by recent surgery | Patients not tested for IT | 9018 | 4.70 (3.15-7.69) | 3.18 (2.73-3.67) | 5.46 (4.78-6.20) | 3.58 (3.22-3.97) | 10.8 (10.2-11.5) | 0.62 (0.48-0.79) | 0.46 (0.34-0.61) | 4.22 (3.83-4.63) | |
| Tested patients (all) | 2075 | 6.41 (4.01-11.0)∗ | 3.72 (2.92-4.69) | 4.63 (3.78-5.62) | 1.21 (0.91-1.58)∗ | 1.79 (1.42-2.22)∗ | 0.07 (0.02-0.19)∗ | 0.07 (0.02-0.19)∗ | 0.93 (0.67-1.25)∗ | ||
| Negative thrombophilia | 1314 | 6.23 (3.91-9.63)∗ | 3.91 (2.82-5.29) | 3.42 (2.57-4.48)† | 1.28 (0.90-1.78)∗ | 1.64 (1.20-2.18)∗ | 0.04 (0.00-0.19)∗ | 0.08 (0.01-0.25)† | 0.84 (0.54-1.25)∗ | ||
| All positive thrombophilia | 761 | 7.00 (4.21-12.6)∗ | 3.51 (2.41-4.95) | 7.25 (5.42-9.50) | 1.10 (0.67-1.70)∗ | 2.03 (1.43-2.80)∗ | 0.12 (0.02-0.39)† | 0.06 (0.00-0.29)† | 1.07 (0.66-1.66)∗ | ||
| Patients with VTE provoked by immobilization ≥4 d | Patients not tested for IT | 19 199 | 4.47 (3.02-7.59) | 3.57 (3.24-3.94) | 8.38 (7.65-9.16) | 5.31 (4.98-5.67) | 25.3 (24.5-26.0) | 1.98 (1.78-2.19) | 1.15 (1.00-1.32) | 5.95 (5.60-6.32) | |
| Tested patients (all) | 3762 | 6.14 (3.61-11.1)∗ | 2.67 (2.15-3.29)‡ | 6.36 (5.48-7.35)† | 1.52 (1.24-1.85)∗ | 3.65 (3.21-4.14)∗ | 0.16 (0.08-0.28)∗ | 0.19 (0.10-0.32)∗ | 0.90 (0.69-1.16)∗ | ||
| Negative thrombophilia | 2449 | 5.82 (3.52-9.03)∗ | 2.26 (1.64-3.03)† | 6.09 (5.06-7.27)† | 1.54 (1.19-1.97)∗ | 3.53 (2.98-4.15)∗ | 0.13 (0.05-0.28)∗ | 0.15 (0.06-0.31)∗ | 0.85 (0.60-1.17)∗ | ||
| All positive thrombophilia | 1313 | 7.03 (4.09-13.4)∗ | 3.25 (2.38-4.33) | 6.95 (5.38-8.83) | 1.48 (1.05-2.04)∗ | 3.86 (3.13-4.70)∗ | 0.21 (0.08-0.46)∗ | 0.25 (0.10-0.52)∗ | 1.00 (0.65-1.46)∗ | ||
| Patients with VTE provoked by estrogen use | Patients not tested for IT | 3466 | 5.22 (3.22-8.54) | 2.55 (1.96-3.26) | 4.61 (3.60-5.83) | 2.12 (1.70-2.62) | 8.89 (7.99-9.86) | 0.46 (0.28-0.71) | 0.31 (0.17-0.52) | 4.25 (3.65-4.94) | |
| Tested patients (all) | 2365 | 6.54 (4.17-11.3)∗ | 1.56 (1.08-2.17)‡ | 2.35 (1.80-3.03)∗ | 0.59 (0.40-0.84)∗ | 0.58 (0.39-0.84)∗ | 0.04 (0.01-0.14)∗ | - | 0.32 (0.19-0.52)∗ | ||
| Negative thrombophilia | 1448 | 6.37 (4.01-9.76)∗ | 1.36 (0.79-2.20)‡ | 2.31 (1.63-3.17)∗ | 0.83 (0.53-1.23)∗ | 0.59 (0.35-0.94)∗ | 0.04 (0.00-0.18)∗ | - | 0.30 (0.14-0.56)∗ | ||
| All positive thrombophilia | 917 | 7.03 (4.57-12.4)∗ | 1.78 (1.07-2.79) | 2.43 (1.56-3.62)† | 0.26 (0.10-0.58)∗ | 0.57 (0.30-0.99)∗ | 0.05 (0.00-0.26)† | - | 0.36 (0.16-0.72)∗ | ||
| Patients with VTE provoked by pregnancy or postpartum | Patients not tested for IT | 676 | 4.67 (3.22-7.20) | 3.28 (1.72-5.70) | 2.53 (1.23-4.63) | 1.29 (0.63-2.38) | 0.99 (0.43-1.97) | 0.14 (0.01-0.70) | 0.14 (0.01-0.70) | 0.14 (0.01-0.70) | |
| Tested patients (all) | 608 | 6.47 (4.17-9.84)∗ | 3.02 (1.75-4.87) | 1.13 (0.55-2.07) | 0.53 (0.23-1.05) | 0.08 (0.00-0.37)† | - | - | 0.08 (0.00-0.37) | ||
