FF-10101 is an irreversible inhibitor of FLT3 active against most types of FLT3 activating mutations.
FF-10101 induced responses in patients who had progressed on other FLT3 inhibitors, as well as in some patients with wild-type FLT3.
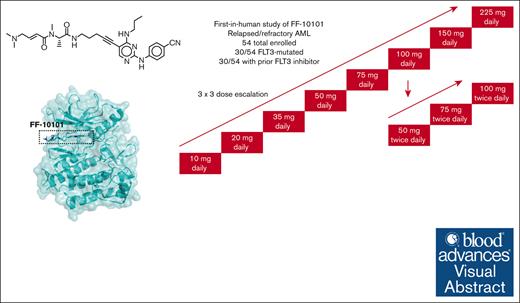
Visual Abstract
FLT3 tyrosine kinase inhibitors (TKIs) have clinical efficacy for patients with FLT3-mutated AML (acute myeloid leukemia), but their impact is limited by resistance in the setting of monotherapy and by tolerability problems when used in combination therapies. FF-10101 is a novel compound that covalently binds to a cysteine residue near the active site of FLT3, irreversibly inhibiting receptor signaling. It is effective against most FLT3 activating mutations, and, unlike other inhibitors, is minimally vulnerable to resistance induced by FLT3 ligand. We conducted a phase 1 dose escalation study of oral FF-10101 in patients with relapsed and/or refractory AML, the majority of whom harbored FLT3-activating mutations and/or had prior exposure to FLT3 inhibitors. Fifty-four participants enrolled in cohorts receiving doses ranging from 10 to 225 mg per day and 50 to 100 mg twice daily (BID). The dose limiting toxicities were diarrhea and QT prolongation. Among 40 response-evaluable participants, the composite complete response rate was 10%, and the overall response rate (including partial responses) was 12.5%, including patients who had progressed on gilteritinib. Overall, 56% of participants had prior exposure to FLT3 inhibitors. The recommended phase 2 dose was 75 mg BID. FF-10101 potentially represents a next-generation advance in the management of FLT3-mutated AML. This trial was registered at www.ClinicalTrials.gov as #NCT03194685.
Introduction
Activating mutations of the FLT3 gene are among the most common genetic perturbations in acute myeloid leukemia (AML) and the most common of these, internal tandem duplication (ITD) mutations, are associated with a worse prognosis.1-3 A number of different FLT3 inhibitors have been shown to improve survival in patients with FLT3-mutated AML when incorporated into induction and consolidation chemotherapy, as monotherapy for relapsed/refractory disease, and as posttransplant maintenance.4-8 Although FLT3 inhibitors are now a part of the standard-of-care approach to AML, responses in the relapsed/refractory setting are still suboptimal.5,9 A number of resistance mechanisms have emerged,10-13 and tolerability (myelosuppression, QT-prolongation, gastrointestinal, and skin toxicities) is still problematic, particularly when the drugs are given in combination therapy.14-17 Given that FLT3 inhibition has clearly improved outcomes in AML, identifying a FLT3 inhibitor that is both more tolerable and less affected by resistance mechanisms offers an opportunity to improve outcomes yet further.
FF-10101 is a novel type 1 FLT3 inhibitor that contains an acryloyl group that forms a covalent bond with cysteine 695 near the adenosine triphosphate–binding pocket of FLT3, irreversibly inhibiting the receptor.18 FF-10101 has potent in vitro inhibitory activity against FLT3 ITD (FLT3-ITD) and FLT3 tyrosine kinase domain (FLT3-TKD) mutations, as well as against the gatekeeper, noncanonical, and extracellular activating mutations that are associated with resistance to other FLT3 inhibitors.19 In addition, its irreversible binding renders the drug minimally affected by the binding of FLT3 ligand to the receptor, another mechanism of resistance to FLT3 inhibitors.18 We conducted a phase 1 first-in-human study to identify a dose of FF-10101 that could achieve sustained in vivo FLT3 inhibition in patients with relapsed and/or refractory (R/R) AML regardless of FLT3 mutation status in a safe and tolerable fashion.
