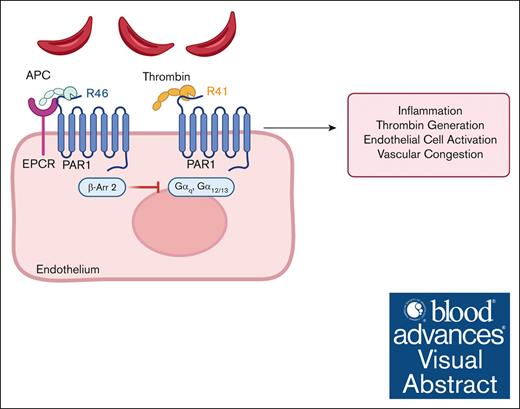
Key Points
APC inhibition exacerbates thrombin generation, inflammation, and end-organ damage in a mouse model of SCD.
APC-PAR1-R46–biased agonism reduces inflammation, suggesting that targeting this pathway may mitigate vascular complications of SCD.
Visual Abstract
Sickle cell disease (SCD) is a hereditary hemoglobinopathy marked by hemolytic anemia and vaso-occlusive events (VOEs). Chronic endothelial activation, inflammation, and coagulation activation contribute to vascular congestion, VOEs, and end-organ damage. Coagulation proteases such as thrombin and activated protein C (APC) modulate inflammation and endothelial dysfunction by activating protease-activated receptor 1 (PAR1), a G-protein–coupled receptor. Thrombin cleaves PAR1 at Arg41, while APC cleaves PAR1 at Arg46, initiating either proinflammatory or cytoprotective signaling, respectively, a signaling conundrum known as biased agonism. Our prior research established the role of thrombin and PAR1 in vascular stasis in an SCD mouse model. However, the role of APC and APC-biased PAR1 signaling in thrombin generation, inflammation, and endothelial activation in SCD remains unexplored. Inhibition of APC in SCD mice increased thrombin generation, inflammation, and endothelial activation during both steady state and tumor necrosis factor α challenge. To dissect the individual contributions of thrombin-PAR1 and APC-PAR1 signaling, we used transgenic mice with point mutations at 2 PAR1 cleavage sites, ArgR41Gln (R41Q) imparting insensitivity to thrombin and Arg46Gln (R46Q) imparting insensitivity to APC. Sickle bone marrow chimeras expressing PAR1-R41Q exhibited reduced thrombo-inflammatory responses compared with wild type PAR1 or PAR1-R46Q mice. These findings highlight the potential benefit of reducing thrombin-dependent PAR1 activation while preserving APC-PAR1 signaling in SCD thromboinflammation. These results also suggest that pharmacological strategies promoting biased PAR1 signaling could effectively mitigate vascular complications associated with SCD.
Introduction
Sickle cell disease (SCD) is the most common inherited hemoglobinopathy worldwide, caused by a single nucleotide mutation in the gene for β-globin.1 This mutation results in a glutamine to valine substitution in the β-globin chain, forming sickle hemoglobin (HbS). Upon deoxygenation, HbS undergoes abnormal polymerization, leading to the sickling of red blood cells (RBC). Sickle RBCs are prone to hemolysis and adhesion to other cells (platelets, neutrophils, and endothelial cells), resulting in hemolytic anemia and vascular stasis, respectively.1,2 A hypercoagulable state and increased risk of venous thrombosis are key characteristics of SCD.3-5 Tissue factor (TF)–dependent activation of extrinsic coagulation cascade contributes to inflammation, end-organ damage, and mortality in mouse models of SCD.6-9 Although nonhematopoietic protease-activated receptor 1 (PAR1) does not contribute to systemic inflammation in a mouse model of SCD at steady state,7 a follow-up study revealed that thrombin-dependent PAR1 signaling promotes vascular stasis in sickle mice through upregulation of von Willebrand factor (VWF) and P-selectin (P-sel) on the endothelium.10
PAR1 is the main thrombin receptor on human platelets but not mouse platelets and is also expressed on leukocytes and endothelial cells.11,12 This G-protein–coupled receptor is activated by proteolytic cleavage of specific amino acids on the extracellular N-terminus, forming tethered ligands that induce signaling. Thrombin cleaves PAR1 at Arg41 (R41), which activates Gαq and Gα12/13 signaling to initiate inflammation, endothelial barrier permeability, adhesion, and cytotoxicity.13,14 PAR1 is also cleaved by activated protein C (APC), although with lower affinity than thrombin.15 Zymogen protein C (PC) binds the endothelial PC receptor (EPCR), which serves 2 purposes. One, it colocalizes PC to thrombomodulin for efficient activation of PC by thrombomodulin-bound thrombin to generate APC. Two, EPCR colocalizes with PAR1 in caveolin-1–positive lipid rafts16 to facilitate APC-mediated cleavage of PAR1 at Arg46 (R46), activating β-arrestin–dependent signaling that is anti-inflammatory and cytoprotective.16-18 Upon dissociation from EPCR and binding to negatively charged phospholipid surfaces, APC is also an endogenous anticoagulant that inactivates FVa and FVIIIa.17 Interestingly, individuals with SCD and sickle cell mice have lower levels of PC and APC activity,19-23 which decrease further during vaso-occlusive events (VOEs) and negatively correlate with markers of coagulation activation.22 APC has beneficial effects in mouse models of sepsis, stroke, and experimental autoimmune encephalitis,24,25 however, its role in SCD has not been characterized. Here, we set out to determine the role of endogenous APC activity and biased PAR1 agonism in a mouse model of SCD.
Methods
Mice
Humanized SCD (Townes) mice express human α-globin and either human sickle β-globin (βSβS, HbSS) or normal adult β-globin (βA/βA, HbAA, or wild type control) and were bred in-house from HbAS breeding pairs. Male and female HbSS and HbAA littermate controls, aged 3 to 4 months were used for these studies. R41Q (thrombin cleavage insensitive) and R46Q (APC cleavage insensitive) transgenic mice26 were used to investigate biased agonism of PAR1 in SCD. C57/Bl6J mice were used as the wild type PAR1 (PAR1-WT) controls. Mice were housed and maintained on a 12-hour light/dark schedule with food and water ad libitum. All animal experiments were approved by the University of North Carolina Institutional Care and Use Committee.
Bone marrow transplantation
HbAA and HbSS mouse bone marrow was transplanted into PAR1-WT, PAR1-R41Q, and PAR1-R46Q recipients as previously described.7 Briefly, recipient mice were placed on acidified (pH 2.6) water containing neomycin (0.1 mg/mL) and polymyxin B sulfate (0.01 mg/mL; Sigma Aldrich) for 1 week before and 2 weeks after transplantation. A total of 1300 Gγ was administered in 2 equal exposures 4 hours apart. Bone marrow nucleated cells were harvested from HbAA and HbSS mice and resuspended in RPMI-1640 with 0.5% bovine serum albumin (Gibco). A total of 5 × 106 donor bone marrow cells were IV injected in recipient mice 1 hour after the last irradiation. Engraftment of donor cells was analyzed using hemoglobin electrophoresis, and only mice expressing human HbAA or HbSS were used for experiments 4 months after transplantation.