| Negative thrombophilia | 313 | 6.34 (4.01-8.77)∗ | 2.95 (1.20-6.14) | 0.23 (0.01-1.11)† | 0.46 (0.12-1.26) | - | - | - | - | ||
| All positive thrombophilia | 295 | 6.64 (4.21-11.1)∗ | 3.07 (1.50-5.63) | 2.27 (1.05-4.31) | 0.60 (0.19-1.45) | 0.15 (0.01-0.73)‡ | - | - | 0.15 (0.01-0.73) | ||
| Unprovoked VTE | Patients not tested for IT | 40 190 | 6.11 (3.42-11.4) | 2.34 (2.18-2.51) | 9.73 (9.22-10.3) | 2.10 (1.97-2.23) | 5.82 (5.61-6.04) | 0.51 (0.45-0.57) | 0.36 (0.31-0.41) | 0.47 (0.41-0.54) | |
| Tested patients (all) | 11 945 | 8.10 (5.22-15.1)∗ | 2.24 (2.00-2.49) | 8.83 (8.22-9.48)‡ | 0.93 (0.81-1.06)∗ | 1.01 (0.90-1.15)∗ | 0.06 (0.03-0.09)∗ | 0.06 (0.04-0.10)∗ | 0.11 (0.08-0.16)∗ | ||
| Negative thrombophilia | 6959 | 7.59 (5.16-13.6)∗ | 2.19 (1.88-2.54) | 8.23 (7.49-9.02)† | 1.02 (0.86-1.20)∗ | 0.99 (0.83-1.16)∗ | 0.06 (0.03-0.11)∗ | 0.06 (0.03-0.11)∗ | 0.13 (0.08-0.20)∗ | ||
| All positive thrombophilia | 4986 | 9.05 (5.39-18.2)∗ | 2.29 (1.95-2.67) | 9.90 (8.83-11.07) | 0.81 (0.65-1.00)∗ | 1.05 (0.87-1.26)∗ | 0.06 (0.02-0.12)∗ | 0.07 (0.03-0.14)∗ | 0.09 (0.05-0.17)∗ | ||
| Patients with splanchnic vein thrombosis | Patients not tested for IT | 611 | 4.67 (2.92-9.33) | 3.26 (1.81-5.43) | 7.39 (4.11-12.32) | 7.85 (5.78-10.5) | 26.6 (22.7-31.0) | 0.17 (0.01-0.84) | 1.52 (0.74-2.80) | 14.7 (11.9-18.1) | |
| Tested patients (all) | 287 | 8.41 (3.75-20.4)∗ | 2.25 (1.10-4.12) | 1.00 (0.17-3.31)† | 1.48 (0.72-2.72)∗ | 1.13 (0.50-2.24)∗ | - | 0.16 (0.01-0.80)† | 0.32 (0.05-1.07)∗ | ||
| Negative thrombophilia | 171 | 6.18 (3.52-16.4)‡ | 2.66 (0.97-5.89) | 1.56 (0.26-5.16)‡ | 0.93 (0.24-2.52)∗ | 0.61 (0.10-2.01)∗ | - | - | 0.61 (0.10-2.01)∗ | ||
| All positive thrombophilia | 116 | 12.0 (4.11-28.8)∗ | 1.88 (0.60-4.54) | - | 2.12 (0.86-4.41)∗ | 1.73 (0.64-3.85)∗ | - | 0.35 (0.02-1.71) | - | ||
| Patients with cerebral sinus vein thrombosis | Patients not tested for IT | 162 | 5.22 (3.24-10.1) | 1.55 (0.26-5.11) | 2.56 (0.43-8.47) | 3.91 (1.82-7.42) | 8.62 (5.27-13.4) | 0.48 (0.02-2.36) | 0.96 (0.16-3.16) | 2.87 (1.16-5.97) | |
| Tested patients (all) | 130 | 9.00 (5.32-15.8)∗ | 1.12 (0.19-3.70) | 1.76 (0.30-5.82) | 1.37 (0.44-3.31) | 2.35 (1.03-4.65)† | - | 0.67 (0.11-2.22) | 1.68 (0.62-3.72) | ||
| Negative thrombophilia | 70 | 10.2 (4.58-15.9)† | 1.20 (0.06-5.94) | 3.06 (0.51-10.10) | 2.03 (0.52-5.51) | 2.60 (0.83-6.26)‡ | - | 0.65 (0.03-3.20) | 1.95 (0.50-5.30) | ||
| All positive thrombophilia | 60 | 8.18 (5.82-14.9)† | 1.05 (0.05-5.17) | - | 0.70 (0.03-3.43) | 2.09 (0.53-5.68)‡ | - | 0.70 (0.03-3.43) | 1.39 (0.23-4.59) |
Patients not tested for IT were used as reference for all comparisons with the remaining subgroups.
DVT, deep venous thrombosis; IQR, interquartile range; PY, patient-years.
P < .001.
P < .01.
P < .05.
VTE provoked by surgery
