Key Points
Controversy exists as to whether HU use is associated with an increased risk of SM in patients with MPN.
Our findings suggest that HU does not increase the risk of SM, including AML/MDS, in this older patient population.
Abstract
Patients with classical Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs), including polycythemia vera (PV), essential thrombocythemia (ET), and primary and secondary myelofibrosis (MF), are known to have an increased risk of second malignancies (SMs). Hydroxyurea (HU) is a guideline-recommended cytoreductive therapy for patients at high risk for MPNs. Controversy exists as to whether HU use is associated with a higher risk of SMs, including acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). We conducted a retrospective cohort study of older patients diagnosed with MPN (age ≥66 years) between 2010 and 2017 and included the data in the Surveillance, Epidemiology, and End Results Medicare-linked database. Multivariable competing risk analyses adjusting for patient characteristics were used to assess the impact of HU on the development of SM. We identified 4023 patients (1688 with PV, 1976 with ET, and 359 with MF) with a median age of 77 (interquartile range [IQR], 71-83) years at the time of MPN diagnosis. After a median follow-up of 3.25 (IQR, 2.10-5.00) years, 489 patients developed an SM (346 solid, 73 lymphoid, and 70 myeloid malignancies). The cumulative incidence probability of SM was 19.88% (95% confidence interval [CI], 17.16%-22.75%) among 2683 HU users and 22.31% (95% CI, 17.51%-27.47%) among 1340 nonusers, respectively (Gray’s test, P < .01). We did not identify significant differences in the incidence of solid or hematologic SMs, including AML/MDS (hazard ratio, 1.33; 95% CI, 0.77-2.29; P = .30), between HU users and nonusers. Our results suggest that the use of HU does not increase the risk of SM in older patients with MPN.
Introduction
The classical Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs) include polycythemia vera (PV), essential thrombocythemia (ET), as well as primary and secondary myelofibrosis (MF).1,2 The median age at diagnosis is 65 to 70 years.3 In addition to phenotypic heterogeneity, MPNs have a different propensity to transform into acute myeloid leukemia (AML), which develops in 1% to 20% of patients during the first 10 years after diagnosis.4,5 Patients with MPNs also bear an increased risk of lymphoproliferative disorders6-8 and solid cancers.9-15 The incidence of these second malignancies (SMs) among patients with MPNs also increases with age.13 However, the etiology of SMs in many of these circumstances remains unclear.
Hydroxyurea (HU), an antimetabolite and nonalkylating myelosuppressive agent, is the most common cytoreductive therapy employed in the management of MPNs. Both the NCCN (National Comprehensive Cancer Network) and the ELN (European LeukemiaNet) guidelines recommend HU as frontline cytoreductive therapy for patients with high-risk ET and PV, as well as hyperproliferative MF.16-18 HU not only reduces the incidence of thrombosis but also improves survival among older patients with ET and PV.19,20 As HU interferes with DNA synthesis, it may have mutagenic and leukemogenic potential, especially among patients with MPNs who already have an inherent risk of leukemic transformation.21 However, there are inconsistent data determining the impact of HU exposure on the risk of SMs, including AML and myelodysplastic syndrome (MDS). Several prospective studies and large registry-based analyses have observed no increased risk of AML development with MPN-directed HU therapy, although they did find an association between patient exposure to radioactive phosphorus (32P) and alkylators and secondary AML.22-24 Conversely, a French study published in 1997 found an increased risk of leukemia transformation among patients with PV treated with 32P and HU maintenance when compared with patients using 32P alone.25 An additional single-center Danish study that used logistic regression analysis found that patients with MPNs treated with HU have a higher risk of SM when compared with patients treated with interferon (IFN)-α2.26 Lastly, HU use has been linked to an increased risk of nonmelanoma skin cancers.15,27-29
Population-based studies evaluating the relationship of HU and SM in patients with MPNs are limited overall and are lacking in the United States. Our study aimed to evaluate the incidence of hematologic and solid SMs as well as the effect of HU on the development of SMs among a large, contemporarily treated group of older patients with MPNs.
