Key Points
PKa deficiency selectively impairs contact pathway–mediated TG in mouse WB.
PKa supports FXII-independent TG in mouse WB, consistent with direct activation of FIX.
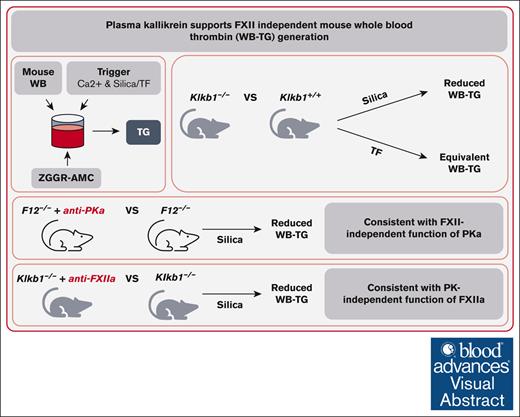
Visual Abstract
Plasma kallikrein (PKa) is an important activator of factor XII (FXII) of the contact pathway of coagulation. Several studies have shown that PKa also possesses procoagulant activity independent of FXII, likely through its ability to directly activate FIX. We evaluated the procoagulant activity of PKa using a mouse whole blood (WB) thrombin-generation (TG) assay. TG was measured in WB from PKa-deficient mice using contact pathway or extrinsic pathway triggers. PKa-deficient WB had significantly reduced contact pathway–initiated TG compared with that of wild-type controls and was comparable with that observed in FXII-deficient WB. PKa-deficient WB supported equivalent extrinsic pathway–initiated TG compared with wild-type controls. Consistent with the presence of FXII-independent functions of PKa, targeted blockade of PKa with either small molecule or antibody-based inhibitors significantly reduced contact pathway–initiated TG in FXII-deficient WB. Inhibition of activated FXII (FXIIa) using an antibody-based inhibitor significantly reduced TG in PKa-deficient WB, consistent with a PKa-independent function of FXIIa. Experiments using mice expressing low levels of tissue factor demonstrated that persistent TG present in PKa- and FXIIa-inhibited WB was driven primarily by endogenous tissue factor. Our work demonstrates that PKa contributes significantly to contact pathway–initiated TG in the complex milieu of mouse WB, and a component of this contribution occurs in an FXII-independent manner.
Introduction
Vascular injury results in activation of the extrinsic pathway of coagulation through the exposure of subendothelial tissue factor (TF) that functions as an essential cofactor for activated factor VII (FVIIa).1,2 The TF/FVIIa complex catalyzes the generation of FXa. Coagulation can also be activated through the contact pathway, in which the exposure of FXII to a negatively charged surface or molecules leads to autoactivation.3-5 Both FXII and plasma prekallikrein (PK) possess inherent proteolytic activity and can reciprocally activate one another to form FXIIa and plasma kallikrein (PKa), in a process enhanced by negatively charged surface and the cofactor high molecular weight kininogen.6-9 FXIIa catalyzes FXIa generation, resulting in subsequent FIXa generation that, in complex with the cofactor FVIIIa, drives FXa generation. FXa, generated by either the extrinsic or contact pathway, complexed with FVa cofactor drives the generation of the terminal coagulation protease thrombin.
A number of other reactions outside the traditional waterfall model of coagulation have been described that enhance thrombin generation (TG). These include TF/FVIIa-mediated activation of FIX, TF/FVIIa/FXa-mediated activation of FVIII, thrombin-mediated activation of FXI, and PKa-mediated activation of FIX.10-13 We previously reported that although TF/FVIIa-mediated activation of FIXa was readily apparent in mouse plasma, there was no evidence of thrombin-mediated activation of FXI.14 Interestingly, however, more recently, we reported on a mouse whole blood (WB) TG assay that was sensitive to both TF/FVIIa-mediated activation of FIX and thrombin-mediated activation of FXI.15 Importantly, this finding more closely resembled the TG phenotype described in human plasma.12
PK and FXI are products of an ancient gene duplication event, leading to the hypothesis that the 2 enzymes may share the ability to cleave and activate FIX.13,16,17 Early studies demonstrated that PKa could indeed directly activate FIX in purified systems.18-21 A series of more recent studies further demonstrated direct FIX activation by PKa in both purified and plasma-based systems.22-24 Although PKa was found to be a less efficient activator of FIX than the canonical activator FXIa, indirect evidence suggests that PKa contributes significantly to FIXa activation and downstream TG independent of FXII and FXI in human plasma in vitro.22-24 The presence of FXI-independent FIXa generation in mice exposed to a contact pathway agonist further suggests that this reaction occurs in vivo.22 Although PKa-mediated activation of FIX was not reported to require cofactors,24 red blood cell (RBC)–derived extracellular vesicles (EVs) were identified as a surface that mediates the action of PKa on FIX.23 The ability of RBC EVs to promote PKa-mediated activation of FIX suggested that this reaction may also contribute to TG in RBC-replete WB.
In establishing the mouse WB TG assay, we observed that FXII- and FXI-deficient, but not FIX-deficient, mouse WB was capable of supporting significant levels of contact pathway–initiated TG.15 This suggested that contact pathway activation could occur absent of FXII and FXI but not FIX. Such an observation was consistent with the ability of PKa to directly activate FIX.22-24 Therefore, we evaluated the contribution of PKa to FXII-independent contact pathway–initiated TG in mouse WB using combinations of genetic and pharmacologic disruption of PKa and FXIIa in our recently established assay.
Methods
Mice
Male and female 8- to 12-week-old Klkb1–/– or F12–/– mice and appropriate wild-type controls, backcrossed 10 generations onto the C57BL/6J background, were used.15,25 Male and female 8- to 12-week-old TFlow mice (mF3–/–;hF3+/+) or TFwt mice (mF3+/+;hF3–/–), backcrossed 6 generations onto the C57BL/6J background, were also used.26 All studies complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were conducted under the approval of the Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Blood collection
WB was collected from the inferior vena cava of anesthetized mice into sodium citrate (final concentration, 0.38% v/v; RICCA Chemical, Arlington, TX) and corn trypsin inhibitor (CTI; final concentration, 50 μg/mL; Haematologic Technologies, Essex Junction, VT). Platelet-poor plasma (PPP) was generated by the centrifugation of WB at 4500g for 15 minutes. Platelet-rich plasma (PRP) was generated by the centrifugation of WB twice at 150g for 5 minutes. Washed RBCs were prepared by collecting 150 μL of packed RBCs after removal of PRP, washing 3 times in 1 mL of wash buffer (1.29 mM sodium citrate, 3.33 mM glucose, 124 mM NaCl, and pH 7.2), and resuspending at 7 × 106/μL in Hepes buffered saline, with cell counts determined using Element HT5 (Heska, Loveland, CO). WB was used within 1 hour of collection, and PPP was aliquoted and frozen at –80°C.