Rates of VTE recurrence on treatment were similar between tested (4.63 [3.78-5.62]) and untested patients (5.46 [4.78-6.20]). After treatment discontinuation, IT-negative patients had significantly lower VTE recurrence rates (3.42 [2.57-4.48]), whereas IT-positive patients had higher rates (7.25 [5.42-9.50]), although not statistically significant when compared with untested patients.
VTE provoked by immobilization for 4 days or more due to nonsurgical reasons
Patients tested for IT and IT-negative patients had lower recurrence rates on anticoagulant treatment than untested patients (2.67 [2.15-3.29] and 2.26 [1.64-3.03] vs 3.57 [3.24-3.94]; P < .05 and P < .01, respectively). After discontinuation of anticoagulant treatment, although tested patients maintained lower recurrence rates, the difference between IT-negative (6.09 [5.06-7.27]) and IT-positive (6.95 [5.38-8.83]) patients was not statistically significant.
VTE provoked by estrogen use, pregnancy, and postpartum
In estrogen-related VTE, tested patients had lower recurrence rates during treatment (1.56 [1.08-2.17]) than untested ones (2.55 [1.96-3.26]; P < .05), with IT-negative patients exhibiting even lower rates (1.36 [0.79-2.20]; P < .05). Posttherapy recurrence rates were similar between IT-negative (2.31 [1.63-3.17]) and IT-positive (2.43 [1.56-3.62]) groups.
In pregnancy or postpartum-related VTE, recurrence rates during treatment were comparable between untested patients and tested patients, regardless of testing results. Upon treatment discontinuation, IT-negative patients had significantly lower recurrence rates (0.23 [0.01-1.11]) as opposed to IT-positive patients (2.27 [1.05-4.31]; P < .01).
Unprovoked DVT and PE
On-treatment VTE recurrence rates did not significantly differ between those tested and untested for IT, as well as between IT-positive and IT-negative patients, yet AT deficiency was specifically associated with the highest on-treatment recurrence rates (3.46 [1.51-6.84]; P < .001). After the discontinuation of anticoagulant treatment, the recurrence rates spiked, highlighting a continuous risk, particularly for IT-positive patients (9.90 [8.83-11.07]).
Unusual site thrombosis
Among patients with splanchnic vein thrombosis, rates of recurrence during anticoagulant treatment did not significantly differ among all groups. After treatment discontinuation, tested patients, irrespective of their IT status, exhibited significantly lower recurrence rates (1.00 [0.17-3.31]) than those untested (7.39 [4.11-12.32]; P < .01), with no reported recurrences in IT-positive patients. Similarly, in patients with cerebral sinus vein thrombosis, rates of on-treatment VTE recurrence were comparable between the groups, and these similarities persisted after treatment discontinuation, although rate of recurrent events was low, with no reported recurrences in IT-positive patients.
