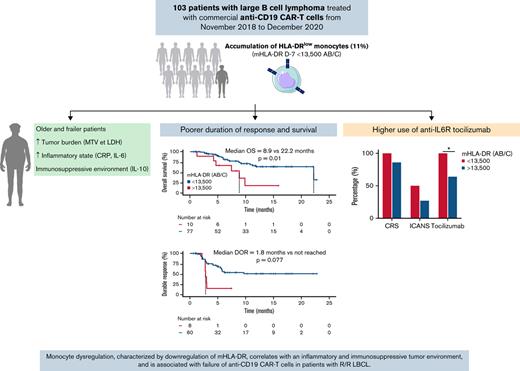
Key Points
Low prelymphodepletion mHLA-DR correlates with older age, poorer ECOG performance status, higher tumor burden, and systemic inflammation.
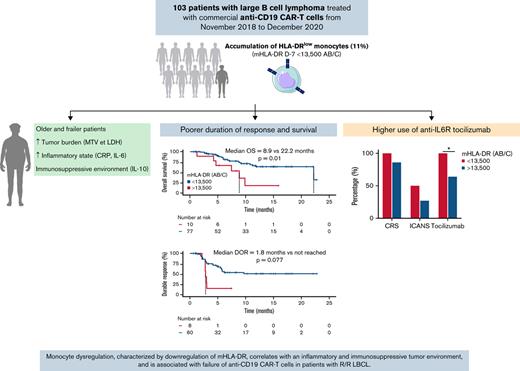
Low prelymphodepletion mHLA-DR is associated with poorer duration of response and survival in LBCL treated with anti-CD19 CAR T cells.
Abstract
Despite their unprecedented success in relapsed/refractory (R/R) large B-cell lymphoma (LBCL), anti-CD19 CAR T cells are associated with significant toxicity, and more than half of patients relapse. As monocytes emerged as key players in CAR therapy, we sought to evaluate the evolution of HLA-DR expression on monocytes (mHLA-DR) before and after commercial anti-CD19 CAR T-cell infusion in a large cohort (n = 103) of patients with R/R LBCL and its association with adverse events and treatment response. Cy-Flu-based lymphodepletion (LD) upregulated mHLA-DR in 79% of the cases, whereas in 2l% of cases (15 patients), the mHLA-DR level decreased after LD, and this decrease was associated with poorer outcome. Low mHLA-DR at day minus 7 (D−7) (<13 500 antibodies per cell) before CAR T-cell infusion correlated with older age, poorer performance status, higher tumor burden, and elevated inflammatory markers. With a median follow-up of 7.4 months, patients with low mHLA-DR D−7 exhibited a poorer duration of response and survival than the higher mHLA-DR D−7 group. For toxicity management, tocilizumab was more frequently used in the low–mHLA-DR D−7 group. These data suggest that monocyte dysregulation before LD, characterized by the downregulation of mHLA-DR, correlates with an inflammatory and immunosuppressive tumor environment and is associated with failure of anti-CD19 CAR T cells in patients with R/R LBCL. Modulation of these myeloid cells represents a promising field for improving CAR therapy.
Introduction
Anti-CD19–directed chimeric antigen receptor T cells (CAR T cells) have emerged as a revolutionary therapy with unprecedented efficacy in the treatment of relapsed/refractory (R/R) large B-cell lymphoma (LBCL). In pivotal trials, durable remissions were achieved in ∼40% of patients1-3 and were subsequently confirmed in real-life studies.4-7 However, despite the clinical success in heavily pretreated patients, up to one-third of ill patients experience significant toxicity (grade 3 or worse cytokine release syndrome [CRS; 2% to 13%] or neurological toxicity [12% to 28%]), and more than half of them progressed or relapsed within 6 months after infusion (51% to 69%).1-3
Several hypotheses underlying CAR failure have emerged based on clinical observation among which are downregulation of CD19 antigen, high target-to-effector ratio, inadequate T-cell fate (effector or exhausted T-cell phenotype), insufficient CAR T-cell persistence, and negative regulation of T-cell functions (regulatory T cells or myeloid-derived suppressor cells),8-14 but the precise mechanisms by which CAR T cells interact with other immune cells remains largely unknown.
Myeloid cells are key players in CAR T-cell therapy and have emerged as the main cellular component that mediates toxicity15-18 and as a potential inhibitor of CAR T-cell efficacy.14,19-22 Indeed, monocytes and macrophages are engaged by activated CAR T cells in a contact-dependent (CD40/CD40L) and -independent (interferon γ [IFNγ], tumor necrosis factor α [TNFα], or granulocyte macrophage colony stimulating factor [GM-CSF]) manner. Activated monocytes secrete inflammatory mediators (including interleukin-1 [IL-1] and IL-6) and chemokines that recruit additional innate immune effectors that are responsible for CRS and neurotoxicity.15-18 In addition, myeloid cells have been shown to limit CAR T-cell activation, expansion, and transduction efficacy in the leukapheresis product,19-21 and suppressive myeloid cells have recently been associated with CAR T-cell failure.14,22
As a reflection of the monocyte activation state, expression of the human leukocyte antigen DR molecules on monocytes (mHLA-DR) has been widely found in acute pathological conditions.23-30 In sepsis, mHLA-DR downregulation is a hallmark of altered immune status that correlates with an excess of myeloid-derived suppressor cells (MDSCs)31,32 and is associated with deleterious clinical outcomes.23-28
We therefore hypothesized that dysregulation of monocytes, measured through mHLA-DR expression on the cell surface, may influence response to CAR T cells and correlate with their toxicity.