Methods
This study leveraged the SEER (Surveillance, Epidemiology, and End Results) Medicare-linked database, which was developed by the National Cancer Institute and the Centers for Medicare and Medicaid Services. This database links patient-level information on incident cancer diagnoses from SEER registries to a master file of Medicare enrollment and claims for inpatient, outpatient, physician services, and prescription drugs. The SEER registries have been shown to be nationally representative and account for approximately 34.6% of the US population.30 Since 2001, SEER registries have been required to report MPN, providing a unique opportunity to access a population-based sample of patients with MPN with an unprecedented size. The Yale Human Investigation Committee determined that this study did not directly involve human subjects.
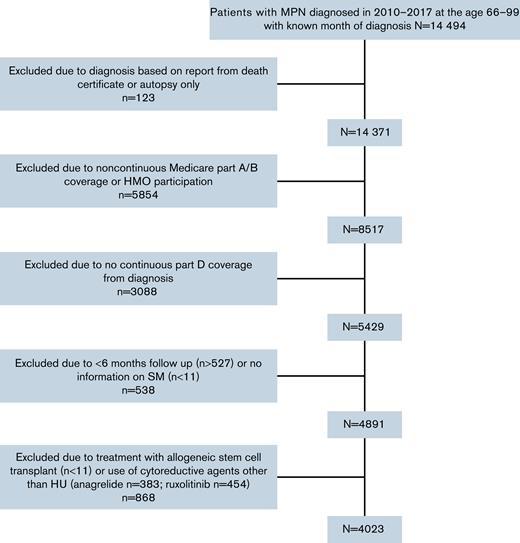
We used the ICD (International Classification of Diseases) for Oncology, third edition (codes 9950, 9961, and 9962) to assemble a retrospective cohort of patients diagnosed with incident MPN between 2010 and 2017. All patients fulfilled the following eligibility criteria: (1) aged 66 to 99 years at diagnosis, (2) had known month of diagnosis, (3) were not reported from autopsy or death certificate only, (4) had continuous Medicare fee-for-service coverage (Parts A and B) and were not enrolled in health maintenance organizations (HMOs) from 12 months before diagnosis to the end of follow-up, and (5) were continuously enrolled in Medicare Part D from diagnosis to the end of study (to enable the assessment of HU use). Patients were excluded if: (1) followed for <6 months, (2) developed an SM within 6 months after diagnosis, or (3) treated with any cytoreductive agent other than HU (including ruxolitinib, busulfan, chlorambucil, and IFNs) or allogeneic stem cell transplant during follow-up. Patients were followed from the diagnosis of MPN through the diagnosis of an SM, death, ending of Medicare coverage, or end of study (31 December 2019), whichever came first.
HU usage after diagnosis was obtained via Medicare Part D claims, and a HU use diary was constructed. To avoid reverse causality, we removed prescriptions in the 6-month period before the occurrence of the second cancer or the end of follow-up. HU proportion of days covered (PDC) was calculated as the ratio of the number of days the patient was covered by HU to the number of days from HU initiation to 6 months before the end of follow-up. In our analysis, a patient who never received HU would have a PDC of 0%, and a patient who had taken HU every day from treatment initiation through 6 months before the end of follow-up would have a PDC of 100%.
Our outcome of interest was first SM (ie, any new malignancy other than MPN and nonmelanoma skin cancer diagnosed ≥6 months after cohort entry. As SEER cancer information was only available up to the end of 2017, we identified SM using SEER data for the years 2010-2017 and Medicare claims for the years 2018-2019. The SM was defined as the first reported malignancy after MPN diagnosis. We used ICD-10 Clinical Modification Codes for a primary malignancy other than nonmelanoma skin cancer and MPN to identify SM in Medicare claims. To be ascertained as an SM, we further required patients to have 1 inpatient claim or 2 outpatient claims ≥30 days apart. As done in previous analyses identifying new myeloid neoplasms in claims,31 we also required patients to have a bone marrow aspirate or biopsy within 60 days before or after the initial myeloid malignancy diagnosis, and ≥1 claim with a myeloid malignancy diagnosis after the bone marrow claim. The date of the first qualifying SM claim was assigned as the SM diagnosis date. We required a 12-month period before this date without any similar claims to ensure SM was newly diagnosed. We categorized an SM as either a solid or hematological (lymphoid and myeloid) malignancy.
