Hispanic patients with RRMM had inferior responses to ide-cel, but racial and ethnic differences in survival were not observed.
Although safety and responses differed based on race and ethnicity, our findings encourage the use of ide-cel for all patients with RRMM.
Visual Abstract
Idecabtagene vicleucel (ide-cel) was the first chimeric antigen receptor T-cell therapy to gain US Food and Drug Administration approval for patients with relapsed/refractory multiple myeloma (RRMM). The clinical outcomes of standard of care (SOC) ide-cel in racially and ethnically diverse populations have been understudied. This study pooled data from 207 patients with RRMM (28% patients of racial and ethnic minority groups) treated with SOC ide-cel across 11 institutions to examine racial and ethnic differences in the incidence of toxicities and adverse events, response to ide-cel, and survival. This study included 22 (11%) Hispanic, 36 (17%) non-Hispanic Black, and 149 (72%) non-Hispanic White patients with RRMM. Compared with Hispanic and non-Hispanic White patients, non-Hispanic Black patients had higher median levels of C-reactive protein (1.0, 0.8, and 3.5 mg/dL, respectively; P = .02) and baseline ferritin (362.0 vs 307.0 vs 680.5, respectively; P = .08) and were more likely to develop cytokine release syndrome (77%, 85%, and 97%, respectively; P = .04). Although best overall response rate was lower among Hispanic patients (59%) than among non-Hispanic Black (86%) and White patients (86%; P = .01), there were no racial and ethnic differences in progression-free or overall survival. We provide, to our knowledge, the first and largest investigation of clinical outcomes of SOC ide-cel by race and ethnicity. Despite differences in safety and response to ide-cel, our findings encourage the use of ide-cel in all patients with RRMM. These findings should be confirmed in larger samples of diverse patients with RRMM, with longer follow-up time.
Introduction
Multiple myeloma (MM) is a clonal malignancy of terminally differentiated plasma cells and the second most common hematologic malignancy in the United States, with >35 000 new diagnoses expected in 2023 alone.1 In the past 20 years, there have been significant advances in the MM treatment paradigm, partly because of an accelerated growth of knowledge about the genomic and molecular characterization of the disease. These advances continue to drive the development of novel therapies, resulting in deeper treatment responses and improved survival. However, the current 5-year survival rate is only 56%, and MM remains incurable, with almost all patients eventually developing relapsed or refractory MM (RRMM).2-4
Recently, chimeric antigen receptor (CAR) T-cell therapy has emerged as a revolutionary cellular immunotherapy for patients with RRMM. In March 2021, idecabtagene vicleucel (ide-cel) became the first B-cell maturation antigen–targeting CAR T-cell therapy to gain US Food and Drug Administration (FDA) approval for patients with RRMM who have received at least 4 prior lines of therapy, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody.5 FDA approval was based on the phase 2 KarMMa trial, which showed an overall response rate (ORR) of 73% and a complete response (CR) or better among 33% of patients.6 More recently, ide-cel has been shown to significantly outperform alternative regimens in a randomized setting in the KarMMa-3 trial7 and has demonstrated durable remission and survival benefits with up to 2-year follow-up,8 a marked improvement in clinical outcomes compared with prior RRMM therapies.9 However, treatment-related toxicity, including cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS), remain a primary concern. Because of these safety concerns, patients with RRMM are closely monitored after ide-cel infusion so that toxicities can be detected and treated promptly.
Racial and ethnic disparities are prevalent in MM. Non-Hispanic Black individuals are more than twice as likely to be diagnosed with MM compared with non-Hispanic White individuals.1,10 Non-Hispanic Black and Hispanic patients with MM have a longer time from diagnosis to initiation of treatment, are underrepresented in clinical trials, and are less likely to receive novel therapies relative to non-Hispanic White patients.11-14 Access to novel therapies, including CAR T-cell therapy, is also reduced for patients of racial and ethnic minority groups and those of a lower socioeconomic status.15,16 Moreover, non-Hispanic Black patients have not experienced the same rate of improvement in survival outcomes over the last 2 decades as non-Hispanic White patients.17 The drivers of these racial and ethnic disparities are likely complex and multifactorial but remain unclear. Nonetheless, studies11,13,14,18 show that with equal access to care and receipt of novel therapies, patients of racial and ethnic minority groups may have similar or better overall survival (OS) than non-Hispanic White patients.
