Key Points
Bleeding manifestations, need for IV iron and blood, and health care utilization rates were greater in age-matched women with HHT vs VWD.
HHT may be the most morbid inherited BD in women at individual and population levels (prevalence-adjusted).
Visual Abstract
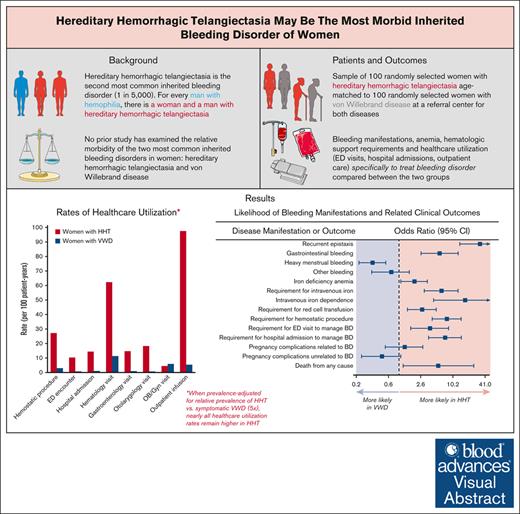
Hereditary hemorrhagic telangiectasia (HHT) is the second-most common inherited bleeding disorder (BD) worldwide and remains without approved therapies. HHT causes serious mucosal bleeding resulting in severe iron-deficiency anemia, major psychosocial complications, and visceral arteriovenous malformations in the brain, lung, and liver, which can cause life-threatening hemorrhagic complications. No study has examined the relative morbidity of HHT and von Willebrand disease (VWD), which is the most common inherited BD in women. We performed an observational cohort study of women with HHT or VWD, comparing a representative sample of 100 randomly selected women with HHT to 100 randomly selected age-matched women with VWD. In HHT vs VWD, recurrent epistaxis and gastrointestinal bleeding were more likely (odds ratio [OR], 32.73 [95% confidence interval, 13.81-71.80]; P < .0001 and 5.69 [2.59-12.89]; P < .0001) and heavy menstrual bleeding was less likely (OR, 0.32 [0.18-0.57]; P < .0001). Iron-deficiency anemia was significantly more likely, and the lowest hemoglobin was significantly lower in HHT than in VWD. The odds of iron infusion dependence, requirement for red cell transfusion, and hemostatic surgical procedures were significantly higher—17-fold, threefold, and eightfold higher, respectively—and hospital admissions to manage disease complications were both ∼14 times more frequent in women with HHT vs those with VWD. In conclusion, much higher disease-related morbidity, mortality, and health care use were observed in women with HHT vs VWD, providing evidence that HHT may be the most clinically significant inherited BD in women. Given the vast gap in research funding for HHT compared with both hemophilia (a disease primarily of men) and VWD, these findings have significant implications for gender equity in hematology.
Introduction
Afflicting 1 in 5000 people, hereditary hemorrhagic telangiectasia (HHT) is the second-most common inherited bleeding disorder (BD) worldwide, second only to von Willebrand disease (VWD) in prevalence.1,2 For every man with hemophilia, there are ∼1 woman and 1 man with HHT. In contrast to VWD and hemophilia, however, HHT remains an understudied and underappreciated inherited BD, with no US Food and Drug Administration (FDA)- or European Medicines Agency (EMA)-approved therapies2 and a small fraction of government and industry research funding for either hemophilia or VWD.3 HHT can cause severe recurrent epistaxis and chronic gastrointestinal (GI) bleeding, resulting in severe iron-deficiency anemia and major psychosocial morbidity.2,4,5 It also results in visceral arteriovenous malformations in the brain, lung, and/or liver in most patients, which can cause life-threatening bleeding, ischemic and hemorrhagic stroke, and other serious complications including cirrhosis, biliary necrosis, pulmonary hypertension, and heart failure.2,4,6 Patients with HHT have significantly reduced overall survival compared with the general population.7 Although HHT prevalence is equal in men and women, many HHT clinical studies provide evidence that both bleeding and visceral disease manifestations are more severe in women.8-10 The reasons for this are not well understood but may be associated with the hormonal impact of estrogens and progestins on the abnormal vascular phenotype.