In this single-center retrospective study, we sought to evaluate the expression of HLA-DR on monocytes over time in a large cohort of commercial anti-CD19 CAR T-cell–treated patients with R/R LBCL and to evaluate its association with treatment responses, survival, and adverse events.
Methods
Study cohort
The study population included all consecutive patients presenting with R/R B-cell lymphoma and treated in the Hematology Department of the Lyon Sud University Hospital. Only patients with LBCL, including diffuse LBCL (DLBCL), primary mediastinal B-cell lymphoma, and transformed indolent non-Hodgkin lymphoma, were considered. Patients without mHLA-DR levels available were excluded from the study. From January 2017 through December 2020, 127 patients were treated with anti-CD19 CAR T cells, and 103 patients who fulfilled the inclusion criteria were enrolled (supplemental Figure 1). Clinical and biological data were retrospectively collected from the patients’ medical charts. The study was conducted in accordance with the Declaration of Helsinki. All patients provided informed consent for CAR T-cell therapy, in accordance with national regulatory rules. This study was approved by the Ethics Committees of Lyon University Hospital and the French authority for the protection of privacy and personal data in clinical research (CNIL, approval 18-076).
Treatment
The choice of commercial anti-CD19 CAR T-cells was dependent only on slot production availability and was not guided by any specific patient characteristic. All patients received lymphodepleting (LD) chemotherapy consisting of a combination of cyclophosphamide and fludarabine (Cy-Flu) from day 5 (D5) to D3 before CAR T-cell infusion. The dosages used were fludarabine 25 mg/m2 for tisagenlecleucel (tisa-cel), and 30 mg/m2 for axicabtagene ciloleucel (axi-cel) and lisocabtagene maraleucel (liso-cel); and cyclophosphamide 250 mg/m2 for tisa-cel, 300 mg/m2 for liso-cel and 500 mg/m2 for axi-cel. Lower dosages were used in cases of kidney function impairment.
Response assessment
Response assessment was performed at month 1 (M1), M3, M6, and M12 after anti-CD19 CAR T-cell infusion, according to the 2014 Lugano classification using positron emission tomography/computed tomography images evaluated by 2 independent expert nuclear medicine physicians.33
Toxicity assessment
Toxicity grading of CRS and immune effector cell–associated neurotoxicity syndrome (ICANS) were assessed according to the consensual ASTCT (American Society for Transplantation and Cellular Therapy) grading scale.34
Standardized measurement of HLA-DR expression on monocytes
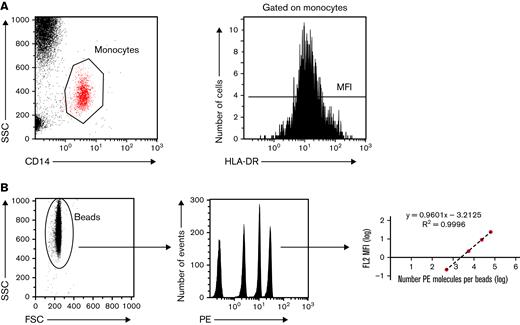
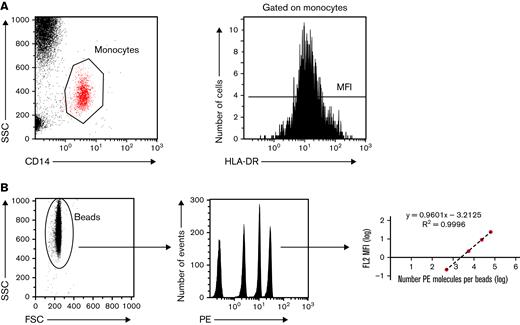
The expression of cell surface HLA-DR on monocytes was assessed in EDTA-anticoagulated blood 7 days before CAR T cell infusion (day minus 7 [D−7]) and on D0, D7, and D14 and M1, M3, M6, M9, and M12 after CAR T-cell infusion (supplemental Figure 2). The number of patients with mHLA-DR among the 103 patients of the cohort who were assessed at each time point is shown in supplemental Figure 2. mHLA-DR measurement was performed using standardized protocols, as previously described, and fulfilling the requirements for accreditation of clinical and diagnostic laboratories, according to the International Organization for Standardization.24,25,35,36 In brief, whole blood (25 μL) was stained with 10 μL QuantiBrite HLA-DR/monocyte mixture at room temperature for 30 minutes in the dark. Samples were then lysed using FACS Lysing Solution (Becton Dickinson, San Diego, CA) for 15 minutes. After a washing step with 3 mL of phosphate-buffered saline, the cell pellet was resuspended in 500 μL of PBS with 1% paraformaldehyde. The samples were run on a Navios flow cytometer, and flow data were analyzed with Navios software (Beckman Coulter, Hialeah, FL). Monocytes were first gated out of the other cells on the basis of CD14 expression, and mHLA-DR expression was then measured on their surface as the median fluorescence intensity related to the entire monocyte population. These results were then transformed into the number of antibodies bound per cell (Ab/c) using calibrated PE beads (BD QuantiBrite PE Beads; Becton Dickinson) that were run on a flow cytometer (Figure 1).