We obtained information on the following patient characteristics: age at diagnosis, sex, race, marital status, SEER region, Elixhauser score for comorbidities,32 cancer history, disability status,33 state buy-in, and census tract Yost index.34,35 The Yost score is a composite index of socioeconomic status based on principal component analysis from variables measuring different socioeconomic status aspects, such as education, income, and occupation, of a census tract.34 High Yost scores indicate high neighborhood socioeconomic status. To construct the Elixhauser score, we searched for ICD-9 and ICD-10 diagnosis codes in the 12 months before MPN diagnosis that appeared on any inpatient claims or ≥2 outpatient and/or physician claims >30 days apart.36 Since performance status is an important factor in clinical decision-making and survival outcomes, we evaluated each patient’s disability status as a claims-based proxy of poor performance status before diagnosis.33 State buy-in was a proxy for individual socioeconomic status.
Patient characteristics were compared between HU users and nonusers using Pearson’s χ2 tests. Medians and interquartile ranges (IQRs) were calculated for continuous variables. Consistent with the SEER–Medicare requirement to preserve confidentiality, the smallest groupings were reported as <11. In this study, death and development of an SM were considered competing events. The cumulative incidence function of SM was computed via a competing risk model. Comparisons of cumulative incidence between treatment groups were performed using Gray’s test.37 Multivariable competing risk regression models were developed using the Fine and Gray method to estimate the adjusted hazard ratios (HRs) for SM. We first analyzed HU as a binary variable, then as a continuous variable by every 10% PDC. HU use was investigated as a time-varying covariate. Patients were initially considered nonusers and then users thereafter for the remainder of follow-up. Type of MPN, age at MPN diagnosis, sex, race, marital status, SEER region, Elixhauser comorbidity score, previous cancer, disability status, state buy-in, and census tract Yost index (in quintile) were adjusted for in the multivariable model. We further assessed the association of HU use with a specific subtype of SM (ie, solid, lymphoid, and myeloid malignancies). For each specific subtype of SM, besides death, other types of SM were also considered as competing events. Sensitivity analyses were conducted by further restricting HU users to have used HU for ≥3 and 6 months, and patients had been followed for 9 and 12 months, respectively. All analyses were 2-sided with a type I error of 0.05 to achieve statistical significance and were conducted with SAS Version 9.4 (SAS Inc., Cary, NC).
Results
The final cohort included 4023 patients (1688 with PV, 1976 with ET, and 359 with MF). A total of 868 patients were excluded because of treatment with non-HU cytoreductive treatments, including 454 and 383 patients receiving ruxolitinib and anagrelide, respectively, or hematopoietic stem cell transplant (Figure 1). The median age at MPN diagnosis was 77 (IQR, 71-83) years. Among these 4023 patients with MPN, 2683 (66.7%) used HU after diagnosis with a median PDC of 83.5% (IQR, 65.9%-95.3%). When compared with patients who did not receive HU, those who used HU were more likely to be older at diagnosis, female, and have state buy-in (Table 1). In addition, more HU nonusers (53.0%) had ≥3 comorbid conditions than HU users (44.7%; P < .01). The study followed patients for up to 10.00 (median, 3.25; IQR, 2.10-5.00) years and 1168 (29.0%; 674 HU users and 494 nonusers) patients died during follow-up. The median follow-up time was longer among HU users (median, 3.58 years; IQR, 2.42-5.33) than that among HU nonusers (median, 2.58 years; IQR, 1.38-4.42).