Mouse TG
WB TG was assessed as previously described.15 In brief, 24 μL of ZGGR-AMC (Z-Gly-Gly-Arg–7-amino-4-methylcoumarin) thrombin substrate (final concentration, 416.7 μM; Bachem, Bubendorf, Switzerland) was added to 72 μL of WB in wells of a round bottom 96-well plate (Caplugs Evergreen, Buffalo, NY) and incubated at 37°C for 10 minutes, after which 48 μL of trigger solution containing CaCl2 (final concentration, 9 mM) and TF (final concentration, 0.05pM or 0.5pM; Innovin, Siemens, Munich, Germany) or silica (final dilution, 1:120; Kontact, Thermo Fisher Scientific, Waltham, MA) was added. The WB solution was mixed gently, and 60 μL was dispensed into a polyvinyl chloride round bottom 96-well plate (Corning, Corning, NY) in duplicate. Fluorescence was measured on a fluorometer (Fluoroskan Ascent, Thermo Fisher Scientific) at an excitation wavelength of 355 nm and an emission wavelength of 460 nm at 37°C, with data acquired using Ascent Software (version 2.6; Thermo Fisher Scientific). To aid with reproducibility, data were acquired for 36 wells with an integration time of 6 seconds to ensure continuous agitation and prevent RBC sedimentation. WB TG parameters were calculated as described previously. Experiments with PRP and RBC-reconstituted PRP were conducted as previously described.15 In brief, washed RBCs at 7 × 106/μL were added to an equal volume of PRP (diluted twofold), with PRP (diluted threefold) used as a comparator. TG was assessed as described for WB above.
In some experiments, samples were supplemented with the small molecule PKa inhibitor berotralstat (MedChem Express, Monmouth Junction, NJ), an anti-FXIIa antibody (garadacimab/CSL-312 bio-similar; ATUM Bio, Newark, CA), an anti-PKa antibody (lanadelumab bio-similar; ProteoGenix USA, Miami, FL), an anti-TF antibody (1H1; Genentech, San Francisco, CA), or appropriate vehicle and immnuoglubulin G (IgG) controls. CSL-312 was produced recombinantly in sterile and low endotoxin conditions using the amino acid sequence detailed in the US patent (US-20170121423-A1). The affinity of CSL-312 for FXIIa was confirmed by homogeneous time-resolved fluorescence and inhibitory activity in activated partial thromboplastin time assays in pooled human plasma (ATUM Bio).
PPP TG was assessed as previously described.14,15 In brief, 10 μL of trigger solution containing phospholipids (final concentration, 4 μM; Synapse Research Institute, Maastricht, The Netherlands) and TF (final concentration, 0.05 pM or 0.5 pM; Innovin, Siemens) or silica (final dilution, 1:120; Kontact, Thermo Fisher Scientific) was added to 40 μL of prediluted PPP (PPP:buffer, 1:3) in a round bottom 96-well plate (Caplugs Evergreen) and incubated at 37°C for 5 minutes, after which 10 μL of a CaCl2 (final concentration, 16.7 mM) and ZGGR-AMC thrombin substrate (final concentration, 416.7 μM; Bachem). Fluorescence was measured on a fluorometer (Fluoroskan Ascent, Thermo Fisher Scientific) at a λex of 390 nm and λem of 460 nm at 37°C and analyzed using Thrombinoscope software (v5.0; Thrombinoscope BV, Maastricht, The Netherlands).
PK activation
The ability of recombinant human PK (50 nM; R&D Systems, Minneapolis, MN) in the presence of recombinant human high molecular weight kininogen (300 nM; R&D Systems) to cleave the chromogenic substrate S2303 (400 μM; DiaPharma, West Chester, OH) in the presence or absence of dextran sulfate (25 μg/mL; 500 kDa; Millipore Sigma, St. Louis, MO) was determined using a spectrophotometer (SpectraMax i3x; Molecular Devices, San Jose, CA).
Statistics
Normality of data was assessed by Shapiro-Wilk tests and parametric or nonparametric tests, selected as appropriate. For 2 group analyses, parametric Student t tests or nonparametric Mann-Whitney U tests were used. Data were analyzed using Prism Software (v9.1; GraphPad, San Diego, CA). A 2-sided P value <.05 was considered statistically significant.