Hemophilia, a once understudied inherited BD afflicting 1 in 10 000 people (or 1 in 5000 men), has been the beneficiary of much-needed and robust government, industry, and foundation funding over the past few decades, producing dozens of FDA-approved therapies, including highly advanced factor products, novel advanced biologic targeted therapeutics, and curative gene therapies. Although advances in VWD management do not reach the level achieved thus far in hemophilia,11 VWD has also benefited from government research funding and industry investment and has multiple FDA-approved therapies available.12 In contrast, government funding for HHT research remains much lower than for either hemophilia or VWD, and no major pharmaceutical company has pursued the development of HHT therapies.
Because of the focus on hemophilia—an X-linked disease largely exclusive to men—in the hemostasis research community, no study has, to date, examined the relative morbidity of the most common inherited BD in women: HHT and VWD. Therefore, the present study examined this question, which has critically important implications for gender equity in hematology, future resource allocation, and a better understanding of current unmet medical needs in the emerging and important hemostasis subfield of women and bleeding.
Methods
This study was approved by the Mass General Brigham Institutional Review Board (2022P000149). We performed an observational study of women with HHT or VWD, cared for at Massachusetts General Hospital, a center with both a Comprehensive Hemophilia and VWD Treatment Center and an HHT Center of Excellence. Using the Mass General Brigham Research Patient Data Registry, database queries were submitted to identify women with a known diagnosis of VWD or HHT (see supplemental Methods for additional details of queries). Diagnosis of HHT or VWD was confirmed (assigned by the treating physician on the basis of the Curacao criteria and/or genetic testing [HHT] or von Willebrand factor studies and/or genetic testing [VWD]) on manual chart review. Women with additional secondary diagnoses of other inherited or acquired bleeding or platelet disorders were excluded. From the query results, a representative sample of 100 randomly selected women from the HHT query meeting the above criteria was identified and age-matched to 100 randomly selected women from the VWD query meeting the eligibility criteria for analysis.
The data collected included demographics, BD diagnosis and classification, and BD complication and treatment history, including sources of bleeding, hemoglobin measurements, requirement for red cell transfusions and IV iron infusions, and outpatient and inpatient encounters to manage BD complications and prevent complications. The symptomatic disease was defined as the presence of recurrent epistaxis, GI bleeding, excessive surgical bleeding, heavy menstrual bleeding, or other manifestations that were judged to be related to the inherited BD by the treating clinician. All data were retrospectively extracted and confirmed using manual chart review by physician authors (E.Z., Z.M.V., and H.A.-S.), including a manual review of all encounters (outpatient, emergency department, and hospital admission) to adjudicate the relatedness between the encounter and the patient’s inherited BD. No scripts or automated chart review software were used in the collection of data.
The rates were calculated as events per 100 patient-years. Bleeding and health care utilization outcomes were analyzed to compare the clinical impact and morbidity of HHT and VWD in women. Continuous variables were compared using a 2-tailed t test (parametric data) or Wilcoxon rank-sum test (nonparametric data). Categorical variables were compared using the χ2 test (used when no cell value was <5) or the Fisher exact test (used when at least 1 cell value was <5). Statistical analysis was performed using GraphPad Prism 9.5.1 (GraphPad, Inc, San Diego, CA).
This study was approved by the Mass General Brigham Institutional Review Board (2022P000149).
Results
The mean (range) age in both groups was 49 years (14-87). The mean body mass index was 27.0 kg/m2 in both groups, and the racial/ethnic breakdown was similar in both groups. Additional details of the demographic and baseline characteristics are listed in Table 1. Mean follow-up durations were 9.07 years per patient in the HHT group and 14.83 years per patient in the VWD group. The VWD group included 83 patients with type 1 VWD and 17 with type 2 VWD (2 with type 2A VWD, 8 with type 2B VWD, and 7 with type 2 VWD without further classification). Ninety-seven of the 100 patients in the HHT group (97%) and 96 of the 100 patients in the VWD group (96%) had symptomatic disease.