Inhibition of endogenous APC activity
Mice were treated with control immunoglobulin G (IgG) or SPC-54 (10 mg/kg, IP)27 24 hours before sample collection.
Tumor necrosis factor challenge
In some studies, mice were challenged with recombinant murine tumor necrosis factor α (TNF-α) to mimic vaso-occlusive crisis. Mice receive TNF-α (2 mg/kg, IP) and samples were collected 5 hours later.
Sample collection
Mice were anesthetized with isoflurane (3% in 100% oxygen), and blood was drawn from the inferior vena cava into syringes containing sodium citrate (3.8%; final ratio, 9:1). Total blood cell count and hematologic profile were determined with a veterinary complete blood count analyzer (HT5, Heska). Plasma was collected by centrifugation at 4000 rotations per minute for 15 minutes at room temperature and stored at –80°C for future analysis.
Analysis of proinflammatory cytokines
Enzyme-linked immunosorbent assays (ELISAs) were used to quantify plasma levels of thrombin-antithrombin complex (TAT; Siemens Healthcare Diagnostics), proinflammatory cytokines interleukin-6 (IL-6), IL-18 (R&D Systems), and high mobility group box 1 (HMGB1; Tecan), and endothelial activation markers soluble vascular cell adhesion molecule 1 (sVCAM-1; R&D Systems), soluble intracellular adhesion molecule (sICAM; R&D Systems), soluble P-sel (sP-sel; R&D Systems), and VWF (Abcam).
Tissue histology
Lungs, kidneys, and livers were harvested from the mice, fixed in 10% formalin, and embedded in paraffin. Livers were sectioned (5 μm) and stained with hematoxylin and eosin (H&E), and congestion and necrosis were evaluated in the entire liver section at 20× magnification by 3 blinded researchers (N.R., K.L., and E.S.) according to the following criteria: for congestion (score 0-4), 0 means no RBCs in sinusoids; 1, RBCs in <10% of sinusoids; 2, RBCs in <25% of sinusoids; 3, RBCs in <50% of sinusoids; and 4, RBCs in >50% of sinusoids, bridging between veins, and RBCs present in parenchymal tissue; for necrosis (score 0-4), 0 means no obvious necrosis; 1, necrosis in <10% of hepatocytes in field; 2, necrosis in <25% of hepatocytes in field; 3, necrosis in <50% of hepatocytes in field; and 4, necrosis in >50% of hepatocytes, bridging between central veins. Liver sections were also stained with Prussian blue, and total Prussian blue area was quantified with Image J. Kidney sections (5 μm) were stained with H&E, and red cell congestion was scored on a scale of 0 to 4 in the glomeruli in 10 high powered fields by 2 blinded researchers (E.S. and N.R.). The lungs were inflated with 10% formalin before collection. Lung sections (5 μm) were stained with H&E or for neutrophils. Before staining for neutrophils, lung sections underwent antigen retrieval (10 mM citrate buffer; pH 6) for 20 minutes at 95°C, and endogenous peroxidases, avidin, and biotin were blocked with hydrogen peroxide (3%) and avidin/biotin blocking kit (Vector Labs), respectively. Neutrophil staining was performed using rat anti-mouse neutrophil monoclonal antibody (1:1000; NIMP-R14; Abcam) and biotinylated anti-rat secondary antibody (Vector Labs). Staining was developed with Vectastain ABC kit and ImmPACT DAB peroxidase substrate (Vector Labs) and counterstained with hematoxylin (Dako). Neutrophils were quantified in 10 high powered fields (400×) as previously described.7
Statistical analysis
Data are presented as a mean ± standard error of the mean. One- or 2-way analysis of variance were performed, with Tukey post hoc test for multiple comparisons. For data that are not normally distributed, Kruskall-Wallis and Dunn multiple comparisons tests were used (GraphPad Prism V9).
Results
Inhibition of endogenous APC exacerbates thromboinflammation in sickle mice at steady state
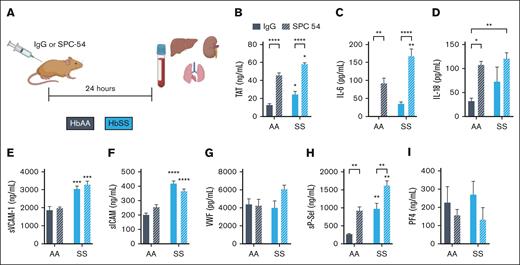
To investigate the role of APC in SCD, HbAA and HbSS mice were treated with control IgG or SPC54, a monoclonal antibody which binds to both APC and protein C and blocks APC’s amidolytic activity27 (Figure 1A). Blood was collected 24 hours after antibody administration. APC inhibition increased TAT in HbAA mice and significantly enhanced the already elevated TAT levels in HbSS mice (Figure 1B). APC inhibition also exacerbated inflammation, measured by plasma levels of IL-6 and IL-18 in both HbAA and HbSS mice (Figure 1C-D). Although APC inhibition did not alter plasma levels of sVCAM1, sICAM, or VWF in HbSS mice (Figure 1E-G), it did significantly increase plasma levels of sP-sel (Figure 1H). P-sel is stored in Weibel-Palade bodies of the endothelium, as well as platelet α-granules. To determine the cellular source of the increased sP-sel in SPC54-treated HbSS mice, plasma levels of platelet factor 4 (PF4) were measured. PF4 is stored in platelet α-granules but is not present in endothelial cells. There were similar levels of PF4 in IgG-treated HbAA and HbSS mice; SPC54 modestly reduced levels of PF4 in both HbAA and HbSS mice, but these were not significant (Figure 1J). This supports the conclusion that the elevation in sP-sel after SPC54 treatment indeed originates from endothelial cells. Moreover, SPC54 significantly reduced circulating platelet counts in HbAA and HbSS mice compared with IgG-treated controls (Table 1), likely due to vascular congestion in the organs. In contrast, SPC54 modestly increased circulating RBC parameters, neutrophils, and neutrophil-to-lymphocyte ratios in HbSS mice (Table 1), indicating that APC inhibition enhances acute inflammatory responses.