Methods
Study design
This was a phase 1, dose-escalation study of oral FF-10101 conducted at 8 centers in the United States. Eligible patients were aged ≥18 years with R/R AML and unlikely to benefit from standard therapy (particularly approved FLT3 inhibitors) according to the local treating physician, and after consensus from all other investigators via teleconferences. The primary objectives were to define the dose-limiting toxicities (DLTs), determine a maximum tolerated dose, and identify a dose or doses for expansion based on in vivo target inhibition, pharmacokinetics, and tolerability. The original design was a phase 1, 3 × 3 dose escalation study in patients with R/R AML, regardless of FLT3 mutation status, in order to identify a recommended phase 2 dose (RP2D). This would be followed by expansion cohorts restricted to patients with FLT3-mutant R/R AML. However, the COVID-19 pandemic began (March 2020) just as the final participants in the dose-escalation phase were enrolled and the RP2D was being identified. The industry sponsor made the decision to halt the study at that time.
The trial schema is shown in Figure 1. Dose escalation of FF-10101 started at a single daily dose of 10 mg and continued up to 225 mg per day in 28-day cycles. If no DLTs were observed in the first 3 patients, the subsequent 3 patients were treated at the next dose level, after agreement between the medical monitor and the participating investigators. If 1 dose-limiting toxicity occurred at a specific dose level, 3 more patients were enrolled at the same dose level. If the 3 additional patients did not experience a dose-limiting toxicity, the next dose level was initiated. If ≥2 DLTs occurred in a cohort of 3 to 6 people, the next lower dose level was declared to be the maximum tolerated dose. A dose-limiting toxicity was defined as any grade ≥3 nonhematologic toxicity considered at least possibly related to FF-10101 and not clearly resulting from the underlying leukemia, except for grade 3 nausea and vomiting that reverted to grade ≤2 within 72 hours, infection, bleeding, or other expected direct complications of cytopenias due to underlying leukemia, grade 3 asthenia, fever, anorexia, or constipation, or alopecia of any grade. In addition, grade ≥3 elevations of creatine kinase (CK) in the absence of clinical signs of myositis were not considered DLTs, as such changes may be due to reduced enzyme clearance caused by the drug inhibiting the function of the CSF1 receptor (like FLT3, a class 3 tyrosine kinase receptor).20-22 To ensure there was no cardiac damage in association with elevated CK, troponin and B-type natriuretic peptide was monitored. All such changes were classified as adverse events (AEs) of special interest for review by the safety committee. Finally, differentiation syndrome was also designated as an AE of special interest (as an on-target effect), not considered a dose-limiting toxicity.
Trial schema. Dose escalation of FF-10101 was carried out in cohorts 3 participants from 10 mg QD up to 225 mg QD. If any participant experienced a grade ≥3 AE that was possibly, probably, or definitely attributed to FF-10101, 3 additional participants were added to the cohort. After 100 mg QD was deemed safe and tolerable, cohorts receiving BID dosing were opened in parallel.
Trial schema. Dose escalation of FF-10101 was carried out in cohorts 3 participants from 10 mg QD up to 225 mg QD. If any participant experienced a grade ≥3 AE that was possibly, probably, or definitely attributed to FF-10101, 3 additional participants were added to the cohort. After 100 mg QD was deemed safe and tolerable, cohorts receiving BID dosing were opened in parallel.
While each dose cohort was assessed for safety and tolerability, each was also evaluated for in vivo FLT3 inhibition using an ex vivo surrogate assay, the plasma inhibitory activity (PIA) assay for FLT3.23 As the trial progressed, in response to PIA assay results and pharmacokinetic data, new cohorts were opened in which participants were dosed twice daily (BID; every 12 hours). The study was conducted in compliance with the Declaration of Helsinki and approved by institutional review boards at each site. All participants provided informed consent. Bone marrow aspirates were collected before start of therapy, and on day 1 of cycle 2 and beyond. Response was evaluated (in participants receiving at least 75% of doses in at least 1 cycle of study drug) using the Revised Recommendations of the International Working Group for AML,24 with the modification that complete remission with incomplete hematologic recovery (CRh), defined as complete remission (CR) but with absolute neutrophil count between 500/μl and 1000/μl and platelets >50 000/ul, was included. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. After data lock, the primary data were directly analyzed by the corresponding author, who then wrote the manuscript without assistance from nonauthors.