Baseline characteristics of the 2 groups
| . | HHT N = 100 . | VWD N = 100 . |
|---|---|---|
| Age in y, mean (range) | 49 (14-87) | 49 (14-87) |
| Gender identity, n (%) | ||
| Female | 97 (97) | 100 (100) |
| Female/nonbinary | 3 (3) | 0 (0) |
| Race, n (%) | ||
| White | 88 (88) | 90 (90) |
| Black | 2 (2) | 3 (3) |
| Asian | 5 (5) | 3 (3) |
| Not available | 5 (5) | 4 (4) |
| Hispanic ethnicity, n (%) | 0 (0) | 5 (5) |
| Disease subtype | HHT-1 31 (31) | Type 1 83∗ |
| HHT-2 34 (34) | Type 2A 2 | |
| Other/no gene testing† 35 (35) | Type 2B 8 | |
| Type 2, NOS 7 | ||
| Body mass index in kg/m2, mean (IQR) | 27.0 (22.1-29.9) | 27.0 (22.9-30.1) |
| . | HHT N = 100 . | VWD N = 100 . |
|---|---|---|
| Age in y, mean (range) | 49 (14-87) | 49 (14-87) |
| Gender identity, n (%) | ||
| Female | 97 (97) | 100 (100) |
| Female/nonbinary | 3 (3) | 0 (0) |
| Race, n (%) | ||
| White | 88 (88) | 90 (90) |
| Black | 2 (2) | 3 (3) |
| Asian | 5 (5) | 3 (3) |
| Not available | 5 (5) | 4 (4) |
| Hispanic ethnicity, n (%) | 0 (0) | 5 (5) |
| Disease subtype | HHT-1 31 (31) | Type 1 83∗ |
| HHT-2 34 (34) | Type 2A 2 | |
| Other/no gene testing† 35 (35) | Type 2B 8 | |
| Type 2, NOS 7 | ||
| Body mass index in kg/m2, mean (IQR) | 27.0 (22.1-29.9) | 27.0 (22.9-30.1) |
NOS, not otherwise specified.
Forty patients with a confirmed diagnosis of VWD did not have a confirmed subtype as specified in the chart. For classification purposes, patients with a VWD:RCo/VWF:Ag ratio <0.6 were grouped with the type 2, NOS subgroup, and those with a VWD:RCo/VWF:Ag ratio >0.6 or an unknown VWD:RCo/VWF:Ag ratio were grouped with the type 1 subgroup.
HHT is diagnosed principally using the Curacao criteria, a set of 4 clinical criteria. All patients included had definite HHT per the Curacao criteria and/or a genetic mutation in a gene known to cause HHT. Genetic testing is not required for HHT diagnosis and may not be performed due to cost concerns.
The bleeding outcomes, pregnancy complications, and mortality are detailed in Table 2. In women with HHT, compared with age-matched women with VWD, recurrent epistaxis and GI bleeding were significantly more likely (odds ratio [OR], 32.73 [95% confidence interval (CI), 13.81-71.80]; P < .0001 and OR, 5.69 [95% CI, 2.59-12.89]; P < .0001, respectively), and heavy menstrual bleeding was significantly less likely (OR, 0.32 [95% CI, 0.18-0.57]; P < .0001). The median (interquartile range) lowest measured hemoglobin was significantly lower in the HHT group than in the VWD group (10.7 [7.9-12.6] g/dL vs 11.4 [9.9-12.5] g/dL; P = .02). Women with HHT had approximately twofold higher odds of iron-deficiency anemia, approximately sixfold higher odds of requiring IV iron infusions, approximately threefold higher odds of requiring red cell transfusions, ∼17-fold higher odds of iron infusion dependence, and approximately eightfold higher odds of requiring hemostatic surgical procedures than women with VWD (Figure 1). Death was more likely in women with HHT than in age-matched women with VWD (OR, 5.44 [95% CI, 1.20-25.23]; P = .03), despite a 39% shorter mean follow-up duration in the HHT group. Complications of pregnancy due to HHT or VWD had similar incidences in each group.
