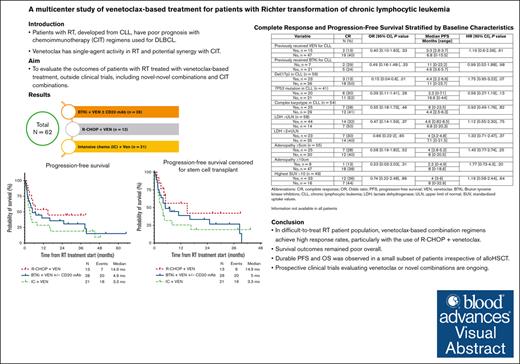
In this retrospective study, venetoclax-based combination regimens led to higher complete remission rates (25%-38%) than historical studies.
The pretreatment characteristic of CLL with del(17p) was associated with a lower complete remission rate than venetoclax-based treatments for RT.
Visual Abstract
Patients with chronic lymphocytic leukemia (CLL) who develop Richter transformation (RT) have a poor prognosis when treated with chemoimmunotherapy regimens used for de novo diffuse large B-cell lymphoma. Venetoclax, a BCL2 inhibitor, has single-agent efficacy in patients with RT and is potentially synergistic with chemoimmunotherapy. In this multicenter, retrospective study, we evaluated 62 patients with RT who received venetoclax-based treatment outside of a clinical trial, in combination with a Bruton tyrosine kinase inhibitor (BTKi; n=28), rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP) (n=13), or intensive chemoimmunotherapy other than R-CHOP (n=21). The best overall and complete response rates were 36%/25%, 54%/46%, and 52%/38%, respectively. The median progression-free and overall survival estimates for the same treatment groups were 4.9/14.3 months, 14.9 months/not reached, and 3.3/9 months, respectively. CLL with del(17p) was associated with a lower complete response rate in the total cohort (odds ratio [OR] 0.15; 95% confidence interval [CI] 0.04-0.6; p=0.01) and venetoclax-naïve subgroup (OR 0.13; 95%CI 0.02-0.66; p=0.01). TP53 mutated CLL was associated with a lower complete response rate (OR 0.15; 95%CI 0.03-0.74; p=0.02) and shorter progression-free survival (hazard ratio 3.1; 95%CI 1.21-7.95; p=0.02) only in venetoclax-naïve subgroup. No other clinical or baseline characteristics, including prior venetoclax treatment for CLL, showed statistically significant association with outcomes. Grade 3-4 neutropenia and thrombocytopenia events were most frequent with intensive chemoimmunotherapy + venetoclax; grade 3-4 infection rates were similar across treatment groups. In this difficult-to-treat RT patient population, venetoclax-based combination regimens achieved high response rates, with durable remission and survival observed in a subset of patients.
Introduction
Richter transformation (RT) is the histologic transformation of chronic lymphocytic leukemia (CLL) into an aggressive lymphoma. Diffuse large B-cell lymphoma (DLBCL) is the most common presentation (90% of cases) with an incidence of 0.5% to 1% per year.1 Unlike de novo DLBCL, this diagnosis carries a poor prognosis, with anthracycline-based chemotherapy regimens delivering a median overall survival (OS) of <12 months and few long-term survivors.2,3 Novel treatment approaches are needed. Venetoclax, an oral B-cell lymphoma 2 protein (BCL2) inhibitor and key agent in the current CLL treatment paradigm, demonstrated single-agent activity in a small cohort (n = 7) of patients with RT, with overall response rate (ORR) of 43%.4 Venetoclax combined with DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) achieved a median OS of 19.6 months.5 The accompanying complete response (CR) rate of 50% is the highest reported in patients with RT and suggests an, at least additive if not synergistic, effect when considering the CR rates of 20% and 0% with EPOCH-R and venetoclax monotherapy, respectively.2,4 The efficacy and tolerability of other venetoclax-containing combinations in the treatment of RT is unknown. In this study, we evaluated the outcomes of patients with RT treated with venetoclax-based regimens, outside clinical trials, including novel-novel combinations and chemotherapy combinations.