Inhibition of endogenous APC by SPC-54 exacerbates the thromboinflammation at steady state in HbSS mice. (A) Four-month-old HbAA (gray) and HbSS (blue) mice were treated with 10 mg/kg (i.p.) IgG (solid bars), or SPC54 (white hashed bars), and samples were collected 24 hours later. Plasma levels of TAT (B), IL-6 (C), IL-18 (D), sVCAM (E), sICAM (F), VWF (G), sP-sel (H), and PF4 (I). Data are represented by mean ± standard error of the mean (SEM) of 5 to 6 mice per group and analyzed by 2-way analysis of variance (ANOVA) and Tukey post hoc test. Asterisks directly above the bars indicate statistical significance of SS mice to AA mice in the same treatment group. Asterisks over brackets indicate difference from IgG-treated mice. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001. AA, controls; i.p., intraperitoneal; SS, sickle mice.
Inhibition of endogenous APC by SPC-54 exacerbates the thromboinflammation at steady state in HbSS mice. (A) Four-month-old HbAA (gray) and HbSS (blue) mice were treated with 10 mg/kg (i.p.) IgG (solid bars), or SPC54 (white hashed bars), and samples were collected 24 hours later. Plasma levels of TAT (B), IL-6 (C), IL-18 (D), sVCAM (E), sICAM (F), VWF (G), sP-sel (H), and PF4 (I). Data are represented by mean ± standard error of the mean (SEM) of 5 to 6 mice per group and analyzed by 2-way analysis of variance (ANOVA) and Tukey post hoc test. Asterisks directly above the bars indicate statistical significance of SS mice to AA mice in the same treatment group. Asterisks over brackets indicate difference from IgG-treated mice. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001. AA, controls; i.p., intraperitoneal; SS, sickle mice.
Complete blood counts from AA and SS mice treated with IgG or SPC-54
| Parameter . | AA/IgG (n = 5) . | AA/SPC-54 (n = 5) . | SS/IgG (n = 5) . | SS/SPC-54 (n = 6) . |
|---|---|---|---|---|
| RBC, 106/μL | 9.33 ± 0.19 | 7.09 ± 0.85∗ | 5.05±0.32∗∗∗∗ | 6.40 ± 0.06∗ |
| Hb, g/dL | 9.56 ± 0.34 | 7.5 ± 0.93∗ | 6.08 ± 0.41∗∗∗ | 8.53 ± 0.24## |
| Hematocrit, % | 31.8 ± 0.88 | 24.1 ± 3.02∗ | 22.92 ± 1.69∗∗ | 31.85 ± 0.72## |
| PMN:lymph | 0.15 ± 0.14 | 1.63 ± 0.34∗∗∗ | 0.28 ± 0.05 | 1.29 ± 0.17## |
| WBC, 103/μL | 8.07 ± 0.74 | 9.21 ± 0.78 | 29.63 ± 6.1∗∗ | 23.21 ± 2.1∗ |
| PMN, 103/μL | 0.98 ± 0.05 | 5.45 ± 0.76∗ | 6.87 ± 2.19∗∗∗ | 12.54±1.11∗∗∗∗,## |
| Lymphocytes, 103/μL | 6.79 ± 0.70 | 3.49 ± 0.37 | 22.01 ± 3.9∗∗∗ | 10.21 ± 1.19## |
| Monocytes, 103/μL | 0.21 ± 0.03 | 0.24 ± 0.04 | 0.57 ± 0.12∗ | 0.35 ± 0.07 |
| Platelet, 103/μL | 895 ± 23.7 | 213.0 ± 16.3∗∗∗ | 814.0 ± 63.7 | 258 ± 18.0### |
| Parameter . | AA/IgG (n = 5) . | AA/SPC-54 (n = 5) . | SS/IgG (n = 5) . | SS/SPC-54 (n = 6) . |
|---|---|---|---|---|
| RBC, 106/μL | 9.33 ± 0.19 | 7.09 ± 0.85∗ | 5.05±0.32∗∗∗∗ | 6.40 ± 0.06∗ |
| Hb, g/dL | 9.56 ± 0.34 | 7.5 ± 0.93∗ | 6.08 ± 0.41∗∗∗ | 8.53 ± 0.24## |
| Hematocrit, % | 31.8 ± 0.88 | 24.1 ± 3.02∗ | 22.92 ± 1.69∗∗ | 31.85 ± 0.72## |
| PMN:lymph | 0.15 ± 0.14 | 1.63 ± 0.34∗∗∗ | 0.28 ± 0.05 | 1.29 ± 0.17## |
| WBC, 103/μL | 8.07 ± 0.74 | 9.21 ± 0.78 | 29.63 ± 6.1∗∗ | 23.21 ± 2.1∗ |
| PMN, 103/μL | 0.98 ± 0.05 | 5.45 ± 0.76∗ | 6.87 ± 2.19∗∗∗ | 12.54±1.11∗∗∗∗,## |
| Lymphocytes, 103/μL | 6.79 ± 0.70 | 3.49 ± 0.37 | 22.01 ± 3.9∗∗∗ | 10.21 ± 1.19## |
| Monocytes, 103/μL | 0.21 ± 0.03 | 0.24 ± 0.04 | 0.57 ± 0.12∗ | 0.35 ± 0.07 |
| Platelet, 103/μL | 895 ± 23.7 | 213.0 ± 16.3∗∗∗ | 814.0 ± 63.7 | 258 ± 18.0### |
PMN, polymorphonuclear lymphocyte (neutrophil); WBC, total white blood cell count.
∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001 vs AA/IgG.
##P < .01 vs SS/IgG.
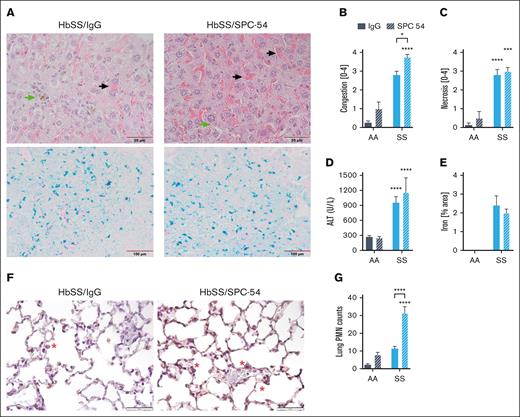
The effects of APC inhibition on organ damage were also evaluated. Histologic evaluation of the livers of SPC54-treated HbSS mice revealed enhanced vascular and sinusoidal congestion of RBCs and the presence of iron-laden macrophages (Figure 2A-B). APC inhibition did not increase hepatic necrosis (Figure 2C) or plasma levels of alanine aminotransferase (Figure 2D) in HbSS mice. Prussian blue staining confirms the presence of iron in the liver tissue of HbSS mice (Figure 2A), and quantification of total Prussian blue area showed that SPC-54 did not alter this (Figure 2E). These data, suggesting that acute vascular congestion and thrombin generation do not exacerbate chronic hepatocyte damage. Lungs were stained for neutrophils (Figure 2F), which were counted in the alveolar spaces and walls. APC inhibition significantly increased the number of neutrophils in the lungs of HbSS mice (Figure 2G), consistent with the notion that inhibition of APC increases acute inflammatory responses. Kidney sections of both HbSS/IgG and HbSS/SPC54 showed congestion of sickled RBCs in glomeruli (supplemental Figure 1A-B). APC inhibition significantly increased glomerular congestion compared with that of IgG-treated HbSS mice (supplemental Figure 1C-D).