Bleeding outcomes, pregnancy complications, and mortality in women with HHT vs VWD
| . | HHT N = 100 . | VWD N = 100 . | OR (95% CI) for incidence in the HHT group relative to the VWD group . | P value . |
|---|---|---|---|---|
| Incidence of bleeding by site | ||||
| Recurrent epistaxis, n (%) | 92 (92) | 26 (26) | 32.73 (13.81-71.80) | <.0001∗ |
| GI bleeding, n (%) | 36 (36) | 9 (9) | 5.69 (2.59-12.89) | <.0001∗ |
| Heavy menstrual bleeding, n (%) | 35 (35) | 63 (63) | 0.32 (0.18-0.57) | <.0001∗ |
| Other bleeding, n (%) | 12 (12) | 16 (16) | 0.72 (0.31-1.57) | .42∗ |
| Hemoglobin, hematologic support requirements, and interventions | ||||
| Lowest measured hemoglobin, median (IQR) | 10.7 (7.9-12.6) | 11.4 (9.9-12.5) | n/a | .02† |
| Iron-deficiency anemia, n (%) | 66 (66) | 50 (50) | 1.94 (1.09-3.41) | .02∗ |
| Requirement for IV iron, n (%) | 41 (41) | 10 (10) | 6.25 (2.99-12.78) | < .0001∗ |
| IV iron dependence‡, n (%) | 26 (26) | 2 (2) | 17.22 (4.49-74.77) | < .0001§ |
| Requirement for red cell transfusion, n (%) | 42 (42) | 21 (21) | 2.72 (1.45-4.99) | .001∗ |
| Requirement for hemostatic procedure||, n (%) | 78 (78) | 31 (31) | 7.89 (4.16-14.60) | <.0001∗ |
| Hemostatic procedures, per 100 patient-years | 27.2 | 3.0 | n/a | <.0001¶ |
| Pregnancy-related complications | ||||
| Complications related to HHT/VWD, n (%) | 16 | 13 | 1.28 (0.56-2.79) | .55∗ |
| Complications unrelated to HHT/VWD, n (%) | 9 | 17 | 0.48 (0.20-1.09) | .09∗ |
| Mortality | ||||
| Death of any cause during follow-up, n (%) | 10 (10) | 2 (2) | 5.44 (1.20-25.23) | .03§ |
| Death due to bleeding complications, n (%) | 3 (3) | 0 (0) | n/a | .25§ |
| Death due to other complications of the disease, n (%) | 4 (4) | 0 (0) | n/a | .12§ |
| . | HHT N = 100 . | VWD N = 100 . | OR (95% CI) for incidence in the HHT group relative to the VWD group . | P value . |
|---|---|---|---|---|
| Incidence of bleeding by site | ||||
| Recurrent epistaxis, n (%) | 92 (92) | 26 (26) | 32.73 (13.81-71.80) | <.0001∗ |
| GI bleeding, n (%) | 36 (36) | 9 (9) | 5.69 (2.59-12.89) | <.0001∗ |
| Heavy menstrual bleeding, n (%) | 35 (35) | 63 (63) | 0.32 (0.18-0.57) | <.0001∗ |
| Other bleeding, n (%) | 12 (12) | 16 (16) | 0.72 (0.31-1.57) | .42∗ |
| Hemoglobin, hematologic support requirements, and interventions | ||||
| Lowest measured hemoglobin, median (IQR) | 10.7 (7.9-12.6) | 11.4 (9.9-12.5) | n/a | .02† |
| Iron-deficiency anemia, n (%) | 66 (66) | 50 (50) | 1.94 (1.09-3.41) | .02∗ |
| Requirement for IV iron, n (%) | 41 (41) | 10 (10) | 6.25 (2.99-12.78) | < .0001∗ |
| IV iron dependence‡, n (%) | 26 (26) | 2 (2) | 17.22 (4.49-74.77) | < .0001§ |
| Requirement for red cell transfusion, n (%) | 42 (42) | 21 (21) | 2.72 (1.45-4.99) | .001∗ |
| Requirement for hemostatic procedure||, n (%) | 78 (78) | 31 (31) | 7.89 (4.16-14.60) | <.0001∗ |
| Hemostatic procedures, per 100 patient-years | 27.2 | 3.0 | n/a | <.0001¶ |
| Pregnancy-related complications | ||||
| Complications related to HHT/VWD, n (%) | 16 | 13 | 1.28 (0.56-2.79) | .55∗ |
| Complications unrelated to HHT/VWD, n (%) | 9 | 17 | 0.48 (0.20-1.09) | .09∗ |
| Mortality | ||||
| Death of any cause during follow-up, n (%) | 10 (10) | 2 (2) | 5.44 (1.20-25.23) | .03§ |
| Death due to bleeding complications, n (%) | 3 (3) | 0 (0) | n/a | .25§ |
| Death due to other complications of the disease, n (%) | 4 (4) | 0 (0) | n/a | .12§ |
Per χ2test (used as no cell value was <5).