Methods
This study was approved by the institutional review boards at each participating institution. We analyzed patients with RT treated with a venetoclax-based regimen at The University of Texas M.D. Anderson Cancer Center (n = 34), the Mayo Clinic (n = 17), The Ohio State University (n = 7), and the Dana-Farber Cancer Institute (n = 4) between March 2012 and March 2021. Patient and disease characteristics from the time of venetoclax-based treatment start were ascertained. CLL characteristics were captured at the time of start of any novel therapy for CLL or the latest time period in case of patients with no prior treatment with novel agents. Chemotherapy regimens considered more intensive than standard R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) in this study included DA-EPOCH-R, R-hyper-CVAD (rituximab, cyclophosphamide, vincristine, doxorubicin, and dexamethasone), high-dose methotrexate and cytarabine, and OFAR (oxaliplatin, fludarabine, cytarabine, and rituximab). Collectively, these regimens are referred to as intensive chemoimmunotherapy + venetoclax in the rest of the manuscript. Most patients treated with intensive chemotherapy or R-CHOP, received venetoclax from cycle 2, with daily ramp up of venetoclax (20, 50, 100, 200, and 400 mg daily), and continued 400 mg daily for 10 days thereafter. There was heterogeneity in the venetoclax ramp-up among patients treated with Bruton tyrosine kinase inhibitor (BTKi) + venetoclax with/without anti-CD-20 antibody, with most patients undergoing accelerated ramp-up aiming to reach a 400 mg daily target dose within 2 weeks. Patients who were on venetoclax before RT did not have dose ramp-up. Retrospective response assessment was as per Lugano 2014 guidelines.6 Toxicity was graded per International Workshop on CLL 2018 guidelines (hematologic toxicity) or Common Terminology Criteria for Adverse Events version 5.0 (nonhematologic toxicity).7 OS was defined as the time from the start of treatment to death, and progression-free survival (PFS) was defined as time between the start of treatment and disease progression or death. Survival outcomes were analyzed using the Kaplan-Meier method, with and without censoring for allogeneic hematopoietic stem cell transplantation (allo-HSCT). Median follow-up was calculated using the reverse Kaplan-Meier method.8 No formal statistical comparisons were made between the different treatment groups given the nonrandomized nature and potential selection biases in treatment allocation. Associations between dichotomized pretreatment patient and disease characteristics and response rates were evaluated using the χ2 method; associations between dichotomized pretreatment characteristics and time-to-event outcomes were evaluated using the log rank test.
Results
In total, 62 patients were identified with a median age of 67 years (range, 43-83 years). High-risk CLL disease characteristics were frequently identified: 52 of 59 (88%) patients with available data had unmutated IGHV; 31 of 59 (53%) had del(17p); 16 of 37 (43%) had TP53 mutation, and 25 of 54 (46%) had complex karyotype (defined as ≥3 abnormalities on CpG-stimulated karyotype). Twelve patients with available data (12 of 14, 86%) had clonally related RT. Among all patients, the median number of prior CLL-directed therapies was 2 (range, 0-7), including prior chemoimmunotherapy (55%), prior BTKi (68%), and prior venetoclax (24%). Of note, in the BTKi + venetoclax group, reason for prior BTKi discontinuation was progressive CLL in 9 patients, RT in 11, and adverse effects in 1 patient. In the overall cohort, 18% of patients had received no prior treatment for CLL and 56% of patients had received no prior RT treatment; 11% of patients were previously untreated for both CLL and RT. Venetoclax-based RT treatment subgroups consisted of BTKi + venetoclax with/without anti-CD20 antibody (n = 28), R-CHOP + venetoclax (n = 13); and intensive chemoimmunotherapy + venetoclax (n = 21); baseline characteristics for each treatment group are shown in Table 1. Median follow-up from the start of venetoclax-based RT treatment was 34 months (95% confidence interval [CI], 27-50).