Characteristics of 4023 patients with MPN, 2010-2017
| . | Overall, n (%) . | Hydroxyurea, n (%) . | ||
|---|---|---|---|---|
| Yes . | No . | P . | ||
| Total | 4023 | 2683 | 1340 | — |
| Type of MPN | ||||
| PV | 1688 (42.0) | 1017 (37.9) | 671 (50.1) | <.01 |
| ET | 1976 (49.1) | 1548 (57.7) | 428 (31.9) | — |
| MF | 359 (8.9) | 118 (4.4) | 241 (18.0) | — |
| Age, y | ||||
| Median (IQR) | 77 (71-83) | 76 (71-83) | 77 (72-83) | — |
| 66-69 | 683 (17.0) | 412 (15.4) | 271 (20.2) | <.01 |
| 70-74 | 927 (23.0) | 601 (22.4) | 326 (24.3) | — |
| 75-79 | 845 (21.0) | 594 (22.1) | 251 (18.7) | — |
| 80-84 | 769 (19.1) | 536 (20.0) | 233 (17.4) | — |
| ≥85 | 799 (19.9) | 540 (20.1) | 259 (19.3) | — |
| Sex | ||||
| Female | 2468 (61.3) | 1771 (66.0) | 697 (52.0) | <.01 |
| Male | 1555 (38.7) | 912 (34.0) | 643 (48.0) | — |
| Race | ||||
| White | 3469 (86.2) | 2323 (86.6) | 1146 (85.5) | .36 |
| Other | 554 (13.8) | 360 (13.4) | 194 (14.5) | — |
| Marital status | ||||
| Single | 1341 (33.3) | 897 (33.4) | 444 (33.1) | .26 |
| Married | 2362 (58.7) | 1560 (58.1) | 802 (59.9) | — |
| Unknown | 320 (8.0) | 226 (8.4) | 94 (7.0) | — |
| Region | ||||
| Northeast | 1794 (44.6) | 1168 (43.5) | 626 (46.7) | <.01 |
| Midwest | 370 (9.2) | 275 (10.2) | 95 (7.1) | — |
| South | 753 (18.7) | 468 (17.4) | 285 (21.3) | — |
| West | 1106 (27.5) | 772 (28.8) | 334 (24.9) | — |
| Elixhauser Comorbidity Index | ||||
| 0 | 506 (12.6) | 344 (12.8) | 162 (12.1) | <.01 |
| 1-2 | 1609 (40.0) | 1141 (42.5) | 468 (34.9) | — |
| ≥3 | 1908 (47.4) | 1198 (44.7) | 710 (53.0) | — |
| Previous cancer | ||||
| No | 3059 (76.0) | 2046 (76.3) | 1013 (75.6) | .64 |
| Yes | 964 (24.0) | 637 (23.7) | 327 (24.4) | — |
| Disability | ||||
| No | 3563 (88.6) | 2392 (89.2) | 1171 (87.4) | .10 |
| Yes | 460 (11.4) | 291 (10.8) | 169 (12.6) | — |
| Yost index | ||||
| Fifth quintile (highest SES) | — | 899 (33.5) | 426 (31.8) | .22 |
| Fourth quintile | 1325 (32.9) | 551 (20.5) | 277 (20.7) | — |
| Third quintile | 828 (20.6) | 424 (15.8) | 223 (16.6) | — |
| Second quintile | 647 (16.1) | 418 (15.6) | 200 (14.9) | — |
| First quintile (lowest SES) | 618 (15.4) | 267 (10.0) | 164 (12.2) | — |
| Unknown | 431 (10.7) | 124 (4.6) | 50 (3.7) | — |
| State buy-in | ||||
| No | 3355 (83.4) | 2263 (84.3) | 1092 (81.5) | .02 |
| Yes | 668 (16.6) | 420 (15.7) | 248 (18.5) | — |
| . | Overall, n (%) . | Hydroxyurea, n (%) . | ||
|---|---|---|---|---|
| Yes . | No . | P . | ||
| Total | 4023 | 2683 | 1340 | — |
| Type of MPN | ||||
| PV | 1688 (42.0) | 1017 (37.9) | 671 (50.1) | <.01 |
| ET | 1976 (49.1) | 1548 (57.7) | 428 (31.9) | — |
| MF | 359 (8.9) | 118 (4.4) | 241 (18.0) | — |
| Age, y | ||||
| Median (IQR) | 77 (71-83) | 76 (71-83) | 77 (72-83) | — |