Cell lines, primary patient samples, and turnover studies
FLT3-ITD–expressing cell lines Molm14 and MV411,25,26 and primary AML samples were cultured as previously described.11,27,28 FLT3 immunoprecipitation and immunoblotting was carried out as previously described.11,27,28 Primary blasts collected from patients with AML were provided by the Sidney Kimmel Comprehensive Cancer Center Hematologic Malignancies Cell Bank Protocol. All participants gave informed consent according to the Declaration of Helsinki under a protocol approved by the institutional review board at Johns Hopkins University. Blasts were isolated and viably cryopreserved as previously described.11,27,28 FF-10101 was provided by FUJIFILM Corporation, and dissolved in dimethyl sulfoxide at stock concentrations of 10 mM. Stocks were aliquoted into 10-μL volumes, stored at −80°C, and thawed immediately before use.
For turnover studies, cell lines or blasts were cultured in the presence or absence of 50 μg/mL cycloheximide (Sigma-Aldrich, St. Louis, MO) before FLT3 immunoblotting analysis.
Pharmacokinetic and pharmacodynamic studies
At predetermined time points during the first and second cycles, whole blood was collected into lithium-heparin tubes. Plasma was separated from the cellular fraction and stored frozen. Plasma samples were analyzed for FF-10101 concentration with a validated method, and, in parallel, for PIA and FLT3 ligand concentration, as previously described.11,27 When feasible, circulating blasts were collected and analyzed for total and phosphorylated FLT3 (P-FLT3) to directly compare with PIA results at simultaneous time points, as previously described.11,27
Statistical analysis
The dose-toxicity association and determination of the maximum tolerated dose (MTD) was determined using a 2-parameter Bayesian logistical regression model.29 All AEs, clinical laboratory data, and pharmacokinetic and pharmacodynamic data were summarized using descriptive statistics.
Results
Trial participants
Between August 2017 and February 2021, 54 participants were enrolled and treated. The database was locked on 27 October 2021. The characteristics of the participants are displayed in Table 1. The median number of prior therapies was 3 (range, 1-7), and 30 of 54 participants (55.6%) harbored a FLT3 mutation at the time of enrollment. An equal proportion (30 of 54; 56.6%) had previously been treated with a FLT3 inhibitor, but in some participants, a previously detected FLT3 mutation was no longer detectable at enrollment. Other than FLT3-ITD and FLT3-TKD (D835) mutations, only 2 noncanonical FLT3 mutations (V491L and A680V) were detected in the participants. No “gatekeeper” mutations (at residue F691) were detected.