The best ORR in all patients was 44%, and CR rate was 34%. Based on the type of treatments received, the ORR/CR rates were 36%/25% with BTKi + venetoclax with/without anti-CD20 antibody, 54%/46% with R-CHOP + venetoclax, and 52%/38% with intensive chemoimmunotherapy + venetoclax. Among patients with available results, the undetectable measurable residual disease rates for coexisting CLL (assessed by flow cytometry at some point during treatment, with a sensitivity of 0.01%, in the blood or bone marrow) were 45% for BTKi + venetoclax with/without anti-CD20 antibody, 55% for R-CHOP + venetoclax, and 67% for intensive chemoimmunotherapy + venetoclax. Considering baseline clinical findings (eg, bulky adenopathy and lactate dehydrogenase elevation) and CLL molecular features (eg, complex karyotype and TP53 aberrations), only the presence of del(17p) in CLL was associated with a lower odds ratio (OR) of 0.15 (95% CI, 0.04-0.60; P = .010) of achieving a CR (Table 2). Only 2 of 15 (13%) CRs were observed in patients who previously received venetoclax for CLL compared with 19 of 47 (40%) in patients who were venetoclax naïve, although this was not statistically significant because of the small sample size (OR, 0.40; 95% CI, 0.10-1.63; P = .33). Among patients who were venetoclax naïve, CR rate was significantly lower in patients with del(17p) (OR, 0.13; 95% CI, 0.02-0.66; P = .01) and TP53-mutated CLL (OR, 0.15; 95% CI, 0.03-0.74; P = .02; supplemental Table 1). Interestingly, similar CR rates were seen among patients treated with BTKi + venetoclax with/without anti-CD20 antibody for RT regardless of prior BTKi exposure for CLL treatment (29% with no prior BTKi vs 24% with prior BTKi; OR, 0.78; 95% CI, 0.11-5.34; P = .58). Of note, 14 patients (14 of 34) treated with a chemotherapy-based regimen (R-CHOP or intensive chemotherapy) achieved a CR. Of them, 9 patients received venetoclax maintenance after the completion of chemotherapy. Five patients did not receive venetoclax maintenance because of disease progression (n = 3) and subsequent allo-HSCT (n = 2). No formal comparison was performed between the 2 groups because of low patient numbers.
A total of 10 patients (16%) proceeded to allo-HSCT after venetoclax-based treatments. Of these patients, best response to venetoclax-based treatment was CR (6 of 10), partial response (n = 1), progressive disease (n = 1), and missing response evaluation (n = 2). The median time from commencing venetoclax to allo-HSCT in patients who had CR or partial response to venetoclax-based treatments was 5 months (range, 4-19 months).
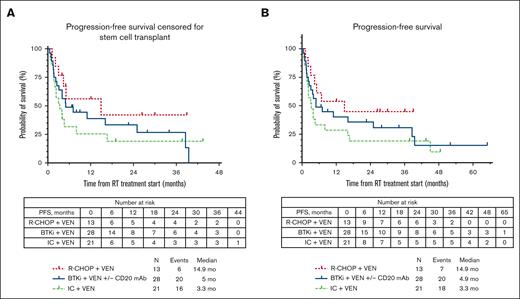
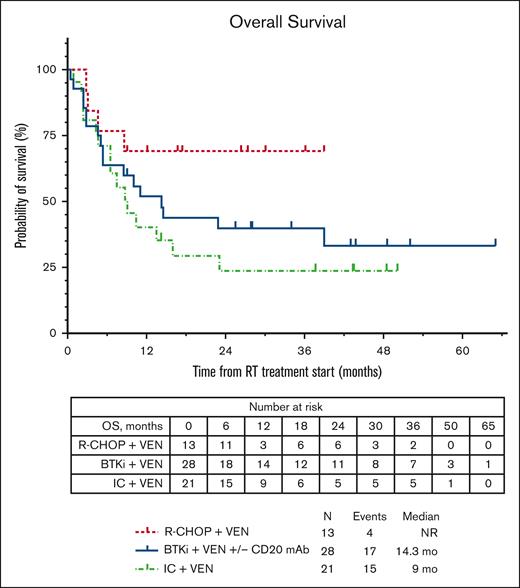
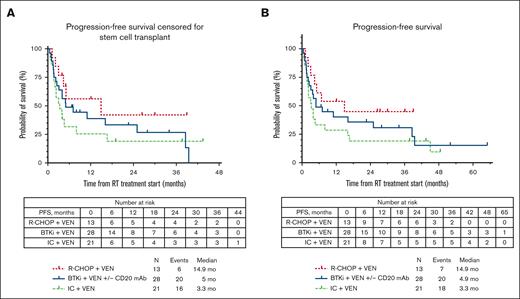
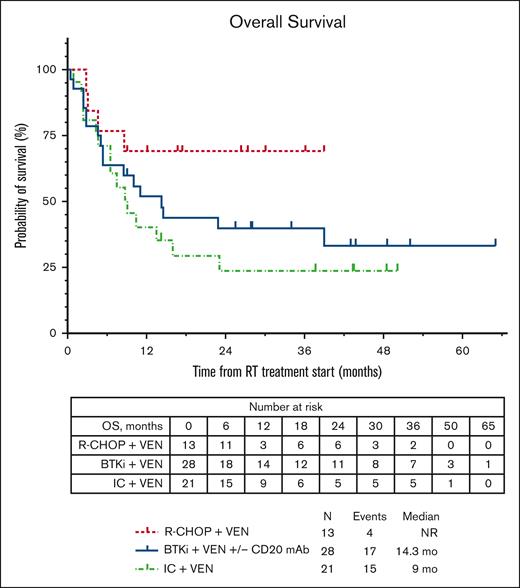