Standardized measurement of HLA-DR expression on monocytes. (A) Monocytes are sorted according to their structure (SSC) and CD14 positivity. Among gated monocytes, the HLA-DR median fluorescence intensity (MFI) is measured and then (B) converted to the number of anti-HLA-DR antibodies bound per monocyte (Ab/c). FSC, forward scattered; MFI, median fluorescence intensity; SSC, side scattered.
Standardized measurement of HLA-DR expression on monocytes. (A) Monocytes are sorted according to their structure (SSC) and CD14 positivity. Among gated monocytes, the HLA-DR median fluorescence intensity (MFI) is measured and then (B) converted to the number of anti-HLA-DR antibodies bound per monocyte (Ab/c). FSC, forward scattered; MFI, median fluorescence intensity; SSC, side scattered.
Determination of the mHLA-DR reference value
Peripheral blood from a cohort of 54 healthy volunteers (HVs) was provided by the French Blood Institute (EFS) in Lyon to define normal values for mHLA-DR. According to EFS standardized procedures for blood donation and to provisions of article R.1243-49 and subsequent ones of the French public health code, written nonopposition to the use of donated blood for research purposes was obtained from the HVs. The blood donors’ personal data were anonymized before transfer to our research laboratory. Monocyte HLA-DR expression was measured in these samples by using a standardized technique, as previously described. Lower and upper values of reference intervals (ie, 13 500 and 35 200 Ab/c) were calculated according to international recommendations as the 5th and 95th percentiles of HV values (supplemental Figure 3), in accordance with previously reported reference values,24,29,35-38 and with the normal values from the routine immunology laboratory (ie, 13 500 Ab/c).39
Cytokine measurement
Whole blood was sampled on EDTA tubes and plasma was frozen at −20°C within 4 hours after blood collection. The cytokine measurement was taken in batches after 1 freeze/thaw cycle, using standardized protocols that fulfilled accreditation requirements for clinical and diagnostic laboratories from the International Organization for Standardization. Plasma concentrations of IL-6, IL-10, and IL-2 soluble receptor (IL-2sR); TNFα; and IFNγ were measured by Simpleplex technology using the ELLA instrument (ProteinSimple, San Jose, CA), according to the manufacturer’s instructions.
Statistical analysis
Standard cutoffs were used to dichotomize continuous variables. For CRP, ferritin, and metabolic tumor volume (MTV), previously reported cutoff values of 30 mg/L, 1 000 μg/L, and 80 mL, respectively, were used.4-6,12,14,40-42 Clinical and biological characteristics were described according to frequency, median and quartiles for each parameter. The D−7 time point referred to the date of hospitalization for CAR T-cell infusion before administration of the LD conditioning regimen. The objective response rate was defined as the proportion of patients who achieved a complete response (CR) or partial response as their best overall response. Overall survival (OS) was defined as the time from CAR T-cell infusion until death from any cause or last follow-up. Progression-free survival (PFS) was defined as the time from CAR T-cell infusion until progression, relapse, death from any cause, or last follow-up. The duration of response (DOR) was defined as the time from the first documented disease response (CR or partial response) to the date of the first documented progression or death due to LBCL. Survival distributions were estimated with the Kaplan-Meier method and compared by log-rank test. A Cox proportional hazards model was used to select prognostic covariates, and for multivariate analysis (MVA), immunologic parameters were adjusted to clinical and biological variables that were significant upon univariate analysis (UVA; P ≤ 0.1). All statistical analyses were performed in GraphPad PRISM 9, SPSS (IBM version 21 for Mac), and R Studio (version 1.3.1073 for Mac).
Results
Patient characteristics
A total of 103 patients who received commercial anti-CD19 CAR T cells for aggressive LBCL in the Hematology Department of the Lyon Sud University Hospital from November 2018 through December 2020 were enrolled in the study (supplemental Figure 1). The clinical and biological characteristics of the patients before the LD regimen are summarized in supplemental Table 1. In brief, the median age at diagnosis was 60 years (range, 51-68 years), and there was a male predominance (63%). Most patients presented with a conserved Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤1 (83%), no B symptoms (90%), and advanced-stage disease (III-IV) (80%). Eighteen patients (17%) received at least 4 prior lines of chemotherapy, and 25 (24%) underwent high-dose chemotherapy followed by autologous stem cell transplantation. Biologically, elevated inflammatory markers, including CRP >30 mg/L and ferritin >1000 μg/L, were observed in 26% (27 of 103) and 23% (23 of 102) of the patients, respectively. The median time from apheresis to infusion was 41 days, and 93% (96 of 103) of the patients received bridging therapy between apheresis and LD (details provided in supplemental Table 2). After the conditioning regimen, commercial anti-CD19 CAR T-cell products were infused, consisting of axi-cel for 53 patients (51%), tisa-cel for 43 patients (42%), and liso-cel for 7 patients (7%). When CAR products were compared, patients treated with tisa-cel underwent prior autologous stem cell transplantation more frequently (37% vs 15%, P = .02) and had a longer time from apheresis to infusion (median, 38 vs 28 days; P < .0001) than those treated with axi-cel (supplemental Table 3).
Kinetics of mHLA-DR expression during CAR T-cell therapy
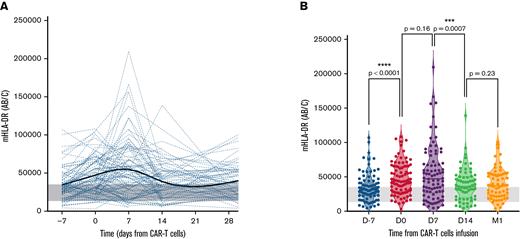
The evolution of mHLA-DR at each time point before and after CAR T-cell infusion is shown in Figure 2 and supplemental Table 4.