Pharmacokinetics and pharmacodynamics
The concentration inhibiting 50% of baseline activity of FF-10101 against FLT3 in 100% human plasma is estimated to be 20 nM (supplemental Figure 1). Pharmacokinetic analysis indicated that after oral dosing, FF-10101 has a mean half-life (t1/2) of ∼10 hours, with absorption unaffected by food. The PIA assay was originally developed as an ex vivo surrogate of FLT3 inhibition using reversible FLT3 inhibitors. Although it is expected to accurately reflect FLT3 inhibition based on the concentration of inhibitor in any given plasma sample, an irreversible inhibitor is expected to have inhibitory effects after the drug has cleared from the system, depending on the turnover or t1/2 of the target receptor. We carried out a series of experiments to determine the t1/2 of both the wild-type and mutant FLT3 receptor in cell lines and in primary blasts isolated from patients (Figure 2A). The t1/2 of FLT3 in Molm-14 and MV4-11 cells, as well as in primary samples ranged from 0.9 to 5.2 hours, which is consistent with previously published studies. The turnover was only modestly decreased in the presence of FLT3 inhibition, with a ∼50% increase in the t1/2 of FLT3 (data not shown). To confirm that the PIA assay was an appropriate surrogate for in vivo FLT3 inhibition by FF-10101, we collected whole blood from a participant receiving 100 mg per day FF-10101 who had circulating blasts at enrollment. We divided the sample in 2, and half was used to directly isolate and analyze P-FLT3 from the circulating blasts whereas the other half was used to isolate plasma for the PIA assay. The results of these 2 parallel assays are shown in Figure 2B. Of note, total FLT3 in the primary blasts was markedly upregulated (more so than in the Molm14 cells), presumably reflecting either increased production, decreased turnover, or both. Regardless, based on the equivalent degree of P-FLT3inhibition in blasts vs in Molm14 cells assessed by PIA (Figure 2B, upper panels), we concluded that the PIA assay was an adequate surrogate for in vivo FLT3 inhibition by FF-10101. Based on the short t1/2 of the FLT3 receptor and the level of P-FLT3 visible at 8 hours after dosing, we also concluded that dosing of FF-10101 needed to be more than once daily (QD) to allow for inhibition of newly synthesized receptors. The protocol was subsequently amended to open additional cohorts with BID dosing.
Turnover of the FLT3 receptor. (A) Molm14 and MV411 cells (each harboring a FLT3-ITD mutation) and primary AML samples (2 from patients with FLT3-ITD mutations, 1 from a patient with wild-type FLT3) were cultured in the presence or absence of 50 μg/mL cycloheximide. At designated time points, cells were harvested and analyzed for total FLT3 expression by immunoblotting. Densitometric results were analyzed by regression analysis after linear conversion to estimate a t1/2. (B) Whole blood was collected at designated time points after dosing from a participant receiving 100 mg per day FF-10101 who had circulating blasts at enrollment. For each time point, half of the sample was used to directly isolate and analyze P-FLT3 from the circulating blasts whereas the other half was used to isolate plasma for the PIA assay using Molm14 cells.
Turnover of the FLT3 receptor. (A) Molm14 and MV411 cells (each harboring a FLT3-ITD mutation) and primary AML samples (2 from patients with FLT3-ITD mutations, 1 from a patient with wild-type FLT3) were cultured in the presence or absence of 50 μg/mL cycloheximide. At designated time points, cells were harvested and analyzed for total FLT3 expression by immunoblotting. Densitometric results were analyzed by regression analysis after linear conversion to estimate a t1/2. (B) Whole blood was collected at designated time points after dosing from a participant receiving 100 mg per day FF-10101 who had circulating blasts at enrollment. For each time point, half of the sample was used to directly isolate and analyze P-FLT3 from the circulating blasts whereas the other half was used to isolate plasma for the PIA assay using Molm14 cells.
PIA assay results for the escalating doses of FF-10101 are displayed in Figure 3A, with representative immunoblots for each dose level shown in supplemental Figure 2. Inhibition of P-FLT3 was most effective at dose levels of 150 mg daily, 225 mg daily, 50 mg BID, and 75 mg BID. The plasma concentration of FF-10101 correlated with the PIA results (Figure 3B), with concentrations >100 ng/mL associated with more complete inhibition. Steady state concentrations of FF-10101 (after dosing on day 1 of cycle 2) are displayed in Figure 3C,D. Maximal trough suppression of P-FLT3 activity (eg, <5% of baseline) was achieved at FF-10101 concentrations >200 ng/ml. Concomitant azole use was allowed after the first week of treatment with FF-10101, and more intensive pharmacokinetic and electrocardiographic monitoring was performed when azoles were used. However, only 10 participants across 7 dosing cohorts were treated with azoles (voriconazole, posaconazole, or isavuconazonium), and so whereas no apparent azole effect on FF-10101 concentration was observed, the sample number is too small to draw any firm conclusions about this drug interaction.