APC inhibition exacerbates hepatic congestion and lung neutrophil accumulation. (A) Representative images of liver sections from HbSS mice treated with IgG or SPC-54 stained with H&E (top) and Prussian Blue (bottom). Black arrow indicates sinusoidal congestion, and green arrow indicates iron-laden macrophages. Quantification of liver congestion (B) and liver necrosis (C) by 3 blinded observers. (D) Plasma levels of alanine aminotransferase (ALT). (E) Quantification of Prussian blue stain per total area. (F) Representative images of lung sections stained for neutrophils (brown). Scale bar, 50 μm; red asterisk (∗) denotes neutrophils. (G) Quantification of neutrophils (PMN) averaged over 10 high powered (40×) fields. Data are represented by mean ± SEM of 5 to 6 mice per group and analyzed by 2-way ANOVA and Tukey post hoc test. Asterisks directly above the bars indicate the statistical significance of SS mice to AA mice in the same treatment group. Asterisks over brackets indicate difference from IgG-treated mice. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001. AA, controls; PMN, polymorphonuclear lymphocytes; SS, sickle mice.
APC inhibition exacerbates hepatic congestion and lung neutrophil accumulation. (A) Representative images of liver sections from HbSS mice treated with IgG or SPC-54 stained with H&E (top) and Prussian Blue (bottom). Black arrow indicates sinusoidal congestion, and green arrow indicates iron-laden macrophages. Quantification of liver congestion (B) and liver necrosis (C) by 3 blinded observers. (D) Plasma levels of alanine aminotransferase (ALT). (E) Quantification of Prussian blue stain per total area. (F) Representative images of lung sections stained for neutrophils (brown). Scale bar, 50 μm; red asterisk (∗) denotes neutrophils. (G) Quantification of neutrophils (PMN) averaged over 10 high powered (40×) fields. Data are represented by mean ± SEM of 5 to 6 mice per group and analyzed by 2-way ANOVA and Tukey post hoc test. Asterisks directly above the bars indicate the statistical significance of SS mice to AA mice in the same treatment group. Asterisks over brackets indicate difference from IgG-treated mice. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001. AA, controls; PMN, polymorphonuclear lymphocytes; SS, sickle mice.
Inhibition of endogenous APC generation exacerbates thromboinflammation in sickle mice during TNF-α challenge
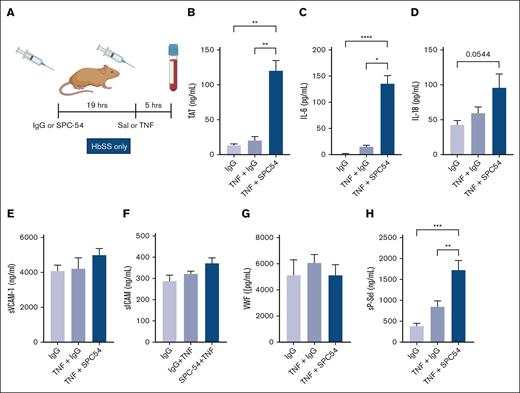
TNF-α administration is a well-characterized model of acute inflammation that induces formation of multicellular aggregates, vascular stasis, and presence of thrombi in organs in HbSS mice.28-31 TNF-α challenge exacerbates thrombin generation and inflammation in HbSS mice.31 To determine the role of APC in TNF-α challenge, HbSS mice were treated with IgG or SPC54 19 hours before TNF-α (2 mg/kg, IP) or saline (as steady state) administration, and samples were collected 5 hours later (Figure 3A). APC inhibition dramatically increased thrombin generation in TNF-α–challenged HbSS mice (Figure 3B). There was a similar pattern in the plasma levels of IL-6 (Figure 3C) and a trend to increased plasma levels of IL-18 in SS mice treated with TNF-α (Figure 3D). In contrast, plasma levels of sVCAM, sICAM, and VWF in HbSS mice were not significantly altered by TNF-α challenge, with or without SPC-54 (Figure 3E-G), yet the endothelial activation marker sP-sel was increased (Figure 3H). This indicates that blocking endogenous APC generation enhances TAT, IL-6, IL-18, and sP-sel in SCD mice after TNF-α challenge. Interestingly, APC inhibition also worsened leukocytosis and caused thrombocytopenia in TNF-α challenged HbSS mice (Table 2).
Inhibition of endogenous APC by SPC54 exacerbates thromboinflammation in HbSS mice after TNF-α challenge. (A) Male and female HbSS mice were treated with IgG or SPC-54 (10 mg/kg, IP) 19 hours before SAL or TNF-α (2 μg/kg, IP) and plasma was collected 5 hours later. Plasma levels of TAT (B), IL-6 (C), IL-18 (D), sVCAM1 (E), sICAM (F), VWF (G), and sP-sel (H). Data represent mean ± SEM mean of 5 to 10 mice per group. Asterisks above brackets indicate statistical significance by 1-way ANOVA with Kruskal-Wallis posttest. ∗P < .05; ∗∗∗P < .001; ∗∗∗∗P < .0001. Sal, saline.
Inhibition of endogenous APC by SPC54 exacerbates thromboinflammation in HbSS mice after TNF-α challenge. (A) Male and female HbSS mice were treated with IgG or SPC-54 (10 mg/kg, IP) 19 hours before SAL or TNF-α (2 μg/kg, IP) and plasma was collected 5 hours later. Plasma levels of TAT (B), IL-6 (C), IL-18 (D), sVCAM1 (E), sICAM (F), VWF (G), and sP-sel (H). Data represent mean ± SEM mean of 5 to 10 mice per group. Asterisks above brackets indicate statistical significance by 1-way ANOVA with Kruskal-Wallis posttest. ∗P < .05; ∗∗∗P < .001; ∗∗∗∗P < .0001. Sal, saline.