The median PFS for the total cohort was similar whether censored at allo-HSCT or without censoring at allo-HSCT (4.9 months; supplemental Figure 1). The median PFS estimates according to therapy received (Figure 1A) were 5 months with BTKi + venetoclax + anti-CD20 antibody, 14.9 months with R-CHOP + venetoclax, and 3.3 months with intensive chemoimmunotherapy + venetoclax. No significant differences were seen in median PFS estimates of individual treatment groups whether censoring was performed at allo-HSCT or not (Figure 1B). We performed a landmark analysis (at 18 weeks from the start of chemotherapy-based regimen time point) in responders, which showed a numerically longer median PFS in patients who had subsequent allo-HSCT (42.1 vs 36.4 months, P = .290; supplemental Figure 2). Similarly, numerically shorter PFS estimates were seen with a number of clinical and baseline CLL characteristics, such as prior receipt of venetoclax or BTKi, and del(17p) and TP53 mutation; however, these differences were not statistically significant (Table 2). Among patients who were venetoclax naïve, presence of TP53 mutation in CLL cells was associated with significantly shorter median PFS (hazard ratio, 3.1; 95% CI, 1.21-7.95; P = .02; supplemental Table 1; supplemental Figure 3). The median OS for the total cohort was 13.5 months (supplemental Figure 1). The estimated median OS according to therapy received (Figure 2) was 14.3 months with BTKi + venetoclax with/without anti-CD20 antibody, not reached with R-CHOP + venetoclax, and 9 months with intensive chemoimmunotherapy + venetoclax. At last follow-up, 26 patients (42%) remained alive. Among the 36 patients who died, the most common cause of death was disease progression (n = 26). Five patients died from infection/sepsis: 3 in the BTKi + venetoclax with/without anti-CD20 antibody group, and 2 in the intensive chemoimmunotherapy + venetoclax group. Among the 5 patients with an infectious cause of death, 2 were receiving the venetoclax-based treatment (both BTKi + venetoclax with/without anti-CD20 antibody) at time of the infection: 1 patient died in CR, and 1 patient died before initial response assessment. One patient treated with R-CHOP + venetoclax died from a subdural hematoma. One patient who received intensive chemoimmunotherapy + venetoclax died from subsequent chimeric antigen receptor T-cell therapy–associated immune effector cell–associated neurotoxicity syndrome. Three patients with no known evidence of disease progression died while on a subsequent line of therapy (1 patient on an unclear clinical trial, 1 who received polatuzumab + rituximab + venetoclax, and 1 who transitioned care to local oncology) without a known cause of death (1 each: lost to follow-up, transitioned care to local physician, and had sudden death).