| 66-69 | 683 (17.0) | 412 (15.4) | 271 (20.2) | <.01 |
| 70-74 | 927 (23.0) | 601 (22.4) | 326 (24.3) | — |
| 75-79 | 845 (21.0) | 594 (22.1) | 251 (18.7) | — |
| 80-84 | 769 (19.1) | 536 (20.0) | 233 (17.4) | — |
| ≥85 | 799 (19.9) | 540 (20.1) | 259 (19.3) | — |
| Sex | ||||
| Female | 2468 (61.3) | 1771 (66.0) | 697 (52.0) | <.01 |
| Male | 1555 (38.7) | 912 (34.0) | 643 (48.0) | — |
| Race | ||||
| White | 3469 (86.2) | 2323 (86.6) | 1146 (85.5) | .36 |
| Other | 554 (13.8) | 360 (13.4) | 194 (14.5) | — |
| Marital status | ||||
| Single | 1341 (33.3) | 897 (33.4) | 444 (33.1) | .26 |
| Married | 2362 (58.7) | 1560 (58.1) | 802 (59.9) | — |
| Unknown | 320 (8.0) | 226 (8.4) | 94 (7.0) | — |
| Region | ||||
| Northeast | 1794 (44.6) | 1168 (43.5) | 626 (46.7) | <.01 |
| Midwest | 370 (9.2) | 275 (10.2) | 95 (7.1) | — |
| South | 753 (18.7) | 468 (17.4) | 285 (21.3) | — |
| West | 1106 (27.5) | 772 (28.8) | 334 (24.9) | — |
| Elixhauser Comorbidity Index | ||||
| 0 | 506 (12.6) | 344 (12.8) | 162 (12.1) | <.01 |
| 1-2 | 1609 (40.0) | 1141 (42.5) | 468 (34.9) | — |
| ≥3 | 1908 (47.4) | 1198 (44.7) | 710 (53.0) | — |
| Previous cancer | ||||
| No | 3059 (76.0) | 2046 (76.3) | 1013 (75.6) | .64 |
| Yes | 964 (24.0) | 637 (23.7) | 327 (24.4) | — |
| Disability | ||||
| No | 3563 (88.6) | 2392 (89.2) | 1171 (87.4) | .10 |
| Yes | 460 (11.4) | 291 (10.8) | 169 (12.6) | — |
| Yost index | ||||
| Fifth quintile (highest SES) | — | 899 (33.5) | 426 (31.8) | .22 |
| Fourth quintile | 1325 (32.9) | 551 (20.5) | 277 (20.7) | — |
| Third quintile | 828 (20.6) | 424 (15.8) | 223 (16.6) | — |
| Second quintile | 647 (16.1) | 418 (15.6) | 200 (14.9) | — |
| First quintile (lowest SES) | 618 (15.4) | 267 (10.0) | 164 (12.2) | — |
| Unknown | 431 (10.7) | 124 (4.6) | 50 (3.7) | — |
| State buy-in | ||||
| No | 3355 (83.4) | 2263 (84.3) | 1092 (81.5) | .02 |
| Yes | 668 (16.6) | 420 (15.7) | 248 (18.5) | — |
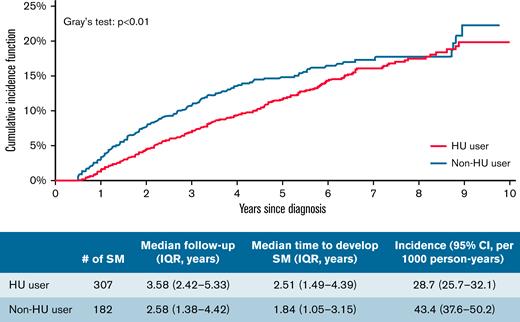
A total of 489 (12.2%) patients (197 with PV, 242 with ET, and 50 with MF) developed an SM (solid: 346; hematologic: 143). The median time to develop an SM was 2.51 (IQR, 1.49-4.39) years among 307 HU users and 1.84 (IQR, 1.05-3.15) years among 182 patients who never received HU. The cumulative incidence probability of SM was 19.88% (95% confidence interval [CI], 17.16%-22.75%) and 22.31% (95% CI, 17.51%-27.47%) for HU users and nonusers, respectively, which was significantly different (Gray’s test, P < .01) (Figure 2). However, in the multivariable competing risk model, after adjusting for sociodemographic characteristics and comorbidities, the risk of all SM was not associated with ever receiving HU (HR, 0.99; 95% CI, 0.82-1.20; P = .92) nor HU PDC (every 10% PDC: HR, 1.01; 95% CI, 0.99-1.03; P = .43) (Table 2).