Hematologic parameters from AA and SS mice after SPC-54 and TNF-α treatment
| Variable . | AA . | SS . | ||
|---|---|---|---|---|
| IgG (n = 3) . | IgG (n = 5) . | IgG/TNF (n = 10) . | SPC-54/TNF (n = 10) . | |
| RBC, 106/μL | 9.50 ± 0.072 | 6.57 ± 0.35∗ | 5.77 ± 0.15∗∗ | 5.62 ± 0.21∗∗ |
| Hematocrit, % | 34.2 ± 2.6 | 34.2 ± 2.55 | 30.56 ± 2.14 | 25.55 ± 1.55$ |
| PMN:lymph | 0.21 ± 0.04 | 0.17 ± 0.05 | 0.53 ± 0.18$ | 1.07 ± 0.12$ |
| WBC, 103/μL | 10.75 ± 1.28 | 31.3 ± 4.42∗ | 25.8 ± 3.59 | 28.21 ± 6.69 |
| PMN, 103/μL | 1. 57 ± 0.25 | 12.37 ± 2.34∗∗ | 28.77 ± 4.95$$ | 49.32 ± 2.36∗∗,$ |
| Lymphocyte, 103/μL | 75.85 ± 2.75 | 77.13 ± 6.52 | 66.23 ± 5.88 | 47.38 ± 2.55 |
| Monocyte, 103/μL | 4.8 ± 1.55 | 3.43 ± 1.68 | 3.8 ± 0.74 | 2.42 ± 0.25 |
| Platelet, 103/μL | 950 ± 82.5 | 919 ± 245 | 903 ± 320 | 268 ± 111.4∗∗,$$ |
| Variable . | AA . | SS . | ||
|---|---|---|---|---|
| IgG (n = 3) . | IgG (n = 5) . | IgG/TNF (n = 10) . | SPC-54/TNF (n = 10) . | |
| RBC, 106/μL | 9.50 ± 0.072 | 6.57 ± 0.35∗ | 5.77 ± 0.15∗∗ | 5.62 ± 0.21∗∗ |
| Hematocrit, % | 34.2 ± 2.6 | 34.2 ± 2.55 | 30.56 ± 2.14 | 25.55 ± 1.55$ |
| PMN:lymph | 0.21 ± 0.04 | 0.17 ± 0.05 | 0.53 ± 0.18$ | 1.07 ± 0.12$ |
| WBC, 103/μL | 10.75 ± 1.28 | 31.3 ± 4.42∗ | 25.8 ± 3.59 | 28.21 ± 6.69 |
| PMN, 103/μL | 1. 57 ± 0.25 | 12.37 ± 2.34∗∗ | 28.77 ± 4.95$$ | 49.32 ± 2.36∗∗,$ |
| Lymphocyte, 103/μL | 75.85 ± 2.75 | 77.13 ± 6.52 | 66.23 ± 5.88 | 47.38 ± 2.55 |
| Monocyte, 103/μL | 4.8 ± 1.55 | 3.43 ± 1.68 | 3.8 ± 0.74 | 2.42 ± 0.25 |
| Platelet, 103/μL | 950 ± 82.5 | 919 ± 245 | 903 ± 320 | 268 ± 111.4∗∗,$$ |
PMN, polymorphonuclear lymphocyte (neutrophil); WBC, total white blood cell count.
∗Indicates statistical difference compared with AA IgG (∗P < .05 and ∗∗P < .01).
$Indicates statistical difference compared with SS IgG ($P < .05; $$P < .01).
Role of endogenous thrombin- and APC-biased agonism of PAR1
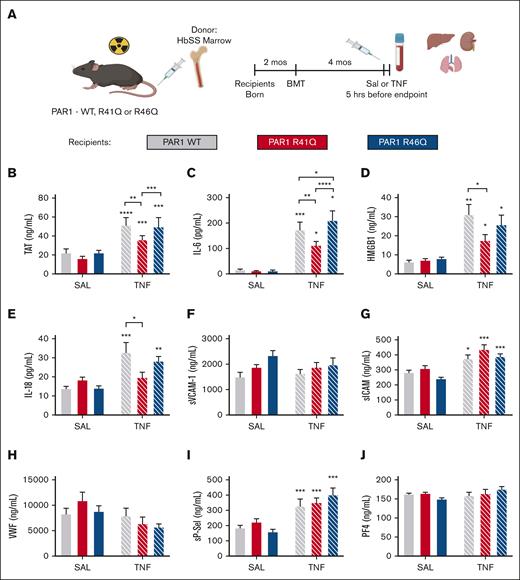
Because SPC54 blocks all APC activities, it was not possible to determine whether the anticoagulant or signaling properties of APC contribute to thrombin generation and inflammation in HbSS mice. To address this, bone marrow from HbSS mice (SSBM) was transplanted into PAR1-modified mice with point mutations in the respective thrombin and APC cleavage sites that make them insensitive to either thrombin (R41Q) or APC (R46Q) cleavage.26 This approach yielded SSBM/WT, SSBM/R41Q, and SSBM/R46Q mice (Figure 4A). Neither the R41Q nor R46Q mutation affected anemia or neutrophilia in SS mice (Table 3).
Effect of PAR1 biased agonism on biomarkers of coagulation and inflammation. SS bone marrow was transplanted into lethally irradiated WT (gray), R41Q (red), and R46Q (blue) mice. Four months later, mice were treated with SAL (steady state, solid bars) or TNF-α (2 mg/kg, IP) (white hashed bars), and plasma was collected after 5 hours (A). Plasma levels of TAT (B), IL-6 (C), HMGB1 (D), IL-18 (E), sVCAM-1 (F), sICAM (G), VWF (H), sP-sel (I), and PF4 (J). Data represent mean ± SEM for 6 to 8 mice (SAL, steady state) and 15 to 17 mice per group (TNF-α challenge). Asterisks above bar represent statistical significance vs SAL-treated mice of same genotype by 2-way ANOVA and Tukey post hoc test. Asterisks above brackets indicate comparison. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001. SAL, saline.
Effect of PAR1 biased agonism on biomarkers of coagulation and inflammation. SS bone marrow was transplanted into lethally irradiated WT (gray), R41Q (red), and R46Q (blue) mice. Four months later, mice were treated with SAL (steady state, solid bars) or TNF-α (2 mg/kg, IP) (white hashed bars), and plasma was collected after 5 hours (A). Plasma levels of TAT (B), IL-6 (C), HMGB1 (D), IL-18 (E), sVCAM-1 (F), sICAM (G), VWF (H), sP-sel (I), and PF4 (J). Data represent mean ± SEM for 6 to 8 mice (SAL, steady state) and 15 to 17 mice per group (TNF-α challenge). Asterisks above bar represent statistical significance vs SAL-treated mice of same genotype by 2-way ANOVA and Tukey post hoc test. Asterisks above brackets indicate comparison. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001. SAL, saline.