PFS estimates according to therapy received for RT. (A) PFS estimates according to therapy received for RT, censored for allo-HSCT; (B) PFS estimates according to therapy received for RT, uncensored for allo-HSCT.
PFS estimates according to therapy received for RT. (A) PFS estimates according to therapy received for RT, censored for allo-HSCT; (B) PFS estimates according to therapy received for RT, uncensored for allo-HSCT.
Grade 3/4 neutropenia and thrombocytopenia (89% and 95%, respectively) were more common with intensive chemoimmunotherapy + venetoclax (Table 3) and R-CHOP + venetoclax (78% and 56%, respectively) than BTKi + venetoclax with/without anti-CD20 antibody (46% and 39%, respectively). Febrile neutropenia was most frequently observed in patients receiving intensive chemoimmunotherapy + venetoclax (48%) and was similar between those treated with R-CHOP + venetoclax (23%) and BTKi + venetoclax with/without anti-CD20 antibody (29%). The rates of grade 3/4 infection were similar across these 3 treatment groups: 43%, 31%, and 36%, respectively. Patients receiving R-CHOP + venetoclax were more likely to be able to complete 6 cycles of combination therapy compared with those receiving intensive chemoimmunotherapy + venetoclax (OR, 0.07; 95% CI, 0.01-0.88; P = .02 supplemental Table 2). The total duration of venetoclax in patients responding in each combination subgroup is shown in supplemental Table 3.
Discussion
Low CR rates and short survival with standard DLBCL treatment regimens demand investigation of novel approaches and a separate treatment paradigm for RT.9 This multicenter, retrospective study provides, to our knowledge, the largest assessment to date of the efficacy and tolerability of venetoclax-based treatment in patients with RT. Here, in a contemporary cohort with many patients exposed to novel agent, we observed CR in 1 of 4 patients treated without cytotoxic chemotherapy (BTKi + venetoclax with/without anti-CD20 antibody) and nearly half of patients receiving R-CHOP + venetoclax. The median PFS appeared poor overall, at <6 months; however, estimates ranged up to 15 months in the R-CHOP + venetoclax subgroup, and some long-term survivors, even without allogeneic stem cell transplant consolidation, were observed across all treatments. Although a subset of patients experienced durable remissions with these venetoclax-based approaches, subsequent allo-HSCT should be considered in eligible patients, until sufficient experience is accrued with larger cohorts of patients treated with venetoclax who did not undergo transplantation demonstrating a cure fraction approaching that which would be expected after allo-SCT.
Limited prospective data for R-CHOP in patients with RT come from a phase 2 trial conducted in the 2000s by the German CLL Study group including 15 patients with RT. The ORR was 67% with 1 (7%) CR (by 1999, computed tomography-based response assessment) and median PFS and OS were 10 and 21 months, respectively.10,11 In this study, the highest CR rate and longest survival estimates were in the 13-patient subgroup treated with R-CHOP + venetoclax. However, among this study’s overall cohort, the R-CHOP + venetoclax subgroup had a lower rate of TP53 aberrations and a higher percentage of previously untreated patients, both of which have been previously associated with improved outcomes.12,13
Intensification of chemotherapy to address inadequate results with R-CHOP has had limited success. Historically, intensive anthracycline-containing (eg, hyper-CVAD and DA-EPOCH-R) and platinum-containing (eg, OFAR) regimens have mostly achieved low CR rates (≤20%) and short median OS (<12 months).2,14-16 A phase 2 study of DA-EPOCH-R + venetoclax including 26 patients with RT appeared to improve upon these outcomes with a 50% CR rate and a 19.6-month median OS5 but at a cost of a high incidence of grade 3/4 hematologic toxicity and risk of infection. Similarly, intensive chemoimmunotherapy + venetoclax achieved responses in over half of patients and a 38% CR rate in this study. Yet, the survival outcomes for this subgroup did not replicate the DA-EPOCH-R + venetoclax trial outcomes but rather remain in line with prior intensive chemoimmunotherapy data. We acknowledge that direct comparison to the phase 2 study of DA-EPOCH-R + venetoclax is problematic because (1) this regimen was grouped with other intensive chemoimmunotherapy regimens in this study, and (2) the current cohort was more heavily pretreated both in regard to prior CLL treatment (2 vs 1 median prior lines; more novel-agent exposed) and more patients had had prior RT-directed treatment (56% vs 93% with no prior RT treatment).