Discussion
In this study, we report the differences in characteristics, treatment, and VTE outcomes between patients enrolled in the large prospective RIETE registry who were tested for IT and those who were not. Approximately one-fifth of RIETE patients were tested for IT, at their physician’s discretion. These patients were generally younger and more likely to present with unusual site thrombosis, events provoked by estrogen exposure, or pregnancy-related events or have prior VTE events. IT testing was less frequent among patients with VTE with prothrombotic risk factors such as recent surgery, immobilization, or active cancer. The impact of IT testing results on posttreatment outcomes varied across subgroups, depending on the risk factors leading to the initial VTE event, highlighting a complex interplay between IT testing and patient outcomes that extends beyond mere diagnostic assessment.
Untested patients showed higher rates of VTE recurrence, major bleeding, and mortality during anticoagulant treatment and after its discontinuation. Furthermore, untested patients consistently showed higher rates of cancer-related mortality, underscoring the profound impact of cancer on their outcomes. Notably, most RIETE patients (91.5%) with cancer were not tested for IT. Specifically, guidelines on VTE management recognize active cancer as a persistent risk factor for VTE, thus warranting long-term anticoagulant treatment and reducing the need for IT testing.12-14
Similarly, patients with unprovoked VTE have a substantial risk of recurrent thrombotic events and are likely to benefit from extended anticoagulant treatment.15,16 Only 22.9% of RIETE patients with unprovoked VTE underwent IT testing. The utility of IT testing in these patients is reduced because although patients with IT exhibit higher rates of VTE recurrence after treatment discontinuation, those without IT also continue to face a consistently high risk of recurrence. Guidelines on VTE management typically advise indefinite anticoagulant treatment for most patients with unprovoked VTE,6-8 reflecting a broad approach that prioritizes long-term prevention of recurrence over selective testing outcomes. In such cases, a strategy of thrombophilia testing followed by tailored treatment may result in fewer patients receiving indefinite anticoagulation but potentially increases the risk of recurrent VTE in patients discontinued based on negative IT results.9
Surgical and immobilization-related VTE showed little variance in recurrence rates across IT testing groups during anticoagulant treatment. Posttreatment recurrence rates were lower in IT-negative patients for surgical VTEs, whereas the effect of IT appeared less pronounced in patients for whom the VTE was triggered by prolonged immobilization. These findings only partially align with the ASH guideline panel’s recommendations, which suggest against IT testing in patients who have completed primary treatment for surgically provoked VTEs, but suggest IT testing in patients with VTE provoked by a nonsurgical major transient risk factors, and prescribe indefinite anticoagulant treatment only to those testing positive.9
Nevertheless, although most patients with VTE provoked by temporary risk factors are recommended to discontinue anticoagulant therapy after primary treatment, testing for thrombophilia would lead to indefinite anticoagulant treatment for patients with IT, increasing bleeding risk and potentially reducing overall clinical benefit. Reflecting a similar trend in clinical practice, only 18.7% of RIETE patients with VTE after recent surgery and 16.4% with VTE after immobilization were tested for IT. This pattern in the RIETE cohort, predating the 2023 guidelines, suggests a longstanding clinical inclination toward selective IT testing in these patient categories. It should be noted, however, that IT-positive patients still exhibited considerable recurrence rates after treatment discontinuation, therefore suggesting that in cases of a known thrombophilia, extending anticoagulant treatment indefinitely may be considered, diverging from current management guidelines.6-8
In RIETE, 40.5% of women with estrogen-associated VTE and 47.3% with pregnancy-associated VTE or VTE during the postpartum period were tested for IT, highlighting a focus on testing in these specific patient groups. Additionally, IT-negative women with pregnancy or postpartum-related VTE experienced significantly lower recurrence rates after treatment discontinuation, indicating a potential to safely stop anticoagulant treatment in this subgroup. However, IT-positive women maintained higher recurrence rates, supporting the need for continued anticoagulation. These findings align with ASH guidelines, which suggest IT testing for women with estrogen, pregnancy, or postpartum-related VTE after completing primary treatment and indefinite anticoagulant treatment for those diagnosed with an IT.9
This approach underscores the value of IT testing in personalizing treatment plans, potentially enhancing safety and efficacy by adjusting the duration of therapy based on IT testing.