Hematologic parameters from SSWT, PAR1R41Q, and PAR146Q mice with bone marrow transplanted from SS mice
| Variable . | SS at steady state . | SS after TNF-α challenge . | ||||
|---|---|---|---|---|---|---|
| WT . | PAR141Q . | PAR146Q . | WT . | PAR141Q . | PAR146Q . | |
| RBC, 106/μL | 5.49 ± 0.08 | 5.65 ± 0.15 | 5.65 ± 0.17 | 4.98 ± 0.21 | 4.95 ± 0.15 | 5.13 ± 0.17 |
| Hematocrit, % | 26.46 ± 0.44 | 27.32 ± 0.68 | 27.24 ± 0.74 | 24.1 ± 0.77 | 23.69 ± 0.69 | 24.03 ± 0.65 |
| PMN:lymph | 0.21 ± 0.002 | 0.21 ± 0.002 | 0.21 ± 0.01 | 0.21 ± 0.01 | 0.21 ± 0.004 | 0.22 ± 0.003 |
| WBC, 103/μL | 31.34 ± 1.71 | 30.81 ± 1.62 | 30.16 ± 1.67 | 14.59 ± 1.38 | 15.31 ± 1.14 | 13.65 ± 1.44 |
| Neutrophil, 103/μL | 3.73 ± 0.41 | 3.6 ± 0.29 | 4.78 ± 0.47 | 3.23 ± 0.29 | 3.36 ± 0.16 | 3.41 ± 0.43 |
| Lymphocyte, 103/μL | 17.74 ± 3.08 | 21.42 ± 2.78 | 20.41 ± 2.85 | 9.05 ± 1.33 | 10.72 ± 1.37 | 8.99 ± 1.3 |
| Monocyte, 103/μL | 0.6 ± 0.07 | 0.72 ± 0.15 | 1.06 ± 0.23 | 0.14 ± 0.03 | 0.19 ± 0.04 | 0.21 ± 0.07 |
| Platelet, 103/μL | 748 ± 43.9 | 721 ± 33.7 | 618 ± 47.6 | 589 ± 62.5 | 598.9 ± 45.9 | 486.8 ± 35.6 |
| Variable . | SS at steady state . | SS after TNF-α challenge . | ||||
|---|---|---|---|---|---|---|
| WT . | PAR141Q . | PAR146Q . | WT . | PAR141Q . | PAR146Q . | |
| RBC, 106/μL | 5.49 ± 0.08 | 5.65 ± 0.15 | 5.65 ± 0.17 | 4.98 ± 0.21 | 4.95 ± 0.15 | 5.13 ± 0.17 |
| Hematocrit, % | 26.46 ± 0.44 | 27.32 ± 0.68 | 27.24 ± 0.74 | 24.1 ± 0.77 | 23.69 ± 0.69 | 24.03 ± 0.65 |
| PMN:lymph | 0.21 ± 0.002 | 0.21 ± 0.002 | 0.21 ± 0.01 | 0.21 ± 0.01 | 0.21 ± 0.004 | 0.22 ± 0.003 |
| WBC, 103/μL | 31.34 ± 1.71 | 30.81 ± 1.62 | 30.16 ± 1.67 | 14.59 ± 1.38 | 15.31 ± 1.14 | 13.65 ± 1.44 |
| Neutrophil, 103/μL | 3.73 ± 0.41 | 3.6 ± 0.29 | 4.78 ± 0.47 | 3.23 ± 0.29 | 3.36 ± 0.16 | 3.41 ± 0.43 |
| Lymphocyte, 103/μL | 17.74 ± 3.08 | 21.42 ± 2.78 | 20.41 ± 2.85 | 9.05 ± 1.33 | 10.72 ± 1.37 | 8.99 ± 1.3 |
| Monocyte, 103/μL | 0.6 ± 0.07 | 0.72 ± 0.15 | 1.06 ± 0.23 | 0.14 ± 0.03 | 0.19 ± 0.04 | 0.21 ± 0.07 |
| Platelet, 103/μL | 748 ± 43.9 | 721 ± 33.7 | 618 ± 47.6 | 589 ± 62.5 | 598.9 ± 45.9 | 486.8 ± 35.6 |
PMN, polymorphonuclear lymphocyte (neutrophil); WBC, total white blood cell count.
There were no statistical differences in hematologic parameters in any of the conditions.
At steady state, there was no difference in TAT in SSBM/R41Q or SSBM/R46Q mice compared with SSBM/WT mice. TNF-α challenge enhanced TAT levels in SSBM/WT mice, which was significantly attenuated in SSBM/R41Q mice. Although TAT levels were higher in SSBM/R46Q mice than SSBM/R41Q, they were not elevated compared with SSBM/WT mice (Figure 4B), indicating that thrombin-mediated PAR1 activation contributes to enhanced thrombin generation. Similarly, the inflammatory cytokine IL-6 was not affected by the PAR1 point mutations at steady state. In contrast, the elevated levels of IL-6 observed in TNF-α–treated SSBM/WT mice were attenuated in SSBM/R41Q mice and further exacerbated in SSBM/R46Q mice (Figure 4C). Plasma levels of HMGB1 were elevated by TNF-α challenge and were attenuated in SSBM/R41Q compared with SSBM/WT or SSBM/R46Q mice (Figure 4D). The inflammatory cytokine IL-18 was significantly upregulated in SSBM/R46Q mice at steady state, yet it was not further increased by TNF-α challenge (Figure 4E). These results suggest that thrombin-mediated PAR1 cleavage fuels inflammation, whereas APC–mediated PAR1 activation attenuates inflammation.
The endothelial activation markers sVCAM and VWF were not altered in SSBM/R41Q and SSBM/R46Q mice compared with SSBM/WT controls at steady state or during TNF challenge (Figure 4F-G). In contrast, TNF-α challenge increased the levels of sP-sel and sICAM in all SS mice (Figure 4G,I), however, these markers were not different from SSBM/WT in either SSBM/R41Q or SSBM/R46Q mice. Neither PAR1 expression nor TNF-α challenge affected plasma levels of PF4 (Figure 4I), suggesting that endothelial cells, rather than platelets, are the source of sP-sel after TNF-α treatment.