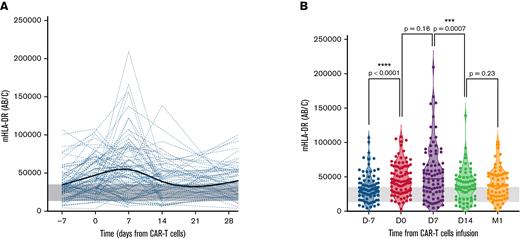
Evolution of mHLA-DR values before and after anti-CD19 CAR T-cell infusion. (A) Individual patient time course showing the evolution of mHLA-DR levels during the first month after infusion. The bold trend curve is the cubic spline representation. (B) Violin plot showing the distribution of mHLA-DR at each time point for 1-year after infusion. The Wilcoxon matched-pairs test was used to calculate P values. The gray band delimits the 5th and 95th percentiles of normal values (ie, 13 500 and 35 200 Ab/c).
Evolution of mHLA-DR values before and after anti-CD19 CAR T-cell infusion. (A) Individual patient time course showing the evolution of mHLA-DR levels during the first month after infusion. The bold trend curve is the cubic spline representation. (B) Violin plot showing the distribution of mHLA-DR at each time point for 1-year after infusion. The Wilcoxon matched-pairs test was used to calculate P values. The gray band delimits the 5th and 95th percentiles of normal values (ie, 13 500 and 35 200 Ab/c).
Before LD, the median mHLA-DR D−7 was 31 013 Ab/c, which was significantly higher than in the HV cohort (21 776 Ab/c; P < .0001; supplemental Figure 3). The LD conditioning regimen led to a significant increase in mHLA-DR with a median at D0 of 43 357 Ab/c (P < .0001, when compared with the median D−7, Wilcoxon matched-pairs test) and was more than doubled in 12 patients (12 of 73; 16%). However, in 15 patients (15 of 73; 21%), mHLA-DR expression decreased after LD (supplemental Figure 4).
At D7 after CAR T-cell infusion, the median mHLA-DR peaked (47 295 Ab/c), but with no significant difference compared with the D0 level (P = .159). On that day, 10 of 86 patients (12%) exhibited a major increase in mHLA-DR D7 (>100 000 Ab/c), along with increased ferritin (median, 1621 μg/L; range: 431-29 151 μg/L, including 2 of 10 patients with ferritin >10 000 μg/L) and IL-2sR (median, 8665 pg/mL; range, 1 350-32 270 pg/mL). After this peak, mHLA-DR rapidly decreased at D14 (median, 35 881 Ab/c; P = .0007, when compared with the median D7).
Correlation between mHLA-DR and patient characteristics before LD
Based on mHLA-DR at D−7 before CAR T-cell infusion, we defined 2 distinct groups of patients, using the threshold of 13 500 Ab/c, which was determined as the fifth percentile in a HV cohort and in accordance with the literature (supplemental Figure 3).24,29,35-39 Among the 103 patients, only 87 had mHLA-DR measured at D−7, and 10 (11%) had a low mHLA-DR D−7 (<13 500 Ab/c), whereas 77 (89%) had an mHLA-DR D−7 ≥13 500 Ab/c. We then compared these 2 groups of patients, considering clinical and biological values at D−7 before infusion. Clinically, patients with low mHLA-DR D−7 were significantly older (mean age, 65.5 years; 95% confidence interval [CI] 59.1-71.3 vs 56.6 years, 95% CI, 53.9-59.3; P = .02), frailer (ECOG PS ≥2, 50% vs 13%; P = .01), and had a higher tumor burden (median MTV, 114.4 vs 35.6 mL; P = .02) than those with mHLA-DR D−7 ≥13 500 Ab/c (Table 1). Despite their more severe features, patients with low mHLA-DR D−7 did not need more frequent bridging therapies or more intensive bridging regimens, compared with those with higher mHLA-DR D−7 (90% vs 92%; P = .59 and 67% vs 52%; P = .45 respectively, supplemental Table 5; supplemental Figure 5).
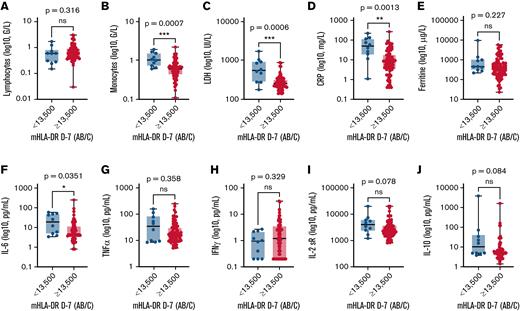
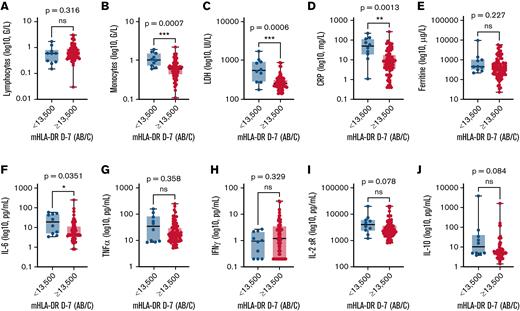
When biological values were compared, patients with low mHLA-DR D−7 had elevated inflammatory markers with higher CRP (>30 mg/L, 60% vs 21%; P = .01) and IL-6 levels (median, 19.05 vs 4.70 pg/mL; P = .04) than patients with mHLA-DR ≥13 500 Ab/c (Figure 3). Interestingly, they also had higher monocyte counts (median, 1.01 × 109/L vs 0.59 × 109/L; P = .0007, Figure 3), because mHLA-DR D−7 level and the monocyte count correlated negatively (r = −0.2653; P = .0141; supplemental Figure 6).