Results
Both PK and FXII contribute to contact pathway–initiated mouse TG
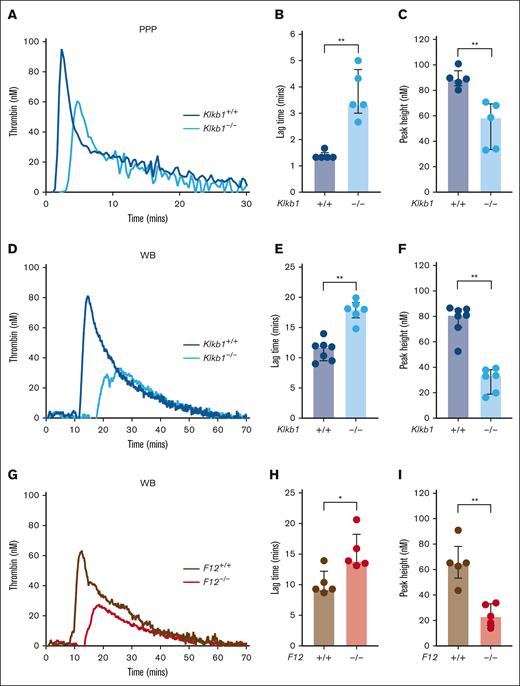
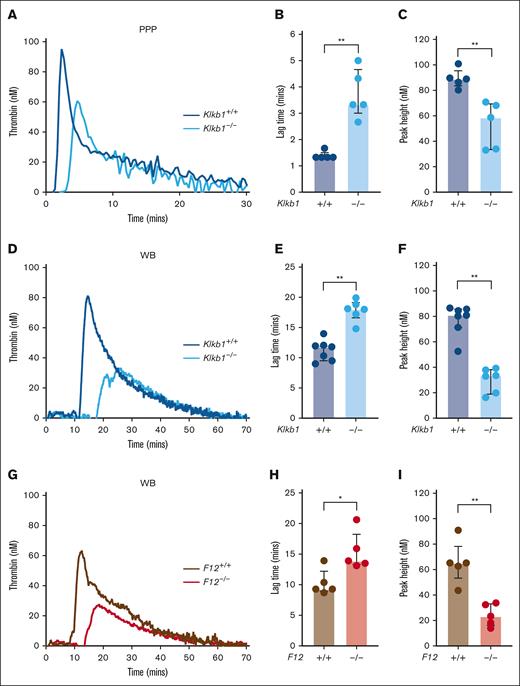
To evaluate the relative contribution of PK and FXII to contact pathway–initiated coagulation in mice, we assessed silica-initiated TG in WB from PK-deficient mice, FXII-deficient mice, and appropriate wild-type controls. In contrast to previous findings in PPP from FXII-deficient mice,15 PPP from Klkb1–/– mice supported detectable silica-initiated TG, although the TG lag time was significantly prolonged and the TG peak significantly reduced compared with Klkb1+/+ control PPP (Figure 1A-C). Silica-initiated TG in Klkb1–/– mouse WB was markedly reduced, with TG lag time significantly prolonged by ∼50% and peak TG significantly reduced by ∼60% compared with WB from Klkb1+/+ control mice (Figure 1D-F). Silica-initiated TG in F12–/– mouse WB was also markedly reduced, with TG lag time significantly prolonged by ∼50%, whereas peak TG was significantly reduced by ∼65% compared with WB from F12+/+ control mice (Figure 1G-I).
Effect of PK and FXII deficiency on contact pathway–initiated TG. (A-C) Representative silica-initiated TG curves in Klkb1+/+ and Klkb1–/– mice PPP (A) with quantification of TG lag time (B) and peak TG (n = 5 per group) (C). (D-F) Representative silica-initiated TG curves in Klkb1+/+ and Klkb1–/– mouse WB (D) with quantification of TG lag time (E) and peak TG (n = 6-7 per group) (F). (G-I) Representative silica-initiated TG curves in F12+/+ and F12–/– mouse WB (G) with quantification of TG lag time (H) and peak TG (n = 5 per group) (I). Data are presented as individual values with median and interquartile range. Data are analyzed with Mann-Whitney U tests; ∗P < .05; ∗∗P < .01.
Effect of PK and FXII deficiency on contact pathway–initiated TG. (A-C) Representative silica-initiated TG curves in Klkb1+/+ and Klkb1–/– mice PPP (A) with quantification of TG lag time (B) and peak TG (n = 5 per group) (C). (D-F) Representative silica-initiated TG curves in Klkb1+/+ and Klkb1–/– mouse WB (D) with quantification of TG lag time (E) and peak TG (n = 6-7 per group) (F). (G-I) Representative silica-initiated TG curves in F12+/+ and F12–/– mouse WB (G) with quantification of TG lag time (H) and peak TG (n = 5 per group) (I). Data are presented as individual values with median and interquartile range. Data are analyzed with Mann-Whitney U tests; ∗P < .05; ∗∗P < .01.
PKa contributes to contact pathway–initiated mouse WB TG in a partially FXII-independent manner
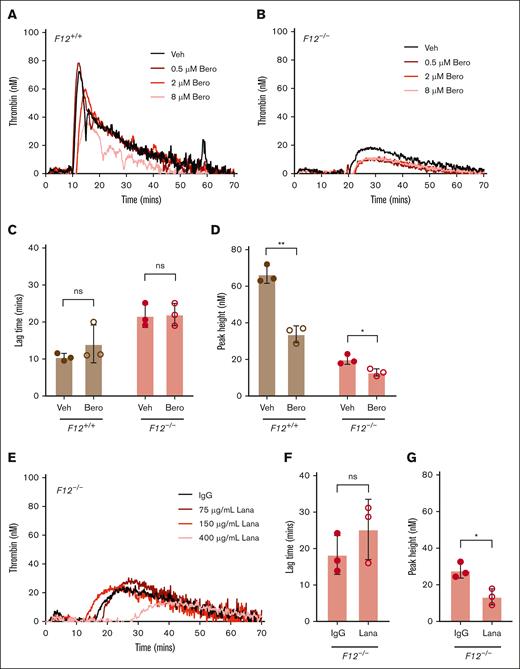
To evaluate the contribution of PKa to FXII-independent contact pathway–mediated TG, silica-initiated TG was evaluated in WB from F12+/+ or F12–/– mice in the presence or absence of the PKa inhibitor berotralstat. Initial experiments indicated that 8 μM of berotralstat was sufficient to partially inhibit silica-initiated TG in WB from F12+/+ and F12–/– mice (Figure 2A-B), and this concentration was used for subsequent experiments. PKa inhibition had no significant effect on silica–initiated TG lag time in WB from F12+/+ or F12–/– mice (Figure 2C). PKa inhibition significantly reduced silica-initiated peak TG in WB from F12+/+ mice (Figure 2D). Importantly, the PKa inhibitor berotralstat also significantly reduced silica-initiated peak TG in WB from F12–/– by ∼35% compared with vehicle control (Figure 2D). Berotralstat had no observable effect on WB TG initiated with 0.05 pM TF (data not shown).
Effect of PKa inhibition on contact pathway–initiated TG in FXII-sufficient and -deficient WB. Representative curves of silica-initiated TG in F12+/+ (A) and F12–/– (B) mouse WB in the presence of increasing concentrations of the PKa inhibitor (PKai) berotralstat or vehicle control. Quantification of TG lag time (C) and peak TG (D) in F12+/+ or F12–/– WB in the presence of 8 μM berotralstat or vehicle control (n = 3 per group). (E) Representative curves of silica-initiated TG in F12–/– mouse WB in the presence of increasing concentrations of the inhibitory anti-PKa antibody lanadelumab or IgG control. Quantification of TG lag time (F) and peak TG (G) in F12–/– WB in the presence of 400 μg/mL lanadelumab or IgG control (n = 3 per group). Data presented as individual values with mean ± standard deviation (SD). Data analyzed with paired Student t tests comparing samples with and without inhibitor; ∗P < .05; ∗∗P < .01. Bero, berotralstat; Lana, lanadelumab; ns, not significant; Veh, vehicle.