The relationship between IT testing and subsequent treatment outcomes in patients with unusual site thromboses is more complex. Although on-treatment recurrence rates showed no differences across IT testing groups, posttreatment outcomes were inferior for untested patients, suggesting the impact of comorbidities, especially among patients with splanchnic vein thrombosis. Tested patients generally had better outcomes, with even IT-positive individuals having no recurrences, although the overall number of events was low. Therefore, although the definitive role of IT testing in guiding treatment duration for unusual site thrombosis is still unclear, it is advisable that patients with persistent risk factors and those with unprovoked thromboses at unusual sites might be considered for indefinite anticoagulant treatment.17,18 In patients with unusual site thrombosis planning to discontinue anticoagulant treatment, the ASH guidelines suggest testing for IT as well as acquired types of thrombophilia, with indefinite anticoagulation in patients testing positive.9
RIETE data indicate comparable treatment outcomes between the subgroup of patients who tested negative for IT and the broader group who underwent IT testing, suggesting that comorbidities and treatment protocols may affect outcomes more than IT itself.
Furthermore, VTE treatment across patient groups was consistent, irrespective of IT status, thereby suggesting that IT detection may not substantially alter VTE management.
This study presents several strengths, including a comprehensive, prospective data collection from the large RIETE cohort, providing insights into the implications of IT testing during and after anticoagulant therapy. The inclusion of patients with all types of IT and the high rates of successful follow-up while reflecting real-world management strategies contribute to the study's external validity. Limitations include the observational nature of the registry, with IT testing reported by individual centers without central verification and the absence of central adjudication for events and outcomes.
This study uniquely demonstrates the complex interplay between IT testing and VTE recurrence in diverse patient subgroups, offering valuable real-world evidence that may inform future revisions of clinical guidelines and practices. Overall, our findings highlight an intricate role of IT testing in tailoring VTE management strategies, indicating that knowledge of IT status may occasionally assist clinicians in stratifying patients more precisely by their risk of recurrence after treatment discontinuation. This underscores the need for a thoughtful approach to IT testing, one that is adapted to specific patient groups in which it can meaningfully affect management decisions and outcomes, given the critical importance of VTE risk factors and underlying conditions.
Acknowledgments
The authors express their gratitude to Sanofi and ROVI for supporting this registry with an unrestricted educational grant. The authors also thank the RIETE Registry Coordinating Center and S&H Medical Science Service for their quality control data and logistic and administrative support and Salvador Ortiz, Universidad Autónoma Madrid, statistical advisor in S&H Medical Science Service for the statistical analysis of the data presented in this study.
RIETE is an investigator-initiated registry. It has been supported by Red Respira from the Instituto Carlos III, Spain (Red Respira-ISCiii-RTIC-03/11), by Sanofi and ROVI. None of these sponsors have had any role in the design of the registry nor do they have rights to access the database, review or comment on prepublished studies from RIETE.
Authorship
Contribution: L.W.R., O.C., and M.M. contributed to study conception and design; L.W.R. and O.C. contributed to the drafting of the manuscript; and all authors contributed to interpretation of the data, contributed to critical revision of the manuscript, and provided final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the Registro Informatizado de Enfermedad TromboEmbólica group appears in “Appendix.”
Correspondence: Omri Cohen, National Hemophilia Center & Institute of Thrombosis & Hemostasis, Sheba Medical Center Tel Hashomer Derech Sheba 2, Ramat Gan 52621, Israel; email: omricmd@gmail.com.
Appendix
Coordinator of the RIETE Registry: Manuel Monreal.
RIETE Steering Committee Members: Paolo Prandoni, Benjamin Brenner and Dominique Farge-Bancel.
RIETE National Coordinators: Raquel Barba (Spain), Pierpaolo Di Micco (Italy), Laurent Bertoletti (France), Sebastian Schellong (Germany), Inna Tzoran (Israel), Abilio Reis (Portugal), Marijan Bosevski (R. Macedonia), Henri Bounameaux (Switzerland), Radovan Malý (Czech Republic), Peter Verhamme (Belgium), Joseph A. Caprini (United States), Hanh My Bui (Vietnam).