Venetoclax has been studied as an adjunct to chemotherapy regimens in DLBCL and double-hit lymphoma (high-grade B-cell lymphoma with rearrangements of MYC, BCL2, and/or BCL6). DA-EPOCH-R + venetoclax showed promising results (97% ORR, 93% CR rate, 83% 2-year PFS) in a phase 1 study with a high-risk cohort including 50% patients with double-hit lymphoma.17 However, the subsequent Alliance A051701 study, which randomized patients to DA-EPOCH-R with/without venetoclax, demonstrated excess treatment-related mortality in the venetoclax arm, which led to early discontinuation of the study.18 R-CHOP + venetoclax, evaluated in the phase 1b/2 CAVALLI study, appeared to strike a better balance between aggressiveness of therapy and acceptable toxicity, particularly in patients with BCL2 overexpression by immunohistochemistry.19 When evaluating the potential risk-to-benefit ratio of adding venetoclax to chemoimmunotherapy, one must consider that the outcomes of chemoimmunotherapy in RT are far inferior to those of chemoimmunotherapy in double-hit lymphoma, so the benefit of improving remission rates is more likely to outweigh the risks of increased infection-related morbidity and mortality in RT than in double-hit lymphoma. Infection-related mortality present in this retrospective series warrants consideration (5 deaths total, 2 during therapy) and likely reflects both the challenging nature of this patient population and cumulative therapy-related myelosuppression. Thus, although venetoclax appears to have activity in combination with other agents in RT, new approaches are needed to improve tolerability in patients with RT, who are often older, frail, and heavily pretreated. In this study, with the caveats that this was a nonrandomized comparison and numbers were small, R-CHOP + venetoclax and BTKi + venetoclax with/without anti-CD20 antibody appeared more deliverable than intensive chemoimmunotherapy + venetoclax, with lower rates of febrile neutropenia than intensive chemoimmunotherapy + venetoclax, which recapitulates data from de novo DLBCL. Considering delivery of chemotherapy cycles in responding patients only, proportionally more patients treated with R-CHOP + venetoclax were able to receive 6 cycles of chemotherapy than those treated with intensive chemoimmunotherapy + venetoclax (6 of 7 vs 3 of 10). Additionally, there were no infection-related deaths in the 13 patients treated with R-CHOP + venetoclax. A prospective, multicenter study evaluating R-CHOP + venetoclax (ClinicalTrials.gov identifier: NCT03054896) is ongoing, with initial results also suggesting less toxicity compared with intensive chemotherapy + venetoclax, and similar efficacy.20 In a patient fit for a chemotherapy + venetoclax approach, R-CHOP + venetoclax is likely preferred over intensive chemotherapy + venetoclax; however, greater clarity around this will be provided by the full results from the trial by Davids et al.