Per 2-tailed t test (parametric data).
Defined as a requirement for ≥2000 mg of elemental iron infused over any contiguous 12-month period.
Per Fisher exact test (used as 1 or more cell values were <5).
Included surgical or other interventional procedures in the uterus to manage heavy menstrual bleeding (eg, hysterectomy), nasal cavity to manage epistaxis (eg, nasal cautery), GI tract to manage GI bleeding (eg, endoscopy), or other interventional procedures done to manage bleeding at any site.
Per Wilcoxon rank-sum test (nonparametric data).
Likelihood of bleeding manifestations and related clinical outcomes in women with HHT vs VWD. Forest plot demonstrating the likelihood of bleeding manifestations and related clinical outcomes in women with HHT vs those with VWD. IV iron dependence was defined as the requirement for ≥2000 mg of elemental iron infused over any contiguous 12-month period.
Likelihood of bleeding manifestations and related clinical outcomes in women with HHT vs VWD. Forest plot demonstrating the likelihood of bleeding manifestations and related clinical outcomes in women with HHT vs those with VWD. IV iron dependence was defined as the requirement for ≥2000 mg of elemental iron infused over any contiguous 12-month period.
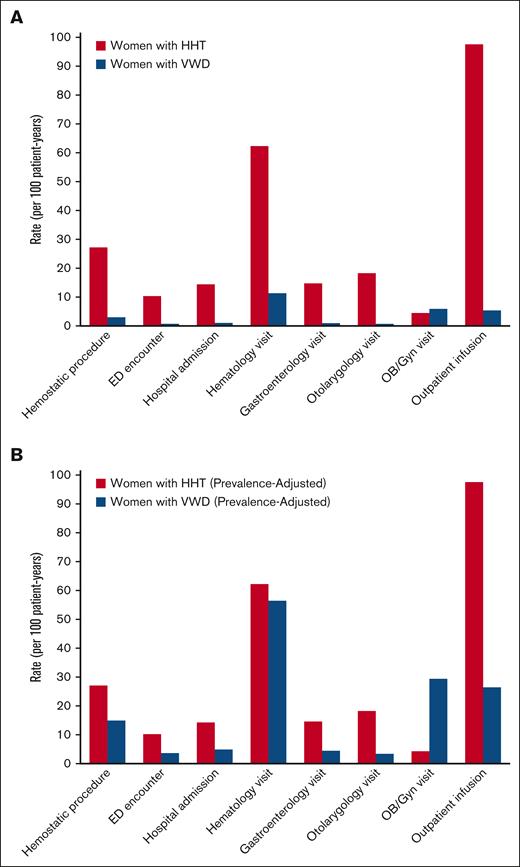
The health care utilization outcomes are detailed in Table 3. In women with HHT, compared with age-matched women with VWD, emergency department (ED) visits and hospital admissions specifically to manage disease complications were significantly more likely (OR, 3.83 [1.67-8.48]; P = .001 and 7.33 [3.57-14.73]; P < .0001, respectively). Rates of ED visitation, admission, and outpatient encounters specifically for HHT or VWD disease management were significantly higher in women with HHT than in women with VWD: ED visits, 10.3 per 100 patient-years vs 0.74 per 100 patient-years, P = .001; admissions, 14.4 per 100 patient-years vs 1.0 per 100 patient-years, P < .0001; outpatient provider encounters, 386.5 per 100 patient-years vs 43.7 per 100 patient-years, P < .0001. Rates of outpatient subspecialty provider encounters specifically for HHT or VWD management with hematology, pulmonology, cardiology, gastroenterology, otolaryngology, medical genetics, and dermatology, as well as outpatient infusion and radiology, were all significantly higher in women with HHT vs women with VWD (Table 2); rates of obstetrics-gynecology visits were significantly higher in women with VWD than women with HHT. Figure 2A compares the rates of hemostatic surgical procedures, ED visits to manage disease manifestations, hospital admissions to manage disease manifestations, and outpatient encounter specialties most relevant to the management of bleeding manifestations in women with HHT vs those with VWD.