Pharmacodynamics and pharmacokinetics. (A) PIA results (expressed as the percentage of P-FLT3 relative to baseline) from trough (before dose) time points on days 8 and 15 of cycle 1 are plotted according to dose level. The solid black line indicates the mean. (B) PIA results plotted against plasma concentration of FF-10101. Whole blood collected at designated time points throughout cycle 1 of each dose level was analyzed for both FF-10101 concentration by mass spectrometry as well as to quantify the percentage of P-FLT3 relative to baseline using the PIA assay. (C) Plasma concentration of FF-10101 after dosing on cycle 2 day 1 for daily dosing cohorts. (D) Plasma concentration of FF-10101 after dosing on cycle 2 day 1 for BID dosing cohorts.
Pharmacodynamics and pharmacokinetics. (A) PIA results (expressed as the percentage of P-FLT3 relative to baseline) from trough (before dose) time points on days 8 and 15 of cycle 1 are plotted according to dose level. The solid black line indicates the mean. (B) PIA results plotted against plasma concentration of FF-10101. Whole blood collected at designated time points throughout cycle 1 of each dose level was analyzed for both FF-10101 concentration by mass spectrometry as well as to quantify the percentage of P-FLT3 relative to baseline using the PIA assay. (C) Plasma concentration of FF-10101 after dosing on cycle 2 day 1 for daily dosing cohorts. (D) Plasma concentration of FF-10101 after dosing on cycle 2 day 1 for BID dosing cohorts.
Safety and tolerability
Overall, 53 of 54 (98.1%) participants reported at least 1 treatment emergent AE (TEAE). The most frequent related TEAEs are summarized in Table 2, and supplemental Table 1 displays all grade ≥3 TEAEs reported for all cohorts. Six participants died while on study treatment from causes deemed by the local investigator to be unrelated to study drug: 4 from sepsis/infection, 1 from intracranial hemorrhage, and 1 from pulmonary embolus, all reported as not related to study drug. Differentiation syndrome, which has been reported in association with FLT3 inhibitor therapy,30,31 occurred in 4 participants: 2 in the 75-mg BID cohort (1 of whom had wild-type FLT3), 1 in the 100 mg QD cohort, and 1 in the 100 mg BID cohort.
CK elevation was an AE of special interest and occurred in 16 (29.6%) participants. In no case of CK elevation was there any sign of myositis. Clearance of the CK enzyme is thought to be cleared, at least in part, by hepatic Kupffer cells (monocyte derived) that are in turn regulated by the CSF1R receptor.21 CSF1R is a type 3 receptor tyrosine kinase,20 structurally homologous to FLT3. Elevation of CK appears to be a class effect of type 3 receptor tyrosine kinase inhibitors, and has been observed in clinical studies of other FLT3 inhibitors.32,33 Given the prevalence of elevated CK, as an additional safety precaution the protocol was amended to monitor cardiac ejection fraction throughout therapy. Echocardiograms were obtained at baseline and at the start of each subsequent cycle of therapy. Follow-up echocardiograms were obtained from 38 of 54 (70%). The most common reason for no follow-up echocardiogram was failure to complete 1 cycle of therapy. Ten of 38 participants were admitted with AEs (9 of 10 for fever/sepsis, and 1 with hemorrhage and differentiation syndrome) when the follow-up echocardiogram was obtained, so these were not included in this evaluation. Of the 28 participants with follow-up echocardiograms obtained on day 1 of cycle 2, ejection fractions fluctuated above and below the baseline value (supplemental Figure 3). A single participant, an 84-year-old male with a prior history of coronary artery disease, atrial fibrillation, and daunorubicin exposure, experienced a decrease in ejection fractions to <50%.
Diarrhea is a common AE in any phase 1 AML trial. However, there was a clear association between FF-10101 dose and diarrhea, in that 1621 (76.2%) participants treated with ≥100 mg/dose (eg, cohorts 100 mg QD, 150 mg QD, 225 mg QD, and 100 mg BID) reported grade 1-3 diarrhea, whereas only 733 (21.2%) participants who received a dose of <100 mg/dose reported diarrhea. The frequency with which diarrhea was reported as an AE according to dose level is depicted in supplemental Figure 4. Although only 4 participants had grade 3 diarrhea, this AE rendered these higher doses poorly tolerated over weeks of therapy.