Effect of PKa inhibition on contact pathway–initiated TG in FXII-sufficient and -deficient WB. Representative curves of silica-initiated TG in F12+/+ (A) and F12–/– (B) mouse WB in the presence of increasing concentrations of the PKa inhibitor (PKai) berotralstat or vehicle control. Quantification of TG lag time (C) and peak TG (D) in F12+/+ or F12–/– WB in the presence of 8 μM berotralstat or vehicle control (n = 3 per group). (E) Representative curves of silica-initiated TG in F12–/– mouse WB in the presence of increasing concentrations of the inhibitory anti-PKa antibody lanadelumab or IgG control. Quantification of TG lag time (F) and peak TG (G) in F12–/– WB in the presence of 400 μg/mL lanadelumab or IgG control (n = 3 per group). Data presented as individual values with mean ± standard deviation (SD). Data analyzed with paired Student t tests comparing samples with and without inhibitor; ∗P < .05; ∗∗P < .01. Bero, berotralstat; Lana, lanadelumab; ns, not significant; Veh, vehicle.
To independently confirm the contribution of PKa to FXII-independent contact pathway–mediated TG, additional experiments were conducted in WB from F12–/– mice with the anti-PKa antibody lanadelumab. Initial experiments indicated that 400 μg/mL of lanadelumab was sufficient to inhibit silica-initiated TG in WB from F12–/– mice, and this concentration was used for subsequent experiments (Figure 2E). Although landelumab did not significantly alter TG lag time, peak TG was significantly reduced by ∼50% in WB from F12–/– mice (Figure 2F-G), a finding similar to that using berotralstat.
The FXII-independent contribution of PK to TG indicates that PK activation can occur without FXII. To investigate this, the ability of the contact activator dextran sulfate to potentiate proteolytic activity of human recombinant PK zymogen in the presence of recombinant human high molecular weight kininogen was determined in a purified system. Proteolytic activity of recombinant PK zymogen was observed and could be significantly enhanced by dextran sulfate (supplemental Figure 1).
Platelets and RBCs contribute to FXII-independent contributions of PKa in contact pathway–initiated TG
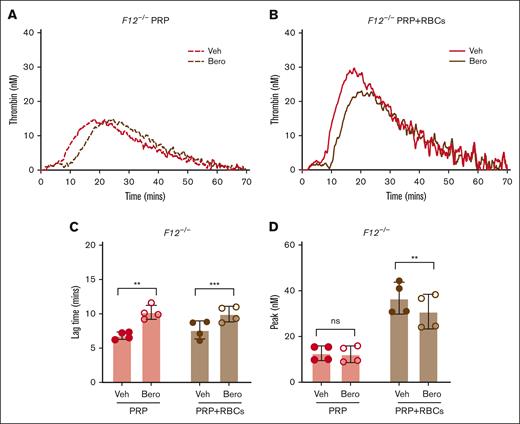
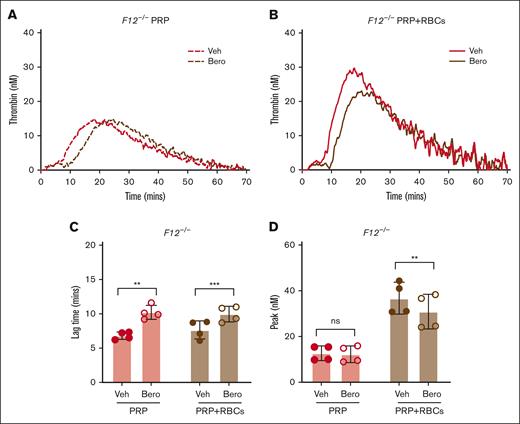
To evaluate the contribution of platelets and RBCs to FXII-independent TG, additional experiments were conducted with PRP and RBC-reconstituted PRP (PRP with RBCs) generated from F12–/– mice. Both F12–/– PRP and PRP with RBCs supported detectable contact pathway–initiated TG in the absence of exogenous lipid; however, TG was markedly more robust in PRP with RBCs, consistent with the procoagulant potential of the RBC surface (Figure 3A-B). Inhibition of PKa using berotralstat significantly prolonged TG lag time in both PRP and PRP with RBCs (Figure 3C). Importantly, however, PKa inhibition with berotralstat reduced peak TG in PRP with RBCs but not in PRP alone (Figure 3D). This indicates that both the platelet and RBC surfaces can contribute to the FXII-independent functions of PKa.
Effect of PKa inhibition on contact pathway–initiated TG in FXII-deficient PRP and RBC-reconstituted PRP. Representative curves of silica-initiated TG in F12–/– PRP (A) and F12–/– PRP reconstituted with RBCs (PRP+RBCs) (B) in the presence of the PKa inhibitor berotralstat or vehicle control. Quantification of TG lag time (C) and peak TG (D) in F12–/– PRP and PRP with RBCs (n = 4 per group). Data presented as mean ± SD with paired t tests comparing samples with and without inhibitor; ∗∗P < .01; ∗∗∗P < .001. Bero, berotralstat; ns, not significant; Veh, vehicle.
Effect of PKa inhibition on contact pathway–initiated TG in FXII-deficient PRP and RBC-reconstituted PRP. Representative curves of silica-initiated TG in F12–/– PRP (A) and F12–/– PRP reconstituted with RBCs (PRP+RBCs) (B) in the presence of the PKa inhibitor berotralstat or vehicle control. Quantification of TG lag time (C) and peak TG (D) in F12–/– PRP and PRP with RBCs (n = 4 per group). Data presented as mean ± SD with paired t tests comparing samples with and without inhibitor; ∗∗P < .01; ∗∗∗P < .001. Bero, berotralstat; ns, not significant; Veh, vehicle.