Health care utilization specifically for HHT or VWD management in women with HHT vs VWD
| . | HHT N = 100 . | VWD N = 100 . | P value∗ . |
|---|---|---|---|
| ED visits and hospital admissions specifically for HHT or VWD management | |||
| Requirement for ED visit†, n (%) | 25 (25) | 8 (8) | .001‡ |
| ED visits, per 100 patient-years | 10.3 | 0.74 | .001 |
| Requirement for hospital admission, n (%) | 50 (50) | 12 (12) | <.0001‡ |
| Hospital admissions, per 100 patient-years | 14.4 | 1.0 | <.0001 |
| Length of hospital stay, mean (range) | 5.5 (1-28) | 4.3 (1-15) | .47 |
| Outpatient encounters§ specifically for HHT or VWD management | |||
| Any outpatient encounter, per 100 patient-years | 386.5 | 43.7 | <.0001 |
| Primary care provider, per 100 patient-years | 37.9 | 19.1 | .28 |
| Hematology, per 100 patient-years | 62.3 | 11.3 | .005 |
| Pulmonology, per 100 patient-years | 36.5 | 0.1 | <.0001 |
| Cardiology, per 100 patient-years | 4.3 | 0.9 | .005 |
| Gastroenterology, per 100 patient-years | 14.7 | 0.9 | <.0001 |
| Otolaryngology, per 100 patient-years | 18.3 | 0.7 | <.0001 |
| Obstetrics and gynecology, per 100 patient-years | 4.4 | 5.9 | .003 |
| Medical Genetics, per 100 patient-years | 7.7 | 0.0 | <.0001 |
| Dermatology, per 100 patient-years | 5.1 | 0.0 | .003 |
| Outpatient infusion, per 100 patient-years | 97.6 | 5.3 | <.0001 |
| Radiology/imaging, per 100 patient-years | 74.0 | 0.3 | <.0001 |
| Other, per 100 patient-years | 23.3 | 1.3 | <.0001 |
| . | HHT N = 100 . | VWD N = 100 . | P value∗ . |
|---|---|---|---|
| ED visits and hospital admissions specifically for HHT or VWD management | |||
| Requirement for ED visit†, n (%) | 25 (25) | 8 (8) | .001‡ |
| ED visits, per 100 patient-years | 10.3 | 0.74 | .001 |
| Requirement for hospital admission, n (%) | 50 (50) | 12 (12) | <.0001‡ |
| Hospital admissions, per 100 patient-years | 14.4 | 1.0 | <.0001 |
| Length of hospital stay, mean (range) | 5.5 (1-28) | 4.3 (1-15) | .47 |
| Outpatient encounters§ specifically for HHT or VWD management | |||
| Any outpatient encounter, per 100 patient-years | 386.5 | 43.7 | <.0001 |
| Primary care provider, per 100 patient-years | 37.9 | 19.1 | .28 |
| Hematology, per 100 patient-years | 62.3 | 11.3 | .005 |
| Pulmonology, per 100 patient-years | 36.5 | 0.1 | <.0001 |
| Cardiology, per 100 patient-years | 4.3 | 0.9 | .005 |
| Gastroenterology, per 100 patient-years | 14.7 | 0.9 | <.0001 |
| Otolaryngology, per 100 patient-years | 18.3 | 0.7 | <.0001 |
| Obstetrics and gynecology, per 100 patient-years | 4.4 | 5.9 | .003 |
| Medical Genetics, per 100 patient-years | 7.7 | 0.0 | <.0001 |
| Dermatology, per 100 patient-years | 5.1 | 0.0 | .003 |
| Outpatient infusion, per 100 patient-years | 97.6 | 5.3 | <.0001 |
| Radiology/imaging, per 100 patient-years | 74.0 | 0.3 | <.0001 |
| Other, per 100 patient-years | 23.3 | 1.3 | <.0001 |
All P values are per the Wilcoxon rank-sum test (except those marked with the ‡ symbol).