BTKis also have activity in RT with responses demonstrated but PFS still being short. Acalabrutinib, a covalent BTKi, achieved a 40% ORR (8% CR) and median PFS of 3.2 months in the RT cohort (n = 25) of the phase 1/2 ACE-CL-001 study.21 Patients with RT (n = 50) receiving the noncovalent BTKi, pirtobrutinib, on the phase 1/2 BRUIN study had a 54% ORR (10% CR), but median PFS was 3.7 months.22 Extensive study of venetoclax + BTKi in patients with CLL support the safe combination of these agents. Our results, including a CR rate of 24% even in the setting of prior BTKi for CLL, support further evaluation of venetoclax + BTKi combinations for treatment of RT. This approach may have added appeal for patients with comorbidities or frailty precluding chemoimmunotherapy-based approaches, or for patients already treated with prior chemoimmunotherapy for either CLL or RT. We await data from prospective trials (ClinicalTrials.gov identifiers: NCT05388006 [acalabrutinib + venetoclax + durvalumab]; and NCT05536349 [pirtobrutinib + venetoclax + obinutuzumab]) to see whether nonchemotherapy-based approaches yield high response rates in patients with RT, especially those with TP53 abnormalities who had inferior outcomes in our study. The tolerability of these regimens relative to chemotherapy + venetoclax regimens will also be important to evaluate.
Nonuniform prognostic and follow-up data are limitations inherent to the retrospective nature of this study. The heterogenous patient populations among the treatment subgroups precludes a fair direct comparison. Nevertheless, the findings from the overall cohort and standalone subgroup analyses provide important insights into the evolving landscape of RT management, emphasizing incorporation of a novel agent. The majority of our cohort had received CLL-directed treatment but had not received venetoclax for CLL. Because venetoclax is now a standard-of-care option for patients with CLL in either the first-line or relapsed setting, this is a limitation of the study. Certainly, from the limited data available in our study, patients with prior venetoclax exposure for CLL had lower likelihood of achieving CR than those who were venetoclax naïve (13% vs 40%), suggesting that alternative treatment approaches may be preferred in such patients. However, given the small numbers in our study, further data are needed to confirm this.
In summary, in a difficult-to-treat RT population, venetoclax-based combination regimens achieved higher CR rates than historical studies using chemotherapy, including a nearly 50% CR rate in the subgroup treated with R-CHOP + venetoclax, but at the cost of considerable myelosuppression and high rates of infection. Survival outcomes remained poor overall; however, durable PFS and OS was observed in a small subset of patients irrespective of allo-HSCT. Prospective studies evaluating venetoclax-based chemotherapy or novel agent combinations actively accruing and in development are expected to further inform the RT treatment paradigm.
Acknowledgments
K.A.R. is a scholar in clinical research of the Leukemia & Lymphoma Society (CDP 2331-20).
This work was supported, in part, by The University of Texas MD Anderson Cancer Center Support Grant CA016672.
Authorship
Contribution: All authors contributed to manuscript review and revision and approved the final version; P.A.T. designed the study; P.J.H., M.S., S.A.P., and P.A.T. acquired data, analyzed the data, interpreted the results, and wrote the report; K.A.R., E.M.P., and J.M.H. acquired data, cared for patients, and interpreted the results; and J.A.B., M.S.D., W.D., A.F., N.J., S.S.K., Y.W., W.G.W., and J.A.W. cared for patients and interpreted the results.