Regarding DLTs, 1 of 7 participants in the 50 mg BID cohort experienced a grade 3 maculopapular rash possibly due to FF-10101. Of 5 participants treated with 100 mg BID, 2 experienced DLTs. The first was a 50-year-old female who developed grade 3 diarrhea, and was subsequently admitted on cycle 1, day 8 with urosepsis and bacteremia. The study drug was stopped at that time. An echocardiogram obtained 7 days later showed a left ventricular ejection fraction of 26% (compared with 52% at baseline). On review, the cardiomyopathy was deemed stress cardiomyopathy in the setting of sepsis. Follow-up echocardiograms showed recovery to a normal ejection fraction. The second participant in the 100 mg BID cohort to experience a dose-limiting toxicity was a 68-year-old woman who reported grade 1 diarrhea and vomiting on cycle 1, day 9. She was noted to have grade 3 QT prolongation, considered probably related to FF-10101, and the study drug was stopped. She also developed grade 3 troponin increase, which was deemed not related to study drug. The participant was admitted with sepsis and progressive disease 2 days later, and expired on cycle 1, day 11. Autopsy revealed extensive extramedullary leukemia but no evidence of cardiomyopathy. The 2 confirmed DLTs for this cohort, therefore, were grade 3 diarrhea and grade 3 QT prolongation, respectively.
The MTD was determined to be 75 mg BID. Per protocol, the 100 mg QD, 150 mg QD, and 225 mg QD dosing levels were tolerated, but from a practical standpoint the investigators concluded that the high prevalence of grade 1-2 diarrhea rendered those dose levels generally less tolerable.
Efficacy
Of 54 total participants, 40 were evaluable for response. Fourteen participants did not receive at least 75% of study drug during the first cycle of therapy and were withdrawn from the study (mostly because of infection or other AEs). These were evaluated only for safety. Among the 40 response-evaluable participants, there was 1 CR (in a FLT3-ITD participant previously treated with midostaurin), 1 CRh (in a participant with wild-type FLT3 never previously treated with a FLT3 inhibitor), 2 complete remission with partial platelet recovery (CRp) (1 in a participant with a FLT3-ITD mutation not previously treated with a FLT3 inhibitor, and 1 in a participant with wild-type FLT3 previously treated with gilteritinib), and 1 partial response (PR; wild-type FLT3, previously treated with gilteritinib). The composite CR rate (CR + CRh + CRp) was 4 of 40 (10%), and the overall response rate (CR + CRh + CRp + PR) was 5 of 40 (12.5%). The overall response rate in participants with FLT3 mutations was 9.5% (2 of 21) and in participants previously treated with a FLT3 inhibitor, it was 13.6% (3 of 22). One participant whose disease lacked a FLT3 mutation and who never previously had FLT3 mutation achieved a CRh. Detailed characteristics of the 5 responding participants are summarized in supplemental Table 2.
Of the 40 response-evaluable participants, 14 had circulating blasts at the end of the first cycle, and an evaluation bone marrow biopsy was deferred. For the remaining 26 participants, the percentage of marrow blasts after the first cycle was compared with the baseline marrow. The results are displayed in Figure 4.
Waterfall plot for best change in marrow blast percentage. Shown are changes in absolute blast percentages in bone marrow aspirates at the time of best response compared with baseline. Note that 14 participants who did not have response assessment marrow biopsy because of circulating blasts are not included in this plot. Participants with no FLT3 mutation who had prior treatment with FLT3 inhibitors (purple bars with stars) had FLT3 mutations detected previously but not at the time of enrollment on this protocol.
Waterfall plot for best change in marrow blast percentage. Shown are changes in absolute blast percentages in bone marrow aspirates at the time of best response compared with baseline. Note that 14 participants who did not have response assessment marrow biopsy because of circulating blasts are not included in this plot. Participants with no FLT3 mutation who had prior treatment with FLT3 inhibitors (purple bars with stars) had FLT3 mutations detected previously but not at the time of enrollment on this protocol.