FXIIa contributes to contact pathway–initiated mouse WB TG in a partially PKa-independent manner
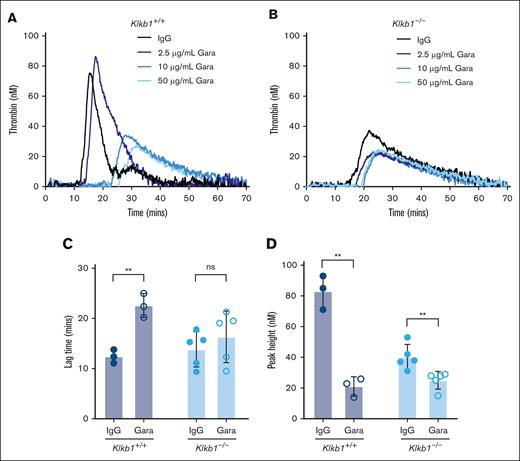
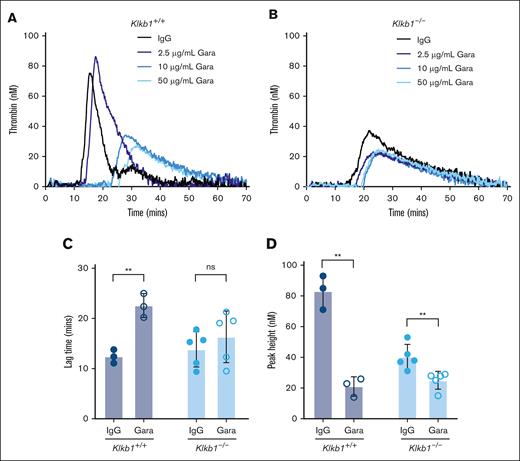
To evaluate the contribution of FXIIa to PKa-independent contact pathway–mediated TG, silica-initiated TG was evaluated in WB from Klkb1+/+ or Klkb1–/– mice in the presence or absence of the anti-FXIIa antibody garadacimab. Initial experiments indicated that 50 μg/mL of garadacimab was sufficient to reduce silica-initiated TG in WB from Klkb1+/+ or Klkb1–/– mice, and this concentration was used for subsequent experiments (Figure 4A-B). As expected, in WB triggered with silica, garadacimab significantly prolonged TG lag time and reduced TG peak in Klkb1+/+ WB compared with IgG control (Figure 4C-D). In Klkb1–/– mouse WB triggered with silica, the anti-FXIIa antibody garadacimab had no significant effect on TG lag time but significantly reduced peak TG (Figure 4C-D).
Effect of FXIIa inhibition on contact pathway–initiated TG in PK-sufficient and -deficient WB. Representative curves of silica-initiated TG in Klkb1+/+ (A) and Klkb1–/– (B) mouse WB in the presence of increasing concentrations of the inhibitory anti-FXIIa antibody garadacimab. Quantification of TG lag time (C) and peak TG (D) in Klkb1+/+ or Klkb1–/– mouse WB in the presence of 50 μg/mL garadacimab or IgG control (n = 3-5 per group). Data presented as individual values with mean ± SD. Data analyzed with paired Student t tests comparing samples with and without inhibitor; ∗∗P < .01. Gara, garadacimab; ns, not signficant.
Effect of FXIIa inhibition on contact pathway–initiated TG in PK-sufficient and -deficient WB. Representative curves of silica-initiated TG in Klkb1+/+ (A) and Klkb1–/– (B) mouse WB in the presence of increasing concentrations of the inhibitory anti-FXIIa antibody garadacimab. Quantification of TG lag time (C) and peak TG (D) in Klkb1+/+ or Klkb1–/– mouse WB in the presence of 50 μg/mL garadacimab or IgG control (n = 3-5 per group). Data presented as individual values with mean ± SD. Data analyzed with paired Student t tests comparing samples with and without inhibitor; ∗∗P < .01. Gara, garadacimab; ns, not signficant.
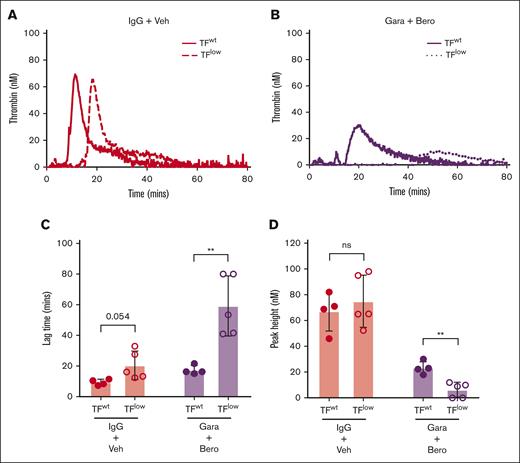
Endogenous TF drives basal TG in contact pathway–inhibited mouse WB
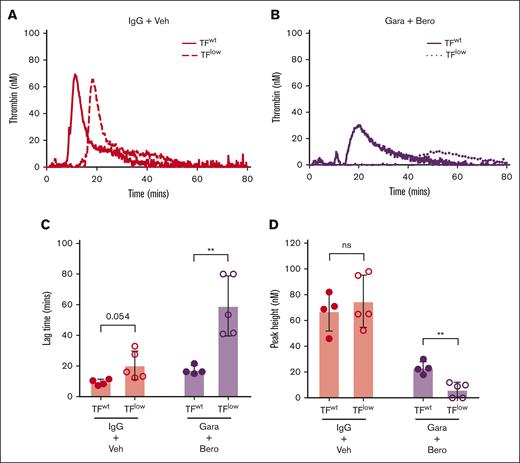
TG was observed in both PKa-inhibited F12–/– mouse WB and FXIIa-inhibited Klkb1–/– mouse WB when triggered with silica. Although it is possible that this could have resulted from incomplete PKa and FXIIa inhibition, we hypothesized that endogenous EV TF present in WB may have contributed to the observed residual TG. To evaluate the influence of endogenous TF in this setting, silica-initiated TG was evaluated in WB from TFlow mice and TFwt controls in the presence or absence of the the PKa inhibitor berotralstat and the anti-FXIIa antibody garadacimab. WB from TFlow mice had a mild impairment in silica-initiated TG with a nonsignificant trend toward reduced TG lag time but no observable effect of peak TG compared with WB from TFwt mice (Figure 5A-D). When both PKa and FXIIa activity were inhibited, TG was markedly reduced in WB from TFlow mice compared with TFwt controls (Figure 5B), with significantly prolonged TG lag time and reduced peak TG (Figure 5C-D). To further investigate the contribution of endogenous TF to basal TG, the effect of the anti-TF antibody 1H1 on TG was evaluated in wild-type mouse WB upon recalcification. Inhibition of TF by 1H1 markedly reduced basal TG with a significant reduction in peak TG, supporting the findings from TFlow mouse WB (supplemental Figure 2).