Cumulative incidence function of SMs among patients with MPN by HU use.
HRs and 95% CIs from multivariable competing risk regression models for SMs among 4023 patients with MPN
| . | Model 1 (HU Yes/No) . | Model 2 (HU PDC) . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Hydroxyurea | ||||
| No | 1.00 | — | — | — |
| Yes | 0.99 (0.82-1.20) | .92 | — | — |
| PDC (per 10%) | — | — | 1.01 (0.99-1.03) | .43 |
| Type of MPN | ||||
| PV | 1.00 | — | 1.00 | — |
| ET | 1.15 (0.94-1.39) | .17 | 1.13 (0.93-1.37) | .22 |
| MF | 1.22 (0.88-1.68) | .23 | 1.24 (0.90-1.71) | .19 |
| Age, y | ||||
| 66-69 | 1.00 | — | 1.00 | — |
| 70-74 | 0.99 (0.76-1.30) | .95 | 0.99 (0.76-1.30) | .95 |
| 75-79 | 0.92 (0.70-1.21) | .56 | 0.91 (0.69-1.21) | .53 |
| 80-84 | 0.76 (0.57-1.03) | .07 | 0.76 (0.56-1.02) | .07 |
| ≥85 | 0.63 (0.46-0.86) | <.01 | 0.63 (0.45-0.86) | <.01 |
| Sex | ||||
| Female | 1.00 | — | 1.00 | — |
| Male | 1.23 (1.01-1.48) | .04 | 1.23 (1.02-1.49) | .03 |
| Race | ||||
| White | 1.00 | — | 1.00 | — |
| Other | 0.76 (0.56-1.03) | .08 | 0.76 (0.56-1.03) | .08 |
| Marital status | ||||
| Single | 1.00 | — | 1.00 | — |
| Married | 0.94 (0.76-1.17) | .60 | 0.95 (0.76-1.18) | .63 |
| Unknown | 1.20 (0.86-1.66) | .28 | 1.20 (0.86-1.66) | .28 |
| Region | ||||
| Northeast | 1.00 | — | 1.00 | — |
| Midwest | 1.13 (0.81-1.56) | .48 | 1.12 (0.80-1.55) | .51 |
| South | 1.03 (0.77-1.38) | .85 | 1.03 (0.77-1.38) | .84 |
| West | 1.06 (0.84-1.34) | .61 | 1.06 (0.84-1.34) | .64 |
| Elixhauser Comorbidity Index | ||||
| 0 | 1.00 | — | 1.00 | — |
| 1-2 | 1.05 (0.79-1.41) | .74 | 1.05 (0.79-1.41) | .73 |
| ≥3 | 1.12 (0.84-1.50) | .43 | 1.13 (0.84-1.51) | .41 |
| Previous cancer | ||||
| No | 1.00 | — | 1.00 | — |
| Yes | 1.20 (0.98-1.47) | .08 | 1.20 (0.98-1.47) | .08 |
| Disability | ||||
| No | 1.00 | — | 1.00 | — |
| Yes | 0.57 (0.39-0.83) | <.01 | 0.57 (0.39-0.84) | <.01 |
| Census tract Yost index | ||||
| Fifth quintile (highest SES) | 1.00 | — | 1.00 | — |
| Fourth quintile | 0.79 (0.61-1.02) | .08 | 0.79 (0.61-1.02) | .08 |
| Third quintile | 0.81 (0.61-1.08) | .15 | 0.81 (0.61-1.08) | .15 |
| Second quintile | 0.91 (0.69-1.20) | .51 | 0.91 (0.69-1.20) | .50 |
| First quintile (lowest SES) | 0.88 (0.61-1.26) | .48 | 0.88 (0.61-1.27) | .49 |
| Unknown | 1.06 (0.68-1.66) | .79 | 1.06 (0.68-1.65) | .81 |
| State buy-in | ||||
| No | 1.00 | — | 1.00 | — |