Ide-cel is now commercially available to patients with RRMM as standard of care (SOC); however, there remains a critical gap in knowledge pertaining to the safety and efficacy of ide-cel among patients of racial and ethnic minority groups with RRMM. Despite the known racial and ethnic disparities in MM, the seminal KarMMa trial publication did not report on the racial and ethnic makeup of the cohort.6 In the recent KarMMa-3 trial,7 only 7% of the patient population treated with ide-cel self-reported a Black race, and Hispanic ethnicity was not reported. Likewise, few studies of CAR T-cell therapy in RRMM or other cancer types have investigated racial and ethnic differences in adverse events and outcomes in the SOC setting.15,16,19-21 To address this limitation, the goal of this study was to evaluate the safety and efficacy of ide-cel by race and ethnicity among patients with RRMM treated with SOC CAR T-cell therapy. To the best of our knowledge, this is the first investigation of potential racial and ethnic differences among recipients of SOC ide-cel CAR T-cell therapy for RRMM.
Methods
Study population
Data from patients with RRMM who underwent leukapheresis for planned SOC ide-cel by 1 May 2022 were pooled across 11 institutions as part of the US Multiple Myeloma Immunotherapy Consortium.22 This work was approved by the relevant institutional review boards or ethics committees at each institution, and either a waiver of informed consent or informed consent was obtained from participants, depending on institutional guidelines.
Statistical analyses
χ2 and Kruskal-Wallis rank-sum tests were used to investigate racial and ethnic differences in patient and clinical characteristics (ie, time of treatment, age at infusion, Eastern Cooperative Oncology Group performance status at lymphodepletion chemotherapy, and presence of extramedullary disease) at baseline, standard inflammatory laboratory values (ie, C-reactive protein [CRP] and ferritin) at baseline, incidence of any grade and severe immune-mediated toxicities (ie, CRS and ICANS) after ide-cel infusion, use of supportive treatments (ie, steroids, tocilizumab, and anakinra) to manage immune-mediated toxicities, incidence of adverse events (ie, cytopenias and infections) after ide-cel infusion, and response to ide-cel (ie, at day 30, day 90, and best response). Ide-cel response was assessed based on the International Myeloma Working Group criteria.23 Patients who died or progressed by the response time point of interest (eg, day 30 or day 90) were included in the response assessment as nonresponders (progressive disease). Patients who were alive and had not yet reached the response time point of interest or had missing responses were considered missing in the response assessment.
Multivariable logistic regression models were used to estimate odds ratios, and 95% confidence intervals (CIs) for the association of race and ethnicity with both best ORR and best CR or better while adjusting for a priori confounders identified by Hansen et al22 as potentially associated with clinical outcomes among patients with RRMM treated with SOC ide-cel (prior B-cell maturation antigen therapy, high-risk cytogenetics, extramedullary disease, Eastern Cooperative Oncology Group performance status at lymphodepletion chemotherapy, penta-refractory status, age at infusion, and number of prior lines of therapy). We also assessed baseline characteristics that differed according to race and ethnicity at P < .1 as potential confounders in the multivariable models. Each baseline characteristic was included in the model, 1 at a time, and if the effect estimate for the association of race and ethnicity with the outcome of interest changed by ≥10%, we included that characteristic as a covariate in the model.
OS was calculated as the time from infusion to death or last contact, and progression-free survival (PFS) was calculated as the time from infusion to disease progression, death, or last contact. Kaplan-Meier survival curves and log-rank tests were used to examine OS and PFS as per race and ethnicity. Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and 95% CIs for the association of race and ethnicity with OS and PFS while adjusting for baseline characteristics using the approach described earlier. The proportional hazards assumption was tested using interaction terms between each covariate and time, individually and collectively, and using Schoenfeld residuals. For OS, high-risk cytogenetics violated the proportional hazards assumption and was included as a strata term for all OS models. No violation of the proportional hazards assumption was observed for the PFS models. A P < .05 was considered statistically significant, but we also discuss a P < .1 cautiously because of the sample size and novelty of these data.