In the original plan of the protocol, once FLT3-inhibitory doses were identified, expansion cohorts of participants with FLT3 mutations were to be treated with the identified doses of FF-10101. This did not take place as the sponsor has suspended development at this time. However, a total of 11 participants with a history of FLT3-mutated AML were treated with doses of FF-10101 determined from PIA analysis to inhibit FLT3 in vivo in sustained fashion (150 mg QD, 225 mg QD, 50 mg BID, and 75 mg BID) and were evaluable for response. Amongst these 10 participants, there was 1 CR, 2 CRp, and 1 PR. Of 4 responders, 3 had been previously treated with FLT3 inhibitors.
Discussion
In this first-in-human phase 1 study, the MTD of FF-10101 was determined to be 75 mg BID. This was also the dosing regimen that yielded the most effective and consistent suppression of FLT3 activity in tolerable fashion, and therefore is the RP2D. In preclinical models, FF-10101 overcomes many of the resistance mechanisms that have been observed with other FLT3 inhibitors,19 and from this initial study, the drug does appear to have, in some cases, clinical activity after the failure of approved FLT3 inhibitors. These data were generated before the widespread use of gilteritinib, and so any conclusions about efficacy in the gilteritinib-resistance setting are very preliminary. This will require a larger study formally designed to test this concept, and clinical resistance to FF-10101 itself would be expected to eventually emerge, most likely from mutation of cysteine 695.19 Importantly, we observed responses in participants with AML lacking detectable FLT3-activating mutations, a phenomenon seen with quizartinib but much less so with gilteritinib.14,34 This may reflect both the irreversibility of FF-10101 as well as its activity against the wild-type receptor, which is frequently overexpressed in AML cells and requires FLT3 ligand for activation.35,36
FLT3 inhibitors are now a component of the standard-of-care management of FLT3-mutated AML at all stages of the disease. There are currently 4 FLT3 inhibitors in common clinical use around the world: sorafenib, midostaurin, gilteritinib, and quizartinib; and a fifth, crenolanib, is in late stages of development.37 Because these drugs have been incorporated into conventional treatment regimens, including combinations with induction chemotherapy, hypomethylating agents, venetoclax, and as maintenance, a number of problems have emerged. Sorafenib does not have regulatory approval for AML, and is often poorly tolerated by patients.38,39 It is also inactive against FLT3-TKD mutations, which act as a mechanism of resistance.12 Midostaurin has no single-agent activity,40 and is likewise poorly tolerated in general, with most patients requiring pretreatment with an anti-emetic before every dose, and with a high cessation rate in maintenance.15,41 Gilteritinib and quizartinib are well tolerated on a day-to-day basis, but both have exceptionally long terminal half-lives in patients.42,43 In the setting of R/R FLT3-mutated AML, this long t1/2 was deemed advantageous for single-agent FLT3 inhibitor therapy, because inhibition of FLT3 needs to be continuous over days in order to induce a meaningful antileukemic effect.27 However, when administered in conjunction with myelosuppressive regimens, both gilteritinib and quizartinib are associated with prolongation of aplasia.17,44,45 With its short terminal t1/2, FF-10101 achieves a potent, sustained inhibition of FLT3 that appears very tolerable and that can be terminated abruptly. This would allow greater flexibility in its incorporation into myelosuppressive treatment regimens. The only consistent toxicity we observed was diarrhea, which only was notable at doses above the RP2D. Thus, although in a narrow sense, FF-10101 might have a role in the treatment of FLT3-mutated AML that is resistant to quizartinib or gilteritinib, its properties of being potent but with a short t1/2 potentially make this a more ideal drug to use in combination regimens. However, a direct head-to-head comparison of FF-10101 with either gilteritinib or quizartinib in the R/R setting, in the form of a phase 2 randomized study, could offer more insight into the potential of this compound for the treatment of FLT3-mutated AML.