Comparison of biological markers according to mHLA-DR D7 before anti-CD19 CAR T-cell infusion. Biological markers included lymphocytes (A), monocytes (B), LDH (C), CRP (D), ferritin (E), IL-6 (F), TNFα (G), IFNγ (H), IL-2sR (I), and IL-10 (J). The Mann-Whitney U test was used to calculate P values.
Comparison of biological markers according to mHLA-DR D7 before anti-CD19 CAR T-cell infusion. Biological markers included lymphocytes (A), monocytes (B), LDH (C), CRP (D), ferritin (E), IL-6 (F), TNFα (G), IFNγ (H), IL-2sR (I), and IL-10 (J). The Mann-Whitney U test was used to calculate P values.
After LD, only MTV was significantly higher in the 15 patients who had downregulated mHLA-DR expression, compared with the other patients (median, 179 vs 35.6 mL; P = .019; supplemental Table 6). Conversely, patients in whom mHLA-DR expression more than doubled after LD exhibited higher lymphocytes at D7 (median, 0.56 × 109/L vs 0.32 × 109/L; P = .048) and the change in mHLA-DR tended to correlate with the lymphocyte count at D7 (r = 0.2993; P = .0573; supplemental Figure 7).
Correlation between mHLA-DR and cytokine levels before and after CAR T-cell infusion
At D−7 before CAR T-cell infusion, levels of the proinflammatory cytokines IL-6 and TNFα were associated with low mHLA-DR (r = −0.4133; P < .0001 and r = −0.2375; P = .0296, respectively; Spearman’s rank-order correlation). IL-2sR and immunosuppressive IL-10 also correlated negatively with mHLA-DR level (r = −0.2673; P = .014 and r = −0.2153; P = .049, respectively). There was a positive association between IFNγ and mHLA-DR (r = 0.3106; P = .004; supplemental Figure 8). Interestingly, along with the mHLA-DR upregulation, the LD conditioning regimen led to an increased level of IFNγ (median D−7, 1.1 vs median D0, 2 pg/mL; P = .0003, Wilcoxon matched-pairs test) and IL-2 (median D−7, 0.3 pg/mL vs median D0, 0.3 pg/mL; P < .0001) after Cy-Flu chemotherapy, whereas TNFα decreased (median D−7, 15.5 pg/mL vs median D0, 13.3 pg/mL; P = .0002; supplemental Figure 9).
At postinfusion D7, mHLA-DR D7 correlated positively with IFNγ (r = 0.2891; P = .0076) and TNFα (r = 0.3337; P = .0019), but not with IL-6, IL-10, or IL-2sR (supplemental Figure 10).
Prognostic value of mHLA-DR before and after LD on response and survival
Among the 103 patients, the overall response rate was 79% (95% CI, 0.71-0.86), with a 58% CR rate as best response. In the low–mHLA-DR D−7 group, the CR rate was 40%, compared with 64% in the other group (P = .18, Fisher’s exact test; supplemental Figure 11).
In the UVA, low mHLA-DR D−7 and tisa-cel were associated with an adverse PFS (hazard ratio [HR], 3.17; 95% CI, 1.51-6.67; P = .002 and HR, 2.04; 95% CI, 1.21-3.45; P = .008 respectively; Table 2). In MVA, tisa-cel and elevated lactate dehydrogenase (LDH) were associated with poorer PFS (HR, 2.55; 95% CI, 1.37-4.76; P = .003 and HR, 2.30; 95% CI, 1.11-4.74; P = .025 respectively; Table 3), whereas the poor prognostic value of low mHLA-DR D−7 was of just below statistical significance (HR, 2.04; 95% CI, 0.92-4.55; P = .079; Table 3). In the UVA for OS, elevated LDH level (HR, 3.39; 95% CI, 1.19-9.67; P = .02), CRP >30 mg/L (HR, 2.20; 95% CI, 1.09-4.42; P = .02), ferritin >1000 μg/L (HR, 2.36; 95% CI, 1.15-4.84; P = .02) and mHLA-DR D−7 <13 500 Ab/c (HR, 3.15, 95% CI, 1.25-7.93; P = .01) were associated with worse outcomes (Table 2). Only mHLA-DR D−7 <13 500 Ab/c (HR, 3.37; 95% CI, 1.16-9.77; P = .03) retained prognostic significance for OS in MVA (Table 3).