Results
At the time of data cut-off (1 May 2022), 235 patients with RRMM had undergone leukapheresis for planned SOC ide-cel (supplemental Figure 1). Twenty patients did not undergo infusion because of either manufacturing failures (n = 5) or death between apheresis and planned infusion (n = 15). There were no differences in the distribution of race and ethnicity by infusion status (P = .4). Of the 215 patients with RRMM who received ide-cel infusion, we excluded 8 patients who self-identified as Asian, Pacific Islander, American Indian, or Alaskan Native because of limitations of sample size and power to investigate racial and ethnic-specific effects within these groups. Of the 207 remaining patients with RRMM for downstream analyses, 72% (n = 149) self-identified as non-Hispanic White, 17% (n = 36) as non-Hispanic Black, and 11% (n = 22) as Hispanic.
The distribution of baseline patient characteristics based on race and ethnicity is provided in Table 1. Hispanic patients were younger at infusion than non-Hispanic Black and non-Hispanic White patients (57.0 vs 64.5 vs 65.0 years; P = .07). Compared with Hispanic and non-Hispanic White patients, non-Hispanic Black patients were less likely to be male (73% vs 61% vs 42%, respectively; P = .04) and had higher median levels of baseline ferritin (362.0 vs 307.0 vs 680.5, respectively; P = .08) and CRP (1.0 vs 0.8 vs 3.5, respectively; P = .02) but lower median levels of albumin before infusion (3.8 vs 3.7 vs 3.5, respectively; P = .04). No other differences in baseline patient characteristics were noted based on race and ethnicity.
There were differences in the development of toxicities and adverse events after ide-cel infusion based on race and ethnicity (Table 2). Non-Hispanic Black patients were more likely to develop any grade CRS compared with Hispanic and non-Hispanic White patients (97% vs 77% vs 85%, respectively; P = .04). There were no differences based on race and ethnicity with regard to the incidence of severe CRS (grade ≥3), incidence of any grade or severe ICANS (grade ≥3), or use of steroids or tocilizumab. However, the prevalence of anakinra use was higher among Hispanic patients than among non-Hispanic Black and non-Hispanic White patients (14% vs 0% vs 5%; P = .06). Non-Hispanic Black patients also had a longer median hospital stay than non-Hispanic White and Hispanic patients (13.5 vs 9.0 vs 8.0 days, respectively; P = .006), but no differences in intensive care unit admission were observed based on race and ethnicity.
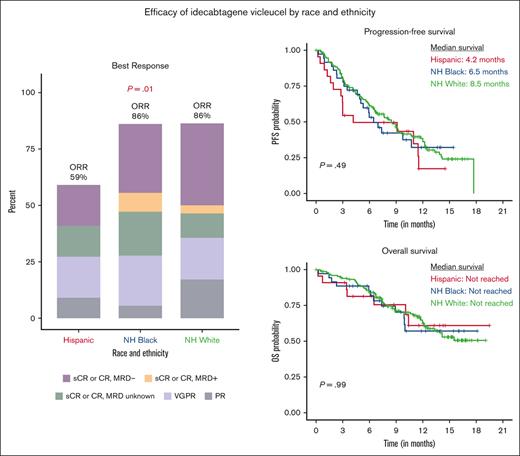
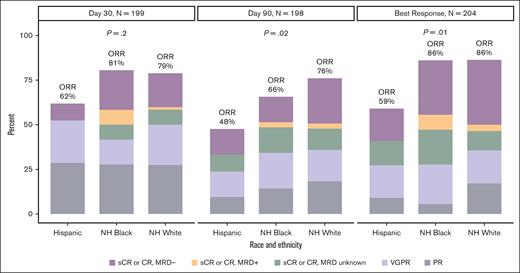
As shown in Figure 1 and supplemental Table 1, the best ORR was lower among Hispanic patients (59%) than among non-Hispanic Black (86%) and non-Hispanic White patients (86%, P = .01). This association remained consistent in multivariable analyses (supplemental Table 2), with Hispanic patients more likely to have an inferior response (best ORR of stable disease or progressive disease) than non-Hispanic White patients (odds ratio, 7.12; 95% CI, 1.97-26.90; P = .003). The prevalence of a best response of CR or better was slightly higher among non-Hispanic Black (58%) and non-Hispanic White (51%) patients than among Hispanic patients (32%, P = .1). In multivariable analyses, race and ethnicity was not associated with achieving a best response of CR or better (supplemental Table 2).
Ide-cel response on day 30 and day 90 and best overall response based on race and ethnicity. MRD, minimal residual disease; NH, non-Hispanic; PR, partial response; sCR, stringent complete response; VGPR, very good partial response.