| Yes | 0.96 (0.72-1.27) | .77 | 0.96 (0.72-1.28) | .78 |
| . | Model 1 (HU Yes/No) . | Model 2 (HU PDC) . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Hydroxyurea | ||||
| No | 1.00 | — | — | — |
| Yes | 0.99 (0.82-1.20) | .92 | — | — |
| PDC (per 10%) | — | — | 1.01 (0.99-1.03) | .43 |
| Type of MPN | ||||
| PV | 1.00 | — | 1.00 | — |
| ET | 1.15 (0.94-1.39) | .17 | 1.13 (0.93-1.37) | .22 |
| MF | 1.22 (0.88-1.68) | .23 | 1.24 (0.90-1.71) | .19 |
| Age, y | ||||
| 66-69 | 1.00 | — | 1.00 | — |
| 70-74 | 0.99 (0.76-1.30) | .95 | 0.99 (0.76-1.30) | .95 |
| 75-79 | 0.92 (0.70-1.21) | .56 | 0.91 (0.69-1.21) | .53 |
| 80-84 | 0.76 (0.57-1.03) | .07 | 0.76 (0.56-1.02) | .07 |
| ≥85 | 0.63 (0.46-0.86) | <.01 | 0.63 (0.45-0.86) | <.01 |
| Sex | ||||
| Female | 1.00 | — | 1.00 | — |
| Male | 1.23 (1.01-1.48) | .04 | 1.23 (1.02-1.49) | .03 |
| Race | ||||
| White | 1.00 | — | 1.00 | — |
| Other | 0.76 (0.56-1.03) | .08 | 0.76 (0.56-1.03) | .08 |
| Marital status | ||||
| Single | 1.00 | — | 1.00 | — |
| Married | 0.94 (0.76-1.17) | .60 | 0.95 (0.76-1.18) | .63 |
| Unknown | 1.20 (0.86-1.66) | .28 | 1.20 (0.86-1.66) | .28 |
| Region | ||||
| Northeast | 1.00 | — | 1.00 | — |
| Midwest | 1.13 (0.81-1.56) | .48 | 1.12 (0.80-1.55) | .51 |
| South | 1.03 (0.77-1.38) | .85 | 1.03 (0.77-1.38) | .84 |
| West | 1.06 (0.84-1.34) | .61 | 1.06 (0.84-1.34) | .64 |
| Elixhauser Comorbidity Index | ||||
| 0 | 1.00 | — | 1.00 | — |
| 1-2 | 1.05 (0.79-1.41) | .74 | 1.05 (0.79-1.41) | .73 |
| ≥3 | 1.12 (0.84-1.50) | .43 | 1.13 (0.84-1.51) | .41 |
| Previous cancer | ||||
| No | 1.00 | — | 1.00 | — |
| Yes | 1.20 (0.98-1.47) | .08 | 1.20 (0.98-1.47) | .08 |
| Disability | ||||
| No | 1.00 | — | 1.00 | — |
| Yes | 0.57 (0.39-0.83) | <.01 | 0.57 (0.39-0.84) | <.01 |
| Census tract Yost index | ||||
| Fifth quintile (highest SES) | 1.00 | — | 1.00 | — |
| Fourth quintile | 0.79 (0.61-1.02) | .08 | 0.79 (0.61-1.02) | .08 |
| Third quintile | 0.81 (0.61-1.08) | .15 | 0.81 (0.61-1.08) | .15 |
| Second quintile | 0.91 (0.69-1.20) | .51 | 0.91 (0.69-1.20) | .50 |
| First quintile (lowest SES) | 0.88 (0.61-1.26) | .48 | 0.88 (0.61-1.27) | .49 |
| Unknown | 1.06 (0.68-1.66) | .79 | 1.06 (0.68-1.65) | .81 |
| State buy-in | ||||
| No | 1.00 | — | 1.00 | — |
| Yes | 0.96 (0.72-1.27) | .77 | 0.96 (0.72-1.28) | .78 |
All variables in the table were mutually adjusted in the model.