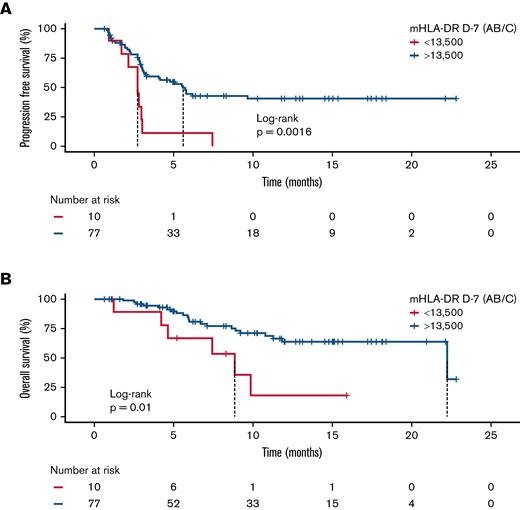
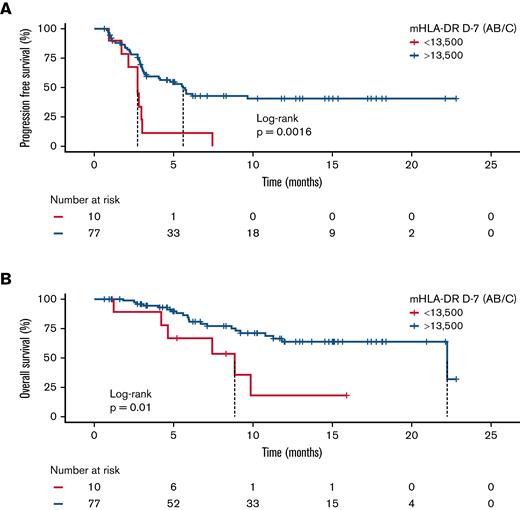
With a median follow-up of 7.4 months after CAR T-cell infusion, the median PFS in the low mHLA-DR D−7 group (<13 500 Ab/c) was 2.8 months (95% CI, 1.9-3.6) vs 5.6 months (95% CI, 4.0-7.3) in the higher mHLA-DR D−7 group (P = .0016). For OS, the median was 8.9 months (95% CI, 4.0-13.8) in patients with low mHLA-DR D−7 vs 22.2 months (95% CI, 7.7-36.8) in patients with mHLA-DR D−7 ≥13 500 Ab/c (P = .01; Figure 4). Finally, the median DOR was only 1.8 months (95% CI, 1.7-2.0) in the low-mHLA-DR D−7 group, whereas it was not reached in the mHLA-DR D−7 ≥13 500 Ab/c group (P = .0023; supplemental Figure 12).
Survival analysis according to mHLA-DR D7 before anti-CD19 CAR T-cell infusion. Kaplan-Meier curves for PFS (A) and OS (B). The log-rank test was used to calculate the P values.
Survival analysis according to mHLA-DR D7 before anti-CD19 CAR T-cell infusion. Kaplan-Meier curves for PFS (A) and OS (B). The log-rank test was used to calculate the P values.
In addition, we also compared the outcome according to mHLA-DR change after LD. The 15 patients who decreased their mHLA-DR expression between D−7 and D0 presented a poorer PFS and OS than those with an increase of mHLA-DR (median PFS, 2.9 months; 95% CI, 2.1-3.4 vs 5.8 months; 95% CI, 4.4-7.2, respectively, P = .0093; median OS, 7.1 months; 95% CI, 4.2-10.1 vs 22 months 95% CI, NA, respectively, P = .013; supplemental Figure 13).
Correlation between mHLA-DR and anti-CD19 CAR T-cell toxicities
In our cohort, 88 patients (85%) developed any-grade CRS with a median time to onset of 3 days, including 3 patients (3%) with severe CRS (grade ≥3). Regarding neurotoxicity, 30 patients (29%) developed ICANS, which was severe (grade ≥3) in 5 cases (5%), with a median time to onset of 7 days.
To assess whether mHLA-DR predicts toxicity, we compared mHLA-DR values at D−7 before anti-CD19 CAR T-cell infusion and showed that the mHLA-DR level did not significantly differ across CRS and ICANS grades (P = .542 for CRS and P = .139 for ICANS; Kruskal-Wallis test; supplemental Figure 14). In patients with low mHLA-DR D−7, there was a trend toward higher CRS and ICANS rates, however (100% vs 86%, P = .34 and 50% vs 27%, P = .16, respectively, Table 1).
We then compared mHLA-DR levels after CAR T-cell infusion according to the presence of CRS or ICANS. For CRS, mHLA-DR levels from D7 to M1 did not differ significantly between patients with CRS and those without. For ICANS, the median mHLA-DR D14 was significantly lower in patients with than in those without (17 415 vs 42 309 Ab/c; P = .0013; supplemental Figure 15).
When the treatments received for toxicity management were examined, the anti-IL6 receptor tocilizumab was initiated after a median time of 4 days from D0 in 69 of the 88 patients who developed CRS (78%), and steroids (dexamethasone) were administered after a median time of 7 days from D0 in 28 of the 30 patients with ICANS (93%). Tocilizumab did not induce significant modifications in mHLA-DR values during the first month after CAR T-cell infusion. For dexamethasone, the median mHLA-DR was significantly lower at D7 (23 177 vs 52 039 Ab/c; P = .042) and D14 (17 408 vs 43 765 Ab/c; P < .0001) in patients who actually received dexamethasone than in those who did not (supplemental Figure 16). Importantly, patients with low mHLA-DR D−7 were treated more frequently with tocilizumab (100% vs 64%; P = .03) and steroids (60% vs 39%, P = .31), even though the difference was not significant compared with treatments of those with higher mHLA-DR D−7 (Table 1).