Ide-cel response on day 30 and day 90 and best overall response based on race and ethnicity. MRD, minimal residual disease; NH, non-Hispanic; PR, partial response; sCR, stringent complete response; VGPR, very good partial response.
Median follow-up of the study population is 9.3 months. There were no statistically significant differences in PFS or OS based on race and ethnicity (P = .49 and P = .99, respectively; Figure 2). The median PFS was 4.2 months for Hispanic patients, 6.5 months for non-Hispanic Black patients, and 8.5 months for non-Hispanic White patients; and the median OS across all racial and ethnic groups was not reached. After adjusting for clinically relevant covariates, race and ethnicity was not associated with PFS (Hispanic vs non-Hispanic White patients: HR, 1.10; 95% CI, 0.52-2.30; and non-Hispanic Black vs non-Hispanic White patients: HR, 1.22; 95% CI, 0.72-2.08) or OS (Hispanic vs non-Hispanic White patients: HR, 1.39; 95% CI, 0.54-3.63; and non-Hispanic Black vs non-Hispanic White patients: HR, 1.13; 95% CI, 0.54-2.38; Table 3).
Progression-free and overall survival by race and ethnicity. Kaplan-Meier curve of (A) PFS and (B) OS based on race and ethnicity. One participant had missing date of death and was excluded from analysis of OS.
Progression-free and overall survival by race and ethnicity. Kaplan-Meier curve of (A) PFS and (B) OS based on race and ethnicity. One participant had missing date of death and was excluded from analysis of OS.
Discussion
To our knowledge, this is the first study to investigate potential racial and ethnic differences in the safety and efficacy of CAR T-cell therapy among patients with RRMM treated with ide-cel in the SOC setting. This is notable because many clinical trials lack diversity because of many reasons, including but not limited to trial availability, lack of education, financial considerations, medical mistrust, and cultural insensitivity.24 Moreover, strict eligibility criteria for clinical trials often inadvertently exclude patients of racial and ethnic minority groups.11 In fact, 75% of our patient population would not have been eligible for the KarMMa clinical trial that led to the FDA approval of ide-cel. Leveraging data from several institutions across the United States that provide CAR T-cell therapy to patients with RRMM, we observed racial and ethnic differences in baseline systemic inflammatory markers and postinfusion toxicity among patients with RRMM treated with SOC ide-cel. In addition, Hispanic patients were more likely to have inferior ide-cel responses. However, both PFS and OS were similar across racial and ethnic groups after adjusting for clinical characteristics and characteristics of patients at high risk for inferior outcomes after CAR T-cell therapy.
We observed racial and ethnic differences in the safety profile of ide-cel, with non-Hispanic Black patients more likely to develop any grade CRS than Hispanic and non-Hispanic White patients. This finding could potentially be explained by an elevated proinflammatory state among non-Hispanic Black patients before ide-cel infusion, with studies showing that patients with a baseline proinflammatory response are more likely to develop CRS.25 Specifically, we observed that non-Hispanic Black patients had higher levels of the systemic inflammatory markers CRP and ferritin before CAR T-cell infusion than both Hispanic and non-Hispanic White patients, which is consistent with studies showing that CRP and ferritin levels are elevated among non-Hispanic Black individuals in the general population.26,27 Additionally, this finding may be because of differences in disease burden by race and ethnicity; however, no racial and ethnic differences in clinical marrow burden (bone marrow plasma cell percentage before lymphodepleting chemotherapy, and baseline β2 microglobulin) were observed. We did not observe any racial and ethnic differences in the grade of CRS, although severe CRS (grade ≥3) was rare in our cohort (3%). Together, these findings have potential clinical implications for outpatient management and early intervention with supportive care for patients of racial and ethnic minority groups after CAR T-cell infusion.
In this study, Hispanic patients had inferior responses to ide-cel compared with non-Hispanic Black and non-Hispanic White patients. This finding persisted even after adjustment for characteristics of patients at high risk that are associated with inferior CAR T-cell therapy responses. A similar finding was observed in a large study of patients with diffuse B-cell lymphoma treated with commercial axi-cel,20 in which Black patients were more likely to have lower ORR and CR rates than White patients, but this did not equate to poorer survival among Black vs White patients. The reason for inferior response among Hispanic patients with RRMM in this study is unclear. It is possible that this finding was a product of the small number of Hispanic patients in our study, unmeasured confounders, or could point to biologic differences across race and ethnicity. Further research is needed to confirm these findings and elucidate potential causes of racial and ethnic differences in CAR T-cell therapy response in larger sample sizes of racially and ethnically diverse patients with RRMM treated with SOC ide-cel.