PDC, proportion of drug coverage.
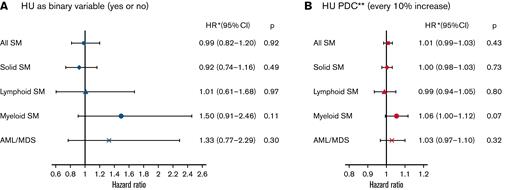
A total of 346 (8.6%) patients developed a solid SM, with 218 having ever received HU. The 3 most common types of solid SM in our analysis were lung and bronchus cancer (n = 72), breast cancer (n = 49), and melanoma (n = 40). The cumulative incidence probability of solid SM was lower among HU users (14.95%; 95% CI, 12.42%-17.70%) than that of nonusers (15.25%; 95% CI, 11.38%-19.65%; Gray’s test, P = .03) (Figure 3). In the multivariable competing risk model, the risk of developing a solid SM was influenced neither by HU ever use (HR, 0.92; 95% CI, 0.74-1.16; P = .49) nor HU PDC (every 10% increase: HR, 1.00; 95% CI, 0.98-1.03; P = .73) (Figure 4).
Cumulative incidence function of SMs among patients with MPN by type and HU use.
Cumulative incidence function of SMs among patients with MPN by type and HU use.
Adjusted HRs and 95% CIs of HU use and types of SMs among 4023 patients with MPN. ∗Adjusted for MPN type, age at MPN diagnosis, sex, race, Elixhauser comorbidity score, marital status, zip code level median household income, eligibility for dual insurance, and SEER region. ∗∗PDC: proportion of days covered with HU prescriptions
Adjusted HRs and 95% CIs of HU use and types of SMs among 4023 patients with MPN. ∗Adjusted for MPN type, age at MPN diagnosis, sex, race, Elixhauser comorbidity score, marital status, zip code level median household income, eligibility for dual insurance, and SEER region. ∗∗PDC: proportion of days covered with HU prescriptions
Only 73 (1.8%) patients developed a lymphoid hematologic SM after MPN diagnosis. Among them, 31 were diagnosed with non-Hodgkin lymphomas. The cumulative incidence probability of lymphoid hematologic SM was 2.51% (95% CI, 1.78%-3.45%) and 4.69% (95% CI, 2.30%-8.35%) for HU users and nonusers, respectively. Results from both Gray’s test (P = .10) (Figure 3) and the multivariable competing risk model (HU ever use: HR, 1.01; 95% CI, 0.61-1.68; P = .97; every 10% increase in HU PDC: HR, 0.99; 95% CI, 0.94-1.05; P = .80) (Figure 4) showed no difference between the 2 groups.
Among 70 (1.7%) patients who developed myeloid hematologic SM, 41 and 15 developed AML and MDS, respectively. The cumulative incidence of myeloid malignancies was 2.42% (95% CI, 1.75%-3.26%) and 2.36% (95% CI, 1.48%-3.58%) for HU users and nonusers, respectively (Gray’s test, P = .71) (Figure 3). In the multivariable competing risk model, the risk of myeloid hematologic SM did not differ by HU status (HU ever use HR, 1.50; 95% CI, 0.91-2.46; P = .11; every 10% increase in HU PDC: HR, 1.06; 95% CI, 1.00-1.12; P = .07) (Figure 4). The cumulative incidence probability of AML/MDS was 1.87% (95% CI, 1.30%-2.62%) and 1.99% (95% CI, 1.18%-3.16%) for HU users and nonusers; this was not significantly different based on Gray’s test (P = .57) (Figure 3) and the competing risk model (HU ever use: HR, 1.33; 95% CI, 0.77-2.29; P = .30; every 10% increase in HU PDC: HR, 1.03; 95% CI, 0.97-1.10; P = .32) (Figure 4).
Patients diagnosed with MPNs at age ≥85 years were less likely