RIETE Registry Coordinating Center: S & H Medical Science Service.
The members of the RIETE Group are Spain: Adarraga MD, Alberich-Conesa A, Aibar J, Alda-Lozano A, Alfonso J, Amado C, Angelina-García M, Arcelus JI, Ballaz A, Barba R, Barbagelata C, Barrón M, Barrón-Andrés B, Beddar-Chaib F, Blanco-Molina A, Caballero JC, Castellanos G, Criado J, De Ancos C, Del Toro J, Demelo-Rodríguez P, De Juana-Izquierdo C, Díaz-Peromingo JA, Dubois-Silva A, Escribano JC, Falgá C, Farfán-Sedano AI, Fernández-Aracil C, Fernández-Capitán C, Fernández-Jiménez B, Fernández-Reyes JL, Fidalgo MA, Francisco I, Gabara C, Galeano-Valle F, García-Bragado F, García-Ortega A, Gavín-Sebastián O, Gil-Díaz A, Gómez-Cuervo C, González-García C, González-Munera A, Grau E, Guirado L, Gutiérrez-Guisado J, Hernández-Blasco L, Herreros M, Jara-Palomares L, Jaras MJ, Jiménez D, Jou I, Joya MD, Lecumberri R, Llamas P, Lobo JL, López-Jiménez L, López-Miguel P, López-Brull H, López-Núñez JJ, López-Ruiz A, López-Sáez JB, Lorenzo A, Lumbierres M, Madridano O, Maestre A, Marchena PJ, Marcos M, Martín del Pozo M, Martín-Martos F, Martínez-Prado R, Maza JM, Mena E, Mercado MI, Moisés J, Molino A, Monreal M, Morales MV, Navas MS, Nieto JA, Núñez-Fernández MJ, Olid M, Ordieres-Ortega L, Ortiz M, Osorio J, Otálora S, Otero R, Pacheco-Gómez N, Pagán J, Palomeque AC, Paredes E, Parra-Caballero P, Pedrajas JM, Pérez-Ductor C, Pérez-Pinar M, Peris ML, Pesce ML, Porras JA, Puchades R, Rivera-Cívico F, Rodríguez-Cobo A, Romero-Brugera M, Ruiz-Artacho P, Ruiz-Giménez N, Ruiz-Ruiz J, Salgueiro G, Sancho T, Sendín V, Sigüenza P, Soler S, Steinherr A, Suárez-Fernández S, Tirado R, Torrents-Vilar A, Torres MI, Trujillo-Santos J, Uresandi F, Valle R, Varona JF, Villalobos A, Villares P, Austria: Ay C, Nopp S, Pabinger I, Belgium: Vanassche T, Verhamme P, Verstraete A, Brazil: Yoo HHB, Colombia: Montenegro AC, Morales SN, Roa J, Czech Republic: Hirmerova J, Malý R, France: Bertoletti L, Bura-Riviere A, Catella J, Chopard R, Couturaud F, Espitia O, Grange C, Le Mao R, Leclercq B, Mahé I, Morange P, Moustafa F, Plaisance L, Sarlon-Bartoli G, Suchon P, Versini E, Germany: Schellong S, Israel: Brenner B, Dally N, Kenet G, Tzoran I, Iran: Sadeghipour P, Rashidi F, Italy: Abenante A, Barillari G, Basaglia M, Bertoni M, Bilora F, Brandolin B, Ciammaichella M, Colaizzo D, Dentali F, Di Micco P, Grandone E, Imbalzano E, Negro F, Pesavento R, Poz A, Prandoni P, Siniscalchi C, Taflaj B, Tufano A, Visonà A, Zalunardo B, Latvia: Skride A, Kigitovica D, Zicans M, Portugal: Fonseca S, Marques R, Meireles J, Pinto S, Republic Of Macedonia: Bosevski M, Trajkova M, Zdraveska M, Switzerland: Bounameaux H, Mazzolai L, United Kingdom: Aujayeb A, United States: Caprini JA, Weinberg I, Vietnam: Bui HM.
References
Author notes
O.C. and L.W.R. contributed equally to this work.
Data are available upon request by email in accordance with RIETE policies.