Despite lower ide-cel response among Hispanic patients, we did not observe differences in OS or PFS based on race and ethnicity among patients with RRMM treated with SOC ide-cel. A few prior studies have examined racial and ethnic differences in CAR T-cell therapy outcomes,15,16,19-21 but the generalizability of their findings to patients with RRMM was limited because of their focus on other hematologic malignancies, pediatric patients, patients treated as part of clinical trials, and/or small samples of patients with RRMM. Most of these past studies that focused on adult patients did not observe racial and ethnic differences in PFS and OS after CAR T-cell therapy, which is consistent with findings in this study. Although further investigation is needed to confirm our findings in a larger sample of diverse patients with longer follow-up time, the collective findings encourage the use of ide-cel for patients with RRMM regardless of race and ethnicity.
An important caveat of this work is that our findings reflect patients with RRMM who had access to and were able to receive ide-cel. Although access to CAR T-cell therapy for RRMM has improved over time, treatment with CAR T-cell therapy is only approved at certain academic centers with limited availability in the community setting. A recent study by Alqazaqi et al28 examined the geographic distribution of clinical trials for CAR T-cell therapy and bispecific antibodies, another emerging novel therapeutic for RRMM, and found that only 36% of Black patients lived in a county with an open trial. Recent studies also show that patients of racial and ethnic minority groups with RRMM are underrepresented in the use of CAR T-cell therapy in both the clinical trial and commercial setting.15,16 Some data suggest that socioeconomic status and insurance coverage contribute to the low representation of patients of racial and ethnic minority groups with RRMM in CAR T-cell therapy clinical trials.15 However, additional research is critically needed to evaluate additional barriers to access to CAR T-cell therapy so that all patients can derive benefit from novel advancements in the management of RRMM.
Our work is strengthened by the use of data from a recently established consortium of institutions that have used CAR T-cell therapy to treat RRMM across the United States (the US Multiple Myeloma Immunotherapy Consortium), improving generalizability of our findings. This allowed us to provide, to our knowledge, the first data on safety and efficacy of SOC ide-cel among racially and ethnically diverse patients with RRMM. Despite these notable strengths, this study is not without limitations. Even with the inclusion of 11 institutions across the United States, our study population of 207 patients with RRMM, including 28% non-White patients, is relatively small, and median follow-up was short at 9 months. However, our sample size and length of follow-up were limited by the recent FDA approval of ide-cel for commercial use (March 2021). Also because of the sample size, we were unable to investigate less common racial and ethnic groups in our cohort as well as ethnic subgroups (eg, Cuban and Puerto Rican), which may mask subgroup heterogeneity in the associations of race and ethnicity with safety and clinical outcomes of patients with RRMM treated with ide-cel. This study used self-reported race and ethnicity and did not have germline genotyping information to characterize genetic ancestry. Lastly, as this was a retrospective study of available clinical data, institutional practices for toxicity management may have differed across centers and correlative biomarkers were not available.
This was, to our knowledge, the first and largest evaluation of racial and ethnic differences in the safety and efficacy of ide-cel CAR T-cell therapy for patients with RRMM treated in a SOC setting. We observed differences in safety and response rates based on race and ethnicity but no differences in PFS or OS. These findings should be investigated in a larger cohort of racially and ethnically diverse patients with RRMM treated with SOC ide-cel with longer follow-up time. With the continued expansion of therapeutic options for patients with RRMM in the SOC setting, continued evaluation of safety and efficacy across diverse patient ethnicities is critical to ensure equity in the improvement of outcomes for all patients with RRMM.
Acknowledgments
This work was supported, in part, by the Moffitt Cancer Center National Cancer Center Institute Core Grant (P30-CA076292) and a generous donation from the Hyer family. F.L.L. is supported by a Scholar in Clinical Research award from the Leukemia and Lymphoma Society. S.S. is supported by Stanford Clinical and Translational Science KL2 Career Development Award program, award number KL2 TR003143, and Stanford Cancer Institute/American Cancer Society Pilot grant 2022. D.K.H. and C.F. are supported by the International Myeloma Society Young Investigator Award for Exemplary Abstract. L.C.P., L.B.O., G.D.A., D.K.H., C.L.F., and M.A. are supported by the Pentecost Family Myeloma Research Center. L.C.P., L.B.O., and D.K.H. are supported by a Moffitt Catchment Area Research Enhancements Award.