Discussion
In this retrospective study, we report on the largest cohort of patients with LBCL treated with commercial anti-CD19 CAR T cells and monitored for HLA-DR expression on monocytes. Before LD, low mHLA-DR D−7 (<13 500 Ab/c) correlated with older age, poorer ECOG PS, higher tumor burden (MTV and LDH), and elevated inflammatory markers (CRP, IL-6, and TNFα). Most important, patients with low mHLA-DR D−7 exhibited poorer DOR and survival than those with higher mHLA-DR D−7. Cy-Flu LD led to upregulated mHLA-DR in 80% of the cases and increased plasma levels of IFNγ and IL-2. However, both a low level of mHLA-DR at D−7 and a decrease after LD were associated with an adverse prognosis. Finally, patients with low mHLA-DR D−7 tended to present higher rates of CRS and ICANS, and they received tocilizumab and steroids more often than the other patients. Our data suggest that monocyte dysregulation before CAR T-cell infusion, characterized by downregulation of mHLA-DR, correlates with a proinflammatory and immunosuppressive tumor environment and is associated with CAR T-cell resistance.
We used the cutoff of 13 500 Ab/c for mHLA-DR D−7 to identify a group of patients at risk for CAR T-cell failure. In the literature, this lower threshold has been largely associated with deleterious prognosis during acute illness (ie, sepsis, polytrauma, and pancreatitis).24,25,19
Low mHLA-DR is known to correlate with monocytic myeloid–derived suppressive cells (M-MDSCs) abundance, defined as HLA-DR−/low monocytes.31,43-46 M-MDSCs arise as the pathologic activation of monocytes during cancer and other chronic inflammatory conditions.45,47 Accumulation of M-MDSCs has been reported in DLBCL, especially in advanced-stage and high-risk disease, and has been associated with poor clinical outcome.48-52
Our findings that a low level of mHLA-DR assessed 7 days before CAR T-cell infusion was associated with a lack of durable response and poorer survival are in accordance with a recent report from Jain and colleagues. In a smaller cohort of LBCL treated with anti-CD19 CAR T cells, they demonstrated an inflammation-mediated resistance to CAR T cells linked to IFN signaling and M-MDSCs.14 Similar findings came from Jaeger et al and Michael, who reported that patients with a highest frequency of CD11b+HLA-DR− cells that represent an MDSC phenotype at baseline were enriched in nonresponders.22 Taken together, these data and ours support the hypothesis of suppressive myeloid cell–mediated resistance to CAR T cells.
We observed that these patients with low mHLA-DR D−7 were older and had a poorer ECOG PS, higher tumor burden (MTV and LDH), and increased systemic inflammation (CRP and IL-6). Previous studies have shown that elevated MTV and LDH and increased CRP are key prognostic factors in CAR T-cell therapy.4-6,12,15,16 However, mHLA-DR D−7 prevailed over these markers in the MVA for OS, suggesting that mHLA-DR downregulation has a proper immunological role and that it is not purely a correlate of more aggressive disease features.
Before the conditioning regimen, we observed that both the proinflammatory cytokines IL-6 and TNFα and the immunosuppressive cytokine IL-10 correlated negatively with mHLA-DR. These data are in accordance with the emerging literature suggesting that the tumor-induced inflammatory environment influences the development and phenotype of monocytes toward protumor and immunosuppressive activities that coincide with the downregulation of HLA-DR.48,53,54 As for the immunosuppressive cytokine IL-10, Xiu et al demonstrated that IL-10 is increased in the serum of patients with B-cell non-Hodgkin lymphoma and that it induces the development of HLA-DRlow monocytes with immunosuppressive effects.55 Interestingly, Lech-Maranda et al showed that the elevation of IL-10 and TNFα is associated with a lower probability of achieving CR and a shorter PFS and OS in a cohort of 106 patients with DLBCL treated with an anthracycline-based regimen.56
Interestingly, we observed that the Cy-Flu LD conditioning regimen led to a significant increase in mHLA-DR, along with increased plasma IFNγ and IL-2 and that patients with a higher increase in mHLA-DR after LD had higher lymphocyte counts at D7. Importantly, patients without an increase in mHLA-DR after LD had with poorer survival.
LD therapy is a well-known inducer of compensatory proliferation of T cells,57-61 and our data suggest a more efficient homeostatic proliferation, and therefore CAR T-cell expansion, in patients with a higher increase in mHLA-DR after LD. This beneficial effect of LD may be mediated, at least in part, by the eradication of suppressive M-MDSCs, as recently reported by Neelapu, leading to increased expansion, function, and persistence of CAR T cells.62 These data suggest that LD may play a key role in depleting suppressive myeloid cells to favor CAR T-cell activation and expansion.