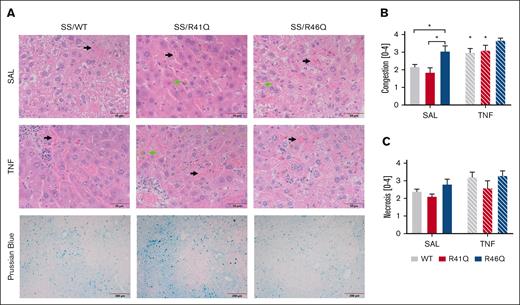
Histological analysis of the livers for vascular congestion revealed vascular congestion of sickled RBCs, the presence of iron-laden macrophages, and necrosis in the livers of all SSBM mice (Figure 5A). When the pathology was scored, we found significantly more RBC-mediated congestion in SSBM/R46Q mice than SSBM/WT or SSBM/R41Q mice at steady state (Figure 5B). Interestingly, TNF-α challenge increased vascular congestion in SSBM/WT and SSBM/R41Q mice compared with their saline-treated counterparts, whereas TNF-α did not further increase vascular congestion in SSBM/R46Q mice (Figure 5B). Neither TNF-α challenge nor PAR1 cleavage site mutations had any significant effect on hepatic necrosis scores (Figure 5C). Prussian blue staining revealed the presence of iron in the liver tissue (Figure 5A), yet quantification of Prussian blue revealed no differences between the PAR1 genotypes. Evaluation of lung pathology revealed the presence of sickled RBCs in the interstitial tissues of all SSBM mice, regardless of PAR1 expression or TNF challenge (supplemental Figure 2). Kidney histopathology revealed increased congestion of sickled RBCs in the SSBM/WT and SSBM/R46Q mice compared with AABM/WT (supplemental Figure 3). Interstitial and glomerular congestion were quantified, but there were no differences between SSBM/WT, SSBM/R41Q, and SSBM/R46Q mice (supplemental Figure 3B-C).
Hepatic congestion is enhanced in SSBM/R46Q mice. (A) Livers were collected and stained with H&E; representative images of livers from SSBM/WT, SSBM/R41Q, and SSBM/R46Q mice during steady state (SAL) and TNF-α challenge; scale bar represents 50 μm. Three blinded observers scored congestion (B) and necrosis (C). Data represent mean ± SEM. Black arrow denotes sinusoidal congestion, and green arrow denotes iron-laden macrophages. There were 6 to 8 mice (SAL, steady state) and 15 to 17 mice (TNF-α challenge) per group. Asterisks above bar represent statistical significance vs SAL-treated mice of same genotype by 2-way ANOVA and Tukey post hoc test. Asterisks above brackets indicate comparison. ∗P < .05. SAL, saline.
Hepatic congestion is enhanced in SSBM/R46Q mice. (A) Livers were collected and stained with H&E; representative images of livers from SSBM/WT, SSBM/R41Q, and SSBM/R46Q mice during steady state (SAL) and TNF-α challenge; scale bar represents 50 μm. Three blinded observers scored congestion (B) and necrosis (C). Data represent mean ± SEM. Black arrow denotes sinusoidal congestion, and green arrow denotes iron-laden macrophages. There were 6 to 8 mice (SAL, steady state) and 15 to 17 mice (TNF-α challenge) per group. Asterisks above bar represent statistical significance vs SAL-treated mice of same genotype by 2-way ANOVA and Tukey post hoc test. Asterisks above brackets indicate comparison. ∗P < .05. SAL, saline.
Discussion
A recent global analysis found that SCD affects ∼8 million people worldwide, with >500 000 new births in 2021.32 It is the 12th leading cause of death for children aged <5 years, and total mortality has increased by 20% since 2000.32 In spite of this, there are only 4 Food and Drug Administation–approved treatment options for patients: hydroxyurea, crizanlizumab, L-glutamine, and voxelotor.1 These therapies only modestly limit the severity and frequency of hemolytic and vascular complications.33-35 Coagulation activation and thrombotic complications are a hallmark of SCD, and an estimated 11% to 27% of patients experience venous thrombosis.5 In addition to thrombotic complications, SCD is characterized by endothelial dysfunction and adhesion, which contributes to vascular stasis and ultimately vaso-occlusive crisis.1,2 APC is uniquely situated in both of these pathways, due to its role as an anticoagulant and in maintaining endothelial homeostasis through biased activation of PAR1.36 However, the role of APC has not been extensively investigated in SCD.
In this study, the role of the endogenous APC system was assessed in HbSS mice by inhibiting APC generation using antibody SPC54. Notably, APC inhibition significantly increased thrombin generation, systemic inflammation, and the release of soluble P-sel in HbSS mice under steady-state conditions. A marked rise in vascular congestion in the liver, heightened neutrophil accumulation in the lungs, and worsened RBC congestion in the kidneys were observed. These findings suggest that the inhibition of APC generation enhances thrombin generation, leading to the hypothesis that this process triggers PAR1-dependent proinflammatory signaling and the release of P-sel from Weibel-Palade bodies at steady state and after TNF-α challenge. Accordingly, a potential protective role of endogenous APC in SCD by limiting thrombin generation is surmised. Importantly, individuals with SCD have decreased PC and APC activity levels compared with healthy controls, with further reductions during VOEs.23,37-40 Furthermore, the decreased APC activity correlates with an increased risk of stroke and thrombosis.21,40
Because APC has both anticoagulant and cytoprotective signaling functions through activation of PAR1, biased agonism of PAR1 by APC in HbSS mice was also evaluated. We previously showed that PAR1 deficiency on nonhematopoietic cells had no effect on thrombin generation or plasma levels of IL-6 at steady state in HbSS mice.7 One interpretation of these results is that PAR1 does not play a role in inflammation and endothelial activation in steady-state disease. Another possibility is that PAR1 deletion removes both detrimental thrombin/PAR1 signaling as well as beneficial APC/PAR1 signaling.18 To address this limitation of gene knockout studies, Sinha et al generated mice with point mutations in PAR1 that are selectively activated by either thrombin or APC to investigate the individual contributions of these 2 pathways.26 Consistent with previous results,7 neither SSBM/R41Q nor SSBM/R46Q mice exhibited differences in thrombin generation, endothelial activation, or systemic inflammation compared with the SSBM/PAR1WT counterparts at steady state. However, hepatic vascular congestion was significantly increased in the SSBM/R46Q mice at steady state compared with SSBM/WT and SSBM/R41Q mice. This observation suggests a protective role for endogenous APC/PAR1 signaling against vascular congestion. Because we previously showed that PAR1 contributes to heme-induced vascular stasis in HbSS mice,10 future studies will determine the individual roles of thrombin- and APC-biased agonism on this complication. It will also be interesting to determine whether long-term biased agonism of either the thrombin/PAR1 or APC/PAR1 pathways contributes to end-organ damage and mortality.
After TNF-α challenge, SSBM/R41Q mice exhibited lower TAT levels than both SSBM/WT and SSBM/R46Q mice, suggesting that thrombin/PAR1 signaling plays a role in the heightened thrombin generation in this model of VOE. We hypothesize that this is an indirect effect, wherein canonical thrombin/PAR1 signaling increases endothelial permeability, thus exposing perivascular TF to the blood to initiate more thrombin generation and resulting in elevated circulating TAT levels. Indeed, our previous work demonstrated that perivascular TF plays a role in thrombin generation in HbSS mice.8 Additionally, these findings suggest that thrombin/PAR1 signaling contributes to endothelial activation, consistent with our previous finding that inhibiting PAR1 with vorapaxar or deficiency in nonhematopoietic PAR1 reduced P-sel expression on the endothelial surface.