Includes only ED visits in which the patient was discharged from the ED without hospital admission. ED visits ending at hospital admission were counted only once, as hospital admissions.
P values are per χ2 test.
All outpatient encounters except for outpatient infusion and radiology/imaging were face-to-face visits with a clinician (physician, nurse practitioner, or physician assistant).
Comparative health care utilization in women with HHT vs VWD. (A) Rates of health care utilization to manage HHT or VWD, including hemostatic surgical procedures, ED encounters to manage BD manifestations, hospital admissions to manage BD manifestations, and outpatient encounter specialties most relevant to management of bleeding manifestations in women with HHT vs those with VWD. (B) Estimated prevalence-adjusted rates of health care utilization to manage HHT or VWD, applying adjustment to the prevalence of HHT and symptomatic VWD in the population (1 in 5000 vs 1 in 1000, so a 5× multiplier was applied to measured VWD rates).
Comparative health care utilization in women with HHT vs VWD. (A) Rates of health care utilization to manage HHT or VWD, including hemostatic surgical procedures, ED encounters to manage BD manifestations, hospital admissions to manage BD manifestations, and outpatient encounter specialties most relevant to management of bleeding manifestations in women with HHT vs those with VWD. (B) Estimated prevalence-adjusted rates of health care utilization to manage HHT or VWD, applying adjustment to the prevalence of HHT and symptomatic VWD in the population (1 in 5000 vs 1 in 1000, so a 5× multiplier was applied to measured VWD rates).
Discussion
In this study, we describe bleeding outcomes, pregnancy complications, health care utilization, and mortality of a representative random sample of women with HHT and compare these to an age–matched random sample of women with VWD at a health system that serves as a tertiary referral center for both diseases. To our knowledge, there is no standard measurement of morbidity validated to compare inherited BDs, and therefore, comparative morbidity is difficult to confirm. Given this, we used the best possible surrogate markers of morbidity for BDs available in an observational cohort study with retrospective data collection, such as outpatient visits to manage the BD, hospitalizations and ED visits for bleeding, and hematologic support requirements with IV iron and red cell transfusion. We found that HHT, a historically neglected BD, was responsible for much higher morbidity and health care utilization (for both bleeding and nonbleeding complications) in women than VWD at the individual patient level. The findings of our study suggest this may also be true at the population level: given that the incidence of HHT is 1 in 5000 persons2 and the incidence of symptomatic VWD (the applicable category for 96% of the VWD group in this cohort) is 1 in 1000 persons,1 even with a fivefold estimated prevalence adjustment of the rates of hemostatic procedures, complications, and health care utilization of many types we describe in our cohort (Figure 2B) nearly all of these rates remain higher in the HHT group, and often markedly so. In addition to being greater than in VWD specifically, the objective impact of HHT generally on women as measured by rates of health care utilization–∼4 outpatient encounters per year for disease management, 1 ED visit or hospital admission every 4 years, and 1 hemostatic surgical procedure every 4 years, on average–is substantial and troubling, appearing comparable with that of men with hemophilia.13,14 Some of these metrics, such as higher rates of ED visits, may also reflect inadequate infrastructure for specialized HHT care (2 HHT centers of excellence vs 11 hemophilia treatment centers in New England, the primary catchment area of our patient population). If so, this reflects another aspect of HHT disease care in need of investment.