Effect of endogenous TF on contact pathway–initiated TG in WB. Representative curves of silica-initiated TG in TFlow or TFwt mouse WB (A) and TFlow or TFwt mouse WB (B) in the presence of 8 μM berotralstat and 50 μg/mL garadacimab with quantification of TG lag time (C) and peak TG (n = 4-5 per group) (D). Data presented as individual values with mean ± SD. Data analyzed with paired Student t tests comparing TFlow and TFwt; ∗∗P < .01. Bero, berotralstat; ns, not significant; Veh, vehicle.
Effect of endogenous TF on contact pathway–initiated TG in WB. Representative curves of silica-initiated TG in TFlow or TFwt mouse WB (A) and TFlow or TFwt mouse WB (B) in the presence of 8 μM berotralstat and 50 μg/mL garadacimab with quantification of TG lag time (C) and peak TG (n = 4-5 per group) (D). Data presented as individual values with mean ± SD. Data analyzed with paired Student t tests comparing TFlow and TFwt; ∗∗P < .01. Bero, berotralstat; ns, not significant; Veh, vehicle.
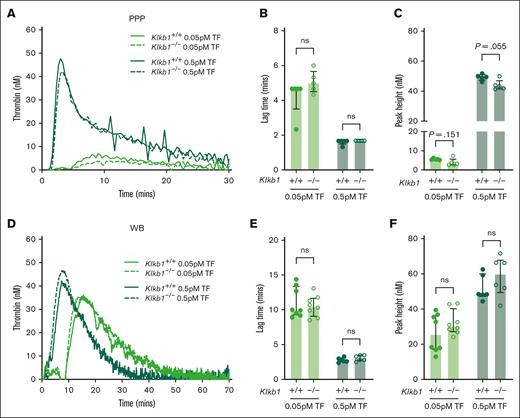
PK deficiency does not significantly alter extrinsic pathway–initiated TG in mouse PPP or WB
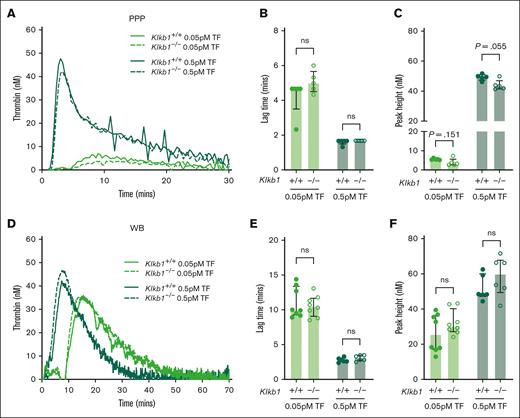
The effect of PK deficiency on extrinsic pathway–initiated TG in mouse PPP and WB was also assessed using low (0.05 pM) and moderate (0.5 pM) doses of TF trigger. When using either dose of TF, no apparent difference in TG was observed in PPP from Klkb1–/– mice compared with Klkb1+/+ controls (Figure 6A). A small but not significant trend toward prolonged TG lag time and reduced peak TG was apparent in PPP from Klkb1–/– mice, compared with Klkb1+/+ controls, when initiated with the low TF dose (0.05 pM; Figure 6B-C). A small but nonsignificant reduction in peak TG was noted in PPP from Klkb1–/– mice, compared with Klkb1+/+ controls, when initiated with the moderate TF dose (0.5 pM; Figure 6C). No spontaneous TG was observed in recalcified PPP from Klkb1–/– mice or Klkb1+/+ controls (data not shown). Similarly, no spontaneous TG was observed in recalcified PPP from F12–/– mice or F12+/+ controls (data not shown). Furthermore, when initiated with low (0.05 pM) or moderate (0.5 pM) doses of TF, TG lag time and peak in WB from Klkb1–/– mice did not differ significantly from Klkb1+/+ controls (Figure 6D-F).
Effect of PK deficiency on extrinsic pathway–initiated TG. (A) Representative TF-initiated TG curves in Klkb1+/+ and Klkb1–/– mouse PPP. Quantification of TG lag time (B) and peak TG (C) in Klkb1+/+ and Klkb1–/– mouse PPP initiated with 0.05 pM and 0.5 pM TF (n = 5 per group). (D) Representative TF-initiated TG curves in Klkb1+/+ and Klkb1–/– mouse WB. Quantification of TG lag time (E) and peak TG (F) in Klkb1+/+ and Klkb1–/– mouse WB initiated with 0.05 pM and 0.5 pM TF (n = 6-8 per group). Data are presented as individual values with median and interquartile range. Data were analyzed with Mann-Whitney U tests comparing Klkb1+/+ and Klkb1–/– samples under the same trigger conditions; ∗∗P < .01.
Effect of PK deficiency on extrinsic pathway–initiated TG. (A) Representative TF-initiated TG curves in Klkb1+/+ and Klkb1–/– mouse PPP. Quantification of TG lag time (B) and peak TG (C) in Klkb1+/+ and Klkb1–/– mouse PPP initiated with 0.05 pM and 0.5 pM TF (n = 5 per group). (D) Representative TF-initiated TG curves in Klkb1+/+ and Klkb1–/– mouse WB. Quantification of TG lag time (E) and peak TG (F) in Klkb1+/+ and Klkb1–/– mouse WB initiated with 0.05 pM and 0.5 pM TF (n = 6-8 per group). Data are presented as individual values with median and interquartile range. Data were analyzed with Mann-Whitney U tests comparing Klkb1+/+ and Klkb1–/– samples under the same trigger conditions; ∗∗P < .01.
Discussion
In this study, we evaluated the contribution of PKa to TG in mouse WB. Results from PK-deficient WB demonstrated that PKa contributes significantly to contact pathway–mediated but not extrinsic pathway–mediated TG. Critically, however, inhibition of PKa significantly and reproducibly attenuated residual TG in FXII-deficient WB. These findings demonstrate that the FXII-independent function of PKa contributes significantly to TG in WB and are consistent with the direct activation of FIX by PKa.