Conflict-of-interest disclosure: K.A.R. received institutional research funding from Genentech, AbbVie, and Novartis; and reports consulting for Genentech, AbbVie, Pharmacyclics, AstraZeneca, Beigene, LOXO@Lilly, and Janssen. M.S.D. serves in an adviser, a consultant, and a speaker role for AbbVie, Adaptive Biotechnologies, Ascentage Pharma, AstraZeneca, BeiGene, Bristol Myers Squibb, Lilly, Genentech, Genmab, Janssen, Merck, MingSight, Ono Pharmaceuticals, Secura Bio, Syros Pharmaceuticals, Takeda, and TG Therapeutics; reports participation in educational programs with Aptitude Health, AXIS Medical Education, Bio Ascend, Curio Science, and Medscape; reports participation in education at PeerView Institute for Medical Education, Physcians’ Education Resource, PlatformQ Health Education, Plexus Communications, and Research to Practice; reports a researcher role with AbbVie, Ascentage Pharma, AstraZeneca, Genentech, Novartis, Secura Bio, and TG Therapeutics; and received royalties from Up-to-Date. W.D. received institutional research funding from Merck, BeiGene, AstraZeneca, AbbVie, DTRM, and Octapharma; received institutional honoraria from Merck, BeiGene, MEI Pharma, Alexion, and Octapharma; and consulted for Merck, BeiGene, MEI Pharma, Alexion, and Octapharma. A.F. received institutional research funding. N.J. received research funding from Pharmacyclics, AbbVie, Genentech, AstraZeneca, Bristol Myers Squibb, Pfizer, ADC Therapeutics, Incyte, Servier, Cellectis, Adaptive Biotechnologies, Precision Biosciences, Aprea Therapeutics, and Fate Therapeutics; served as an adviser for, and received honoraria from, Janssen, Pharmacyclics, AbbVie, Genentech, AstraZeneca, ADC Therapeutics, BeiGene, TG Therapeutics, Servier, Adaptive Biotechnologies, and Precision Biosciences. S.S.K. received institutional research funding from Kite/Gilead, Humanigen, Lentigen, Tolero/Sumtomo, MorphoSys, and LEAHLabs; received institutional honoraria from Kite/Gilead; consulted for, and reports membership on advisory committees or speakers bureau of, Novartis, Kite/Gilead, Humanigen, LEAHLabs, CapstanBio, Torque, and Luminary Therapeutics; holds patents in, and receives royalties from, MustangBio, Sendero, and Mettaforge; and is a current equity holder in private company LEAHLabs. Y.W. received institutional research funding from Incyte, InnoCare, Loxo Oncology, Eli Lilly, MorphoSys, Novartis, Genentech, and Genmab; serves on the advisory board (compensation to institution) of Eli Lilly, LOXO Oncology, TG Therapeutics, Incyte, InnoCare, Kite, Jansen, BeiGene, and AstraZeneca; consulted for (compensation to institution) Innocare and AbbVie; received institutional honorarium from Kite. W.G.W. received research funding from AbbVie Inc, Acerta Pharma, Bristol Myers Squibb, Cyclacel Pharmaceuticals, Genentech (a member of the Roche Group), Gilead Sciences Inc, Janssen Biotech Inc, Juno Therapeutics, Kite Pharma, Loxo Oncology Inc, Oncternal Therapeutics, Pharmacyclics LLC, Sunesis Pharmaceuticals Inc, and Xencor. J.A.W. serves as an adviser to AbbVie, Arqule, AstraZeneca, BeiGene, Janssen, Loxo, Newave, Pharmacyclics; serves as a consultant for AbbVie, Arqule, AstraZeneca, BeiGene, Genentech, Janssen, Loxo, Newave, Pharmacyclics; is a researcher for Karyopharm, MingSight Pharmaceuticals MorphoSys, Schrodinger, and Verastem; and serves as a speaker for AbbVie, Arqule, AstraZeneca, BeiGene, Janssen, Loxo, Newave, and Pharmacyclics. S.A.P. received institutional research funding from Janssen, AstraZeneca, Merck, and Genentech; received institutional honoraria from Pharmacyclics, Merck, AstraZeneca, Janssen, BeiGene, Genentech, Amgen, MingSight Pharmaceuticals, TG Therapeutics, Novalgen Limited, Kite Pharma, and AbbVie. P.A.T. received institutional research funding from AbbVie, Adaptive Biotechnologies, Genentech, Lilly, and Pharmacyclics; and consulted for, and received honoraria from, AbbVie, Adaptive Biotechnologies, Ascentage, AstraZeneca, BeiGene, Genentech, Genmab, Janssen, Lilly, Merck, and Pharmacyclics. The remaining authors declare no competing financial interests.
Correspondence: Mahesh Swaminathan, Department of Leukemia, The University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; email: mswaminathan@mdanderson.org.
References
Author notes
P.J.H. and M.S. contributed equally to this work.
The participants of this study did not give written consent for their data to be shared publicly, so because of the sensitive nature of the research, supporting data are not available.
The full-text version of this article contains a data supplement.