In this study, mHLA-DR peaked at postinfusion D7 and mHLA-DR positively correlated with IFNγ and TNFα levels at that same time. Indeed, it is now well established that, upon target recognition, activated CAR T cells engage monocytes and macrophages in a contact-dependent manner and through cytokine production (IFNγ, TNFα, and GM-CSF), and those cells in turn secrete inflammatory mediators, among which are IL-6 and IL-1, thus exacerbating the cytokine storm.15,16,18 In 12% of the patients, mHLA-DR D7 peaked above 100 000 Ab/c. Interestingly, such elevated values have been observed only in hemophagocytic lymphohistiocytosis so far.63 Both CRS and hemophagocytic lymphohistiocytosis share similar hyperinflammatory conditions with uncontrolled macrophage activation, but it remains unclear how to separate these 2 entities from a treatment perspective.18
Regarding cell-related toxicity, patients with low mHLA-DR D−7 received more often tocilizumab than the others. Earlier use of mitigation strategy with tocilizumab and/or steroids probably explains why no raw difference was observed in terms of CRS/ICANS toxicity between low- or high-mHLA-DR groups. Whether this reflected only the association between low mHLA-DR and adverse prognostic factors (higher MTV or elevated LDH or CRP) or confirms a pathophysiological link between HLA-DR expression on monocytes and CRS or ICANS remains unknown.18 Deng et al recently demonstrated, using single-cell analysis, that a rare cell population found in the infusion product and with monocytelike transcriptional features was associated with high-grade ICANS.13 As for the association between ICANS and a significant decrease in mHLA-DR D14, we hypothesized that this could be in part explained by the use of steroids in 93% of the patients experiencing ICANS, as steroids were also associated with a significant mHLA-DR decrease at D14. Several studies have shown that steroids can negatively regulate HLA-DR transcription by decreasing MHC class II mRNA levels, which may therefore modify monocyte-CAR T-cell interactions.56
In this retrospective study, we provided a comprehensive characterization of mHLA-DR evolution during CAR T-cell procedures and correlated mHLA-DR values with clinical, radiological, and biological data. We described that monocyte dysregulation, measured through the downregulation of mHLA-DR pre-LD, correlated with inflammatory and immunosuppressive tumor environment and was associated with shorter response duration and adverse outcomes. Conversely, LD led to an increase in mHLA-DR in most patients, that was associated with higher lymphocytes proliferation at D7, whereas decreased expression led to poorer outcome.
Altogether, our findings may provide novel insights in some of the mechanisms of CAR T-cell failure. We suggest that downregulation of mHLA-DR corresponds to an excess of M-MDSCs that is found in patients with higher tumor burden and chronic inflammatory state, which in part reflects the severity of the disease, but also underlies immune dysfunction that may contribute to CAR failure.
Better understanding of resistance mechanisms to CAR therapy may pave the way for myeloid cell modulation in vivo or in the leukapheresis product.64 Stroncek and colleagues demonstrated that higher proportions of myeloid cells in the peripheral blood mononuclear cell concentrates are associated with poorer T-cell expansion.20 More recently, Noaks et al showed that monocyte depletion from the leukapheresis product improves the level of T-cell activation, transduction efficacy, and the level of CAR expression, but also CAR T-cell cytotoxicity in a more resting and naive state.19 Similar results came from Wang and colleagues who demonstrated that depletion of high-content CD14+ cells from apheresis products (cutoff set at 40% of peripheral blood mononuclear cells) is critical for successful transduction and expansion of CAR T cells during a large-scale, good manufacturing process.21
Finally, mHLA-DR appears as an interesting composite biomarker, that reflect not only excess of M-MDSCs in its very low values, but also monocyte activation. mHLA-DR measurement has recently been standardized with regard to flow cytometric gating methods, allowing for easier routine use.44,52,53,65
There are limitations in our findings that are related to the monocentric and retrospective nature of this study, the limited number of patients with low mHLA-DR D−7, and the lack of correlation with anti-CD19 CAR T-cell expansion.
In summary, our observations support that dysregulation of monocytes, characterized by downregulation of HLA-DR, correlates with an inflammatory and immunosuppressive tumor environment and is associated with the failure of anti-CD19 CAR T cells in patients with R/R LBCL. Modulation of these myeloid cells may represent a promising way to improve CAR therapy.
Acknowledgments
The authors thank all patients, families, and caregivers who participated in the study.
The study was supported by Hospices Civils de Lyon.
Authorship
Contribution: E. Bourbon, P.S., F.V., G.M., and E. Bachy contributed to the conception and design of the work; E. Bourbon, P.S., E. Bachy, and F.V. wrote the paper; F.V., G.M., and M.G. performed the biological analysis of the data; all authors contributed to data collection; and all authors contributed to drafting and revising the manuscript and approved the final version.
Conflict-of-interest disclosure: P.S. received honoraria from and served in advisory and consultancy roles for Janssen, Roche, Novartis and Kite/Gilead. E.F. received honoraria from and served in advisory and consultancy roles for Kite/Gilead, Janssen, and AbbVie. V.S. received honoraria from Roche. F.W. received honoraria from and served in advisory and consultancy roles for Novartis and Kite/Gilead. E. Bachy received honoraria from and served as a consultant for Gilead, Novartis, Roche, Amgen, Janssen, Sanofi, and AbbVie. H. Ghesquières received honoraria from and served in advisory and consultancy roles for Gilead Sciences, Celgene, Roche, Janssen, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Emmanuel Bachy, Hematology Department, Lyon Sud Hospital, Rue du Grand Revoyet, 69310 Pierre-Bénite, France; e-mail: emmanuel.bachy@chu-lyon.fr.
References
Author notes
Data are not publicly available according to the Hospices Civils de Lyon’s strict institutional policy with regard to public availability of unidentified patient data. However, those data that are minimally required to replicate the outcomes of the study will be made available on reasonable request from the corresponding author (emmanuel.bachy@chu-lyon.fr).
The full-text version of this article contains a data supplement.
E. Bourbon and P.S. contributed equally to this study.
E. Bachy and F.B. contributed equally to this study.