Our study has several strengths. The overall incidence of disease complications in each group was consistent with published literature for both HHT and VWD,15,16 supporting the generalizability of our findings. Data were meticulously collected by physicians for all 200 patients using a manual chart review of several thousand provider notes, which allowed for proper adjudication of whether encounters or complications were related to the BD in question, a task that would have been impossible with any alternative study design (such as analyses of claims or billing databases). Data were collected from patients in both the inpatient and outpatient settings, overcoming certain limitations of prior studies using only an inpatient sample.17 Our study also has important limitations. One such limitation is the possibility that relevant confounders were not adequately addressed by age-matching alone. Additionally, although nearly all patients in each group had symptomatic HHT or VWD, the institution at which the study was performed was a referral center for both diseases, and the mean follow-up duration was long in both groups, there remains the possibility of referral bias in patients with more severe bleeding manifestations and the possibility of chart misdiagnoses in 1 or both groups, a known limitation of retrospective data collection. In addition, although our distribution of VWD subtypes is consistent with the overall prevalence of disease subtypes in the general population, we acknowledge that most of the patients with VWD in our study had type 1 VWD, which often presents more mildly than type 2 VWD. HHT and VWD are both inherited BDs, but HHT generally involves more organ systems, introducing additional complexity in the comparison of bleeding manifestations between these 2 diseases. Lastly, we acknowledge that the lack of treatments for HHT, compared with those for VWD, may play a role in our findings of HHT having higher morbidity and health care utilization rates than VWD. If true, it only emphasizes the need for greater government, industry, and academic investment in research and development for this disease.
In conclusion, our findings suggest that HHT may be the most morbid and clinically relevant inherited BD in women. These findings are critically important in establishing research priorities and achieving gender equity in the hemostasis field, given that both government and industry funding for VWD research is much greater than for HHT research and that such funding for hemophilia research, a disease primarily of men, is at least an order of magnitude greater than that for HHT research.3 Although both hemophilia and VWD have benefited from industry and government research funding, resulting in multiple FDA-approved therapies for each disease, HHT has no FDA-approved therapies. Current drug development in HHT has largely been limited to repurposing efforts conducted by academic clinicians within HHT centers, comprising primarily of retrospective observational studies and small single-institution trials due to funding limitations.18-23 The findings of our study should serve as a call to action by the academic hematology and hemostasis communities, who in general continue to marginalize HHT among inherited BDs, categorizing it with ultrarare coagulation factor deficiencies (with prevalences of <1 per million persons) rather than its peer “common” inherited BDs, hemophilia, and VWD. Continued neglect of academic attention and funding for clinical care and research on HHT will only serve to sustain and exacerbate gender inequities in hematologic diseases and limit meaningful alleviation of the suffering of patients with this morbid inherited BD.
Acknowledgment
H.A.-S. is funded by the National Heart, Lung, and Blood Institute (1K23HL159313).
Authorship
Contribution: E.Z. contributed to concept and design, data collection, data analysis, creation of tables and figures, critical revisions of intellectual content, and final approval; Z.M.V. contributed to data collection, critical revisions of intellectual content, and final approval; J.R.-L. contributed to critical revisions of the intellectual content and final approval; and H.A.-S. contributed to concept and design, data collection, data analysis, writing of the first draft of the manuscript, creation of the tables and figures, critical revisions of intellectual content, and final approval.
Conflict-of-interest disclosure: H.A.-S. received research funding from Agios, Sobi, Novartis, Vaderis, and Amgen, and consultancy from Agios, Sobi, Moderna, Novartis, Rigel, Argenx, Forma, and Pharmacosmos. The remaining authors declare no competing financial interests.
Correspondence: Hanny Al-Samkari, Division of Hematology Oncology, Massachusetts General Hospital, Bartlett Hall Extension Office 133, Boston, MA 02114; email: hal-samkari@mgh.harvard.edu.
References
Author notes
Data are available on request from the corresponding author, Hanny Al-Samkari (hal-samkari@mgh.harvard.edu).
The full-text version of this article contains a data supplement.