To evaluate the contribution of PKa to TG, we used our recently developed mouse WB TG assay.15 We previously demonstrated the sensitivity of this assay to TF/FVIIa-mediated activation of FIX and thrombin-mediated activation of FXI. This mouse WB TG assay allowed for the use of WB from mice completely deficient in either PK or FXII in combination with highly selective small molecule inhibitors. Importantly, during the development of this WB assay residual contact pathway–initiated TG was observed in FXII- and FXI-deficient, but not FIX-deficient, WB.15 These findings were similar to those in a prior report of RBC EV–initiated TG in human plasma that supported a FXII-independent, but FIX-dependent, contribution of PKa to TG.23 The observed residual TG in FXII-deficient mouse WB in our study allowed for the interrogation of FXII-independent contributions of PKa in the complex environment of WB.
Initial evaluation of PK-deficient mouse samples demonstrated a partial defect in contact pathway–initiated TG in both WB and plasma, consistent with a previous report.27 Interestingly, the ability of PK-deficient mouse plasma to support some contact pathway–initiated TG contrasts with the findings in which TG was almost completely absent in FXII-deficient mouse plasma.15,27 This observation suggests that in mouse, plasma FXII is essential, whereas PK is at least partially dispensable. In contrast to the phenotype observed in mouse plasma, PK-deficient and FXII-deficient mouse WB demonstrated comparable partial reductions in contact pathway–initiated TG.15 This phenotype is consistent with PK-independent functions of FXIIa and FXII-independent functions of PKa occurring in WB.
To assess FXII-independent contributions of PK in WB more directly, we determined the effect of PKa inhibition on contact pathway–mediated TG in the presence or absence of FXII. The ability of the PKa inhibitor berotralstat to significantly reduce contact pathway–initiated TG in FXII-deficient WB was indicative of an FXII-independent function of PKa. The related compound avoralstat was previously shown to inhibit FVIIa.28 However, no effect of berotralstat was observed on extrinsic pathway–initiated TG, suggesting that the contact pathway phenotype was unlikely to be an off-target effect of the inhibitor.29 Furthermore, the highly specific anti-PKa antibody lanadelumab resulted in a similar reduction in contact pathway–initiated TG in FXII-deficient WB. Lanadelumab is not reported to have any significant off-target effects when used at concentrations in the low micromolar range.30,31 Taken together, these data provide evidence of an FXII-independent contribution of PKa to contact pathway–initiated TG in mouse WB.
The findings of this study complement the 3 recent reports of FXII-independent functions of PKa.22-24 Both Noubouossie et al and Kearney et al demonstrated that PKa can directly activate FIX in a purified system.23,24 Furthermore, Visser et al, Noubouossie et al, and Kearney et al showed that PKa contributes to contact pathway–initiated TG in a partially FXII- and FXI-independent manner in human plasma.22-24 Our data demonstrate that the FXII-independent functions of PK persist in the complex environment of WB and is consistent with the in vivo phenotype reported by Visser et al.22
Although consistent with the direct activation of FIX by PKa, other FXII-independent mechanisms may have also contributed to the phenotype we observed. As reported by Kearney et al, PKa can also directly activate FII. The relative contribution of PKa-mediated activation of FII vs FIX in WB TG is unclear. Although PKa activates FIX ∼3 times more efficiently than FII, the circulating concentration of FII is >10 times higher than that of FIX. However, FIXa generated by PKa can likely support the activation of multiple molecules of FII, amplifying the contribution of this reaction to TG. Further studies are required to determine the physiological relevance of PKa-mediated activation of FIX and FII.
The findings of this study diverge from that of a previous report on the effect of PK deficiency on TF-induced or spontaneous TG.27 Stavrou et al previously reported that plasma from PK-deficient mice supported markedly reduced TF-initiated TG and spontaneous TF in recalcified plasma in the presence or absence of the FXIIa inhibitor recombinant human albumin conjugated Infestin-4.27 Although we observed small reductions in extrinsic pathway–initiated TG in PK-deficient mouse plasma, using the same concentration of TF trigger as the prior report and a concentration 10-fold lower in the presence of the FXIIa inhibitor CTI, these differences were not significant. No spontaneous plasma TG was observed in the absence of TF trigger when evaluated in the presence of CTI. Furthermore, in WB, no significant effect of PK deficiency on TF-initiated TG was observed in the presence of CTI. We chose to supplement all samples with CTI to prevent autoactivation of the contact pathway that could mask subtle phenotypes. At the concentrations used, the inhibitory effects of CTI could be readily overcome by silica agonist.
In reconstitution experiments, we found that RBCs potentiated TG in the absence of FXII. This finding is consistent with our prior experience with the mouse WB TG assay.15 Interestingly, we observed that the potentiation of TG in this FXII-deficient setting was partially mediated by PKa. It has previously been shown that RBC EVs facilitate PKa-mediated activation of FIX, independent of FXII.23 Our data suggest that the surface of intact RBCs may facilitate PKa-mediated activation of FIX. Intact RBCs are likely to be a physiologically relevant surface, given their abundance in WB relative to other cell types. However, it should be noted that monocytes were not completely removed from the washed RBC preparations, and this may have contributed to the observed enhancement in TG.
It is important to consider how significant amounts of FXIIa might be generated in the absence of PK and likewise how PKa might be generated in the absence of FXIIa. Although PK and FXII reciprocally activate one another, autoactivation of FXII can take place in the absence of PK, and FXII possesses inherent proteolytic activity in zymogen form.6,32-34 Our findings indicate that sufficient amounts of FXIIa are generated in the absence of PK to support some degree of TG in WB. A growing body of literature indicates that PK can also be activated in the absence of FXII. Contemporaneously to the descriptions of PKa-mediated activation of FIX, Ivanov et al elegantly demonstrated that single-chain PK zymogen, such as FXII, possesses inherent proteolytic activity.7,34 In purified systems, PK autoactivation has been found to occur in the presence of the classical contact pathway activator dextran sulfate, in the absence of FXII.35,36 These prior studies of PK autoactivation used plasma purified proteins, leading to speculation that the observed phenotype may have resulted from contamination with trace amounts of PKa, caused by exposure of PK to FXIIa in plasma. Encouragingly, we found that FXIIa-naïve recombinant human PK possessed detectable PKa-like proteolytic activity that could be enhanced in the presence of dextran sulfate. A number of FXIIa-independent activators of PK, including heat shock protein 90 and prolylcarboxypeptidase, have also been described.37-40 Together, this body of evidence indicates that a number of plausible pathways exist for FXIIa-independent activation of PK; however, further work is required to establish whether any play a physiologically relevant role.