Remarkably, SSBM/R41Q mice demonstrated protection against TNF-α–enhanced IL-6, IL-18, and HMGB1 levels, whereas sickle mice expressing SSBM/R46Q exhibited elevated IL-6 levels when compared with both SSBM/WT and SSBM/R41Q mice. These findings strongly suggest that thrombin-PAR1-R41 signaling increases inflammation, and APC-PAR1-R46 signaling blunts inflammation. Thus, biased agonism of PAR1 differentially regulates inflammation in SCD during a VOE-like challenge. These data are consistent with the worsened IL-6 and IL-18 levels observed with SPC-54–mediated APC inhibition. Inflammatory cytokines such as IL-6 and IL-18 can be secreted from activated endothelial cells and leukocytes. In these studies, we can only investigate the role of nonhematopoietic PAR1 signaling due to the nature of the bone marrow transplantation approach. Although the primary source of these cytokines in HbSS mice cannot be identified, our data suggest that endothelial cells contribute to elevated cytokine levels during TNF-α challenge.
This study revealed notable differences in the regulation of sVCAM and sP-sel, 2 markers of endothelial activation, in response to APC inhibition and biased PAR1 signaling. Plasma sVCAM-1 levels were unaffected by APC inhibition or either PAR1 point mutation. In the context of SCD, elevated sVCAM-1 levels are primarily attributed to increased endothelial expression and proteolytic release.41 Our data suggest that this process is not influenced by PAR1 signaling. In contrast, P-sel is stored in Weibel-Palade bodies with VWF. In SCD, thrombin-PAR1-R41 signaling is known to release Weibel-Palade bodies from endothelial cells to increase endothelial surface and plasma levels of VWF and P-sel.10,42,43 Indeed, APC inhibition with SPC-54 significantly raised sP-sel levels, at steady state and during TNF-α challenge in SS mice. To discern the source of sP-sel, considering its potential release from platelet α-granules after thrombin activation of PAR4 in mice,44 we examined plasma levels of PF4, stored in α-granules but not endothelial cells. No increase in PF4 was observed in HbSS mice compared with HbAA, and APC inhibition had no impact on PF4 levels, suggesting that in this context, sP-sel release originated from endothelial cells rather than platelets.
However, the approach of inhibiting APC alone does not elucidate whether the increase in sP-sel is a result of canonical thrombin-PAR1-R41 signaling (due to increased thrombin generation) or inhibition of protective APC-PAR1-R46 signaling. To address that, we evaluated sP-sel levels in our SSBM mice with PAR1 point mutations. Interestingly, sP-sel levels were not different from that of SSBM/WT mice in either SSBM/R41Q or SSBM/R46Q mice at steady state or after TNF-α challenge, suggesting that PAR1 signaling does not contribute to the release of sP-sel in this model. One possible explanation is that PAR1 signaling primarily influences P-sel expression on the surface but not the release of sP-sel into the circulation. Weibel-Palade body release is also stimulated by other agonists such as vascular endothelial growth factor,45 histamine,46 vasopressin, and sphingosine 1 phosphate,47 suggesting that these effectors may play a role in this pathway in this context.
Circulating levels of VWF were modestly increased in HbSS after TNF-α challenge, consistent with previous reports.30 Interestingly, unlike sP-sel, there were no discernable differences in VWF levels between SSBM/WT, SSBM/R41Q, and SSBM/R46Q mice at steady state or after TNF-α challenge, suggesting that biased agonism of PAR1 does not affect VWF release into the circulation. It is possible that although the assay did not detect differences in total VWF among the groups, differences in the size distribution of the VWF multimers may exist. ADAMTS13, a metalloprotease controlling VWF cleavage and ultralarge VWF multimer degradation, exhibits decreased activity in SCD.30 APC has been demonstrated to increase ADAMTS13 messenger RNA in endothelial cells treated with plasma from patients with sepsis. It is possible that APC-PAR1-R46–biased agonism may influence ADAMTS13 activity and thus VWF multimer size, yet this remains to be tested.
In conclusion, our study demonstrates that APC inhibition exacerbates thrombin generation and inflammation both under steady state conditions and during acute TNF-α challenge that models a VOE. Our findings offer insight into the intricate interplay between canonical thrombin-PAR1-R41 signaling, inflammation, and endothelial activation in sickle mice, while underscoring the benefits of APC-PAR1-R46 signaling in reducing inflammation. These results highlight a multifaceted role for PAR1 signaling and APC in SCD. Because APC can limit thrombin generation and promote PAR1-dependent beneficial biased signaling after R46 cleavage, the potential therapeutic implications of leveraging this biased role of PAR1 to mitigate vascular complications associated with SCD is promising and merits further studies. Indeed, these results extend beyond SCD, suggesting a broader relevance to inflammatory conditions in which PAR1 has been implicated, such as cardiovascular disease,48-51 stroke,52 viral infections,53 sepsis,26 and colitis.54 It will be interesting to determine whether PAR1 modulators such as parmodulins or pepducins will be beneficial in this setting.
Acknowledgments
The authors thank Robert Lee and David Paul for helpful conversations about the results.
E.S. is supported by National Institutes of Health (NIH), National Heart Lung and Blood Institute (NHLBI) grant R01 HL155193 and an American Society of Hematology Scholar award. L.O.M. is supported by NIH, NHLBI grants R01HL148096, R01 HL142975, and R01HL104165. R.P. is supported by NIH, NHLBI grant R01 HL157441. J.H.G. is supported by NIH, NHLBI grant R01 HL142975.
Authorship
Contribution: N.R. conducted the experiments, analyzed data, and wrote the manuscript with consultation from E.S. and contribution from all coauthors; K.L., J.D., F.T., and C.F. conducted experiments; J.H.G. provided reagents; L.O.M. provided reagents and consulted on experiments; R.P. consulted on experiments; and E.S. designed the experiments, performed experiments, analyzed the data, wrote the manuscript, and supervised the project.
Conflict-of-interest disclosure: E.S. and R.P. are supported by research funding from CSL Behring for an unrelated project. The remaining authors declare no competing financial interests.
Camille Faes died on 25 May 2020.
Correspondence: Erica Sparkenbaugh, Department of Medicine, Blood Research Center, The University of North Carolina at Chapel Hill, 116 Manning Dr, 8114 Mary Ellen Jones Bldg, Chapel Hill, NC 27599; email: erica_sparkenbaugh@med.unc.edu.
References
Author notes
Data are available upon request from the corresponding author, Erica Sparkenbaugh (erica_sparkenbaugh@med.unc.edu).
The full-text version of this article contains a data supplement.