Authorship
Contribution: L.C.P., L.B.O., D.K.H., S.S., and K.P. conceived and designed the study; L.C.P. conducted the statistical analyses; L.C.P. and L.B.O. drafted the initial version of the manuscript; and all authors contributed to the acquisition, management, and interpretation of data, and critically reviewed, revised, and approved the final version of the manuscript.
Conflict-of-interest disclosure: L.C.P. declares research funding from Bristol Myers Squibb and Karyopharm. D.K.H. declares honoraria from OncLive; a consulting or advisory role and research funding from Bristol Myers Squibb; and research funding from Adaptive Biotech. K.P. declares honoraria from Bristol Myers Squibb, Celgene, Janssen, Merck, Pfizer, Karyopharm, Takeda, Curio Bioscience, and AbbVie, and research funding from Bristol Myers Squibb, Janssen, AbbVie, Nektar, Allogene, Precision Bio, Cellectis, and Takeda. M.A. declares a consulting or advisory role with Bristol Myers Squibb and Janssen. D.W.S. declares a consulting or advisory role with Bristol Myers Squibb, Janssen, Sanofi, Pfizer, AbbVie, and GlaxoSmithKline. B.J.B. declares honoraria from Pfizer, OncLive, Janssen, Oncopeptides, and AbbVie, and serves on the advisory board of Janssen. H.H. declares honoraria from Sanofi and Karyopharm. C.F. declares honoraria from Sanofi and stock ownership in Affimed. J.M. declares consulting for Envision, Novartis, Caribou Bio, and Sana Technologies, and a consulting or advisory board role for Kite, AlloVir, Bristol Myers Squibb, CRISPR, and Nektar. G.K. declares a consulting or advisory board role for Janssen, Bristol Myers Squibb, Sanofi, and Arcellx Cellectar. L.D.A. Jr declares a consulting or advisory board role for Janssen, Celgene, Bristol Myers Squibb, Amgen, GlaxoSmithKline, AbbVie, BeiGene, Cellectar, Sanofi, Karyopharm, Oncopeptides, and Prothena. O.N. declares a consulting or advisory board role with Janssen, Bristol Myers Squibb, Karyopharm, Takeda, GlaxoSmithKline, Adaptive Biotechnologies, GPCR Therapeutics, and Sanofi, and research funding from Takeda and Janssen. C.L.F. declares honoraria from/consulting with Bristol Myers Squibb, Seattle Genetics, Celgene, AbbVie, Sanofi, Incyte, Amgen, and ONK Therapeutics & Janssen; and research funding from Bristol Myers Squibb, Janssen, and Roche/Genentech. F.L.L. declares research support from Bristol Myers Squibb, Kite Pharma, and Novartis; consulting for Gerson Lehrman Group; is an advisory board member for A2, Allogene, Amgen, bluebird bio, Bristol Myers Squibb, Iovance, Kite Pharma, Janssen, Legend Biotech, Novartis, Pfizer, Sana, Takeda, Wugen, and Umoja; and education, speaking, or authorship for Emerging Therapy Solutions, Aptitude Health, American Society of Hematology, BioPharma, Communications CARE Education, Clinical Care Options Oncology, Imedex, and Society for Immunotherapy of Cancer. The remaining authors declare no competing financial interests.
Correspondence: Lauren C. Peres, Department of Cancer Epidemiology, H. Lee Moffitt Cancer Center and Research Institute, 12902 USF Magnolia Dr, Tampa, FL, 33612; email: lauren.peres@moffitt.org.
References
Author notes
∗L.C.P., L.B.O., S.S., D.K.H., and K.P. contributed equally to this work.
Presented in abstract form at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 10-13 December 2022.
Data are available on reasonable request from author, Doris Hansen (Doris.Hansen@moffitt.org).
Data requestors will need to sign a data access agreement and submit a proposal to be reviewed and approved by the US Multiple Myeloma Immunotherapy Consortium Steering Committee.
The full-text version of this article contains a data supplement.