PK-independent contributions of FXIIa to contact pathway–mediated TG in WB were also evaluated by determining the effect of FXIIa inhibition in the presence or absence of PK. FXIIa inhibition with garadacimab significantly reduced contact pathway–mediated TG in both PK-sufficient and -deficient WB. The observed PK-independent function of FXIIa likely results from FXII autoactivation and subsequent FXIa-mediated TG.32,33 Interestingly, FXIIa inhibition in PK-deficient WB impaired TG to a similar extent as PKa inhibition in FXII-deficient WB. This suggests that the contribution of PK-independent FXIIa activity and FXII-independent PKa activity to TG may be similar. Such an apparent equivalence would be surprising given that FXIIa is expected to generate markedly more FIXa than PKa owing to amplification by FXIa. It important to note that, in humans, although PKa is approximately sixfold less potent in activating FIX compared with FXIa, PK zymogen is almost 20 times more abundant than FXI zymogen in plasma, which may contribute to the observed involvement of PKa in FIXa generation.24
In our study, phenotypes consistent with both FXII-independent functions of PKa and PK-independent functions of FXIIa required the use of small molecule and antibody-based inhibitors. In experiments with the PKa inhibitor berotralstat, the dose-dependent inhibition of TG observed in FXII-sufficient WB was not present in FXII-deficient WB. Such a finding might be explained by low levels of PKa generated in the absence of FXII being fully inhibited by the lowest dose of berotralstat used. Likewise, the lack of a dose-dependent effect of garadacimab in PK-deficient WB may be the result of limited generation of FXIIa that was fully inhibited by the lowest dose of garadacimab used.
It was found that even when blocking both FXII and PK activation, some residual persistent TG remained. Data obtained with WB from mice expressing low levels of TF supplemented with PKa and FXIIa inhibitors suggested that endogenous TF is a major source of persistent TG observed in PKa-inhibited FXII-deficient WB and FXIIa-inhibited PK-deficient WB initiated via the contact pathway. The source of this endogenous TF is unclear. It is possible that extravascular TF was incorporated into WB during collection. Alternatively, circulating monocytes or monocyte-derived vesicles may have contributed to the basal level of TF in WB as previously described.41,42 Although the low basal level of TG in WB from mice expressing low levels of TF was not surprising, the attribution of the persistent TG to endogenous TF indicates that other noncanonical reactions, occurring independently of both PK and FXII, are unlikely to contribute to contact pathway–initiated coagulation. The ability to attribute the vast majority of WB TG to given initiators of coagulation indicates that the background noise in the assay is low, providing added confidence in the modest but significant phenotypes presented. Furthermore, the observation that calcified WB TG could be reduced by the anti-TF antibody 1H1 indicates that inhibition of endogenous TF may be useful when studying aspects of contact pathway–initiated TG in WB.
Studies in mice indicate that PK is involved in the pathogenesis of both venous and arterial thrombus formation.27,43,44 It is interesting to consider whether some of the protective effects of PK deficiency and inhibition in these studies may be attributed to reduced PKa-mediated activation of FIX. There is clearly a role for FXII-dependent functions of PKa in thrombosis given that FXII- and FXI-deficient mice are also protected in models of arterial and venous thrombus formation.45-49 To date, there is no direct evidence that FXII-independent functions of PKa are involved in thrombosis in vivo. Further work is required to determine the extent to which direct PKa activation of FIX contributes to thrombotic pathologies.
A better understanding of FXII-dependent and -independent activation of the contact pathway may help inform the implementation of novel anticoagulant approaches. Both PKa and FXIIa targeted therapies have been developed or are under development for the management of hereditary angioedema.50-53 FXIIa targeted therapies are also being evaluated in the setting of artificial device–associated thrombosis.54 It is possible that the use of PKa and FXIIa targeted therapies in combination may more effectively prevent pathological contact pathway activation. Such an approach would be expected to inhibit both FXII-dependent and FXII-independent activation of the contact pathway without altering hemostasis.
The mouse WB TG approach used in our study has some potential inherent limitations. A species-dependent difference in FXI biology between humans and mice has been reported. Murine FXI possesses a basic glycosaminoglycan binding motif, not present in human FXI, that results in a significant pool of vessel-bound FXI and a lower circulating concentration of FXI.55 Low FXI zymogen in mouse WB may make TG more dependent on PKa-mediated activation of FIX. There may also be differences in plasma levels of other coagulation factor zymogens between mice and humans that further contribute to differences in the contributions of specific pathways.
In conclusion, our findings demonstrate that the FXII-independent activity of PKa enhances contact pathway–mediated TG in the complex milieu of mouse WB.
Acknowledgments
The authors thank Ying Zhang for excellent technical assistance.
This work was supported by National Heart, Lung, and Blood Institute of the National Institutes of Health grants K08HL136840 and R03HL162761 (P.K.B.), R01HL126974 (A.S.W.), R01HL157441 (R.P. and N.S.K.), and R35HL155657 (N.M.). S.P.G. was supported by an American Society of Hematology Scholar Award, a University of North Carolina Junior Faculty Development Award, and National Institutes of Health training grant T32HL007149. J.W. was supported by the Natural Science Foundation of the Jiangsu Higher Education Institutions (23KJB310021) and the Natural Science Foundation of China (82370137).
Authorship
Contribution: J.W. conceptualized the study, performed experiments, analyzed and interpreted data, and edited the manuscript; S.D., R.K.K., Y.P., and A.I. performed experiments and edited the manuscript; P.S., M.R., A.S.W., N.S.K., R.P., P.K.B., and N.M. interpreted data and edited the manuscript; and S.P.G. conceptualized the study, conducted experiments, oversaw completion of the study, and wrote the manuscript.
Conflict-of-interest disclosure: M.R. is employed by Synapse Research Institute, a not-for-profit research unit of Diagnostica Stago. The remaining authors declare no competing financial interests.
Correspondence: Steven P. Grover, The University of North Carolina at Chapel Hill Medicine, 8310 Mary Ellen Jones Building CB 7035, 116 Manning Dr, Chapel Hill, NC 27514; email: steven_grover@med.unc.edu.
References
Author notes
Data are available upon reasonable request from the corresponding author, Steven P. Grover (steven_grover@med.unc.edu).
The full-text version of this article contains a data supplement.