In summary, FF-10101 is a well-tolerated, oral, irreversible FLT3 inhibitor that can inhibit FLT3 in patients with AML and has activity in patients previously treated with FLT3 inhibitors. This agent potentially represents the next stage in evolution of clinically important FLT3 inhibitors.
Acknowledgments
This work was supported by FUJIFILM Pharmaceuticals, USA, Inc Cambridge, MA, and funded by the Newly extended TEchnology transfer Program (NexTEP), Japan Science and Technology Agency (JST), grant JPMJTT15N2.
Authorship
Contribution: M.L. designed the study, enrolled trial participants, analyzed the data, and wrote the manuscript; A.P., G.S., A.T.F., G.R., E.S.W., J.A., and C.S. enrolled trial participants and edited the manuscript; T.R. performed laboratory experiments; M.A., T.S., R.A.S., G.M., T.M., M.J., and K.C. contributed to study design, analyzed the data, and edited the manuscript; and M.K. was the medical monitor and edited the manuscript.
Conflict-of-interest disclosure: M.L. received consultancy fees from AbbVie, Astellas, Amgen, Bristol Myers Squibb, Daiichi Sankyo, Jazz, Pfizer, and Kite, and received research support from Fujifilm. G.S. received research support from Fujifilm. A.T.F. received research support from Servier, Bristol Myers Squibb, and AbbVie, and consulting fees from Servier, Bristol Myers Squibb, AbbVie, Genentech, Amge, Takeda, Astellas, Mablytics, EnClear, Orum, Forma, Daiichi Sankyo, Pfizer, Rigel, Autolus, Menarini, and Remix. G.R. received research support from Janssen and consultancy fees from AbbVie, Amgen, Argenx, AstraZeneca, bluebird bio, Blueprint Medicines, Bristol Myers Squibb, Caribou Biosciences, Celgene, Daiichi Sankyo, Ellipses Pharma, GlaxoSmithKline, Janssen, Jasper Pharmaceuticals, Jazz Pharmaceuticals, Molecular Partners, Novartis, Pfizer, Roche, Syndax, and Takeda. E.S.W. received consultancy fees from AbbVie, Astellas, Bristol Myers Squibb, Daiichi Sankyo, Genentech, Gilead, GlaxoSmithKline, Janssen, Jazz, Kite, Kura, Novartis, NuProbe, Pfizer, Rigel, Sellas, and Sumitomo Pharma. A.P. received consultancy fees from AbbVie, Astellas, BerGenBio, Daiichi Sankyo, Genentech, ImmunoGen, Foghorn, Actinium, and Forma, and research support from AbbVie, Astellas, Daiichi Sankyo, Fujifilm, and Syndax. J.A. received consultancy fees from AbbVie, Astellas Pharma, BioSight, bluebird bio, Curio, Daiichi Sankyo, Gilead, Kura Oncology, Kymera, Stemline Therapeutics, and Syros, and research funding from AbbVie, Agios, ALX Oncology, Amgen, Amphivena, Aprea AB, Aptose Biosciences, Astellas Pharma, BioSight, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Cyclacel, Fujifilm, ImmunoGen, Kartos Therapeutics, Kura Oncology, Loxo, and Pfizer. M.A., T.S., R.A.S., G.M., T.M., M.J., K.C., and M.K. are employees of Fujifilm, whose product (FF-10101) is described in this work. C.S. received research funding from Fujifilim (clinical trial), Astellas (clinical trial), AbbVie, Bristol Myers Squibb/Celgene (clinical trial), Erasca, Revolution Medicines, and Zentalis (clinical trial), and has served on a board of advisory committee for Astellas Pharma, Daichi Sankyo, and Genentech. T.R. declares no competing financial interests.
Correspondence: Mark Levis, Johns Hopkins University, 1650 Orleans St, Room 2M44, Baltimore, MD 21287; email: levisma@jhmi.edu.
References
Author notes
Access to deidentified participant data is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement (fphucontact@fujifilm.com). Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. The earliest availability of data will be 2 years after first regulatory approval of the initial indication.
The full-text version of this article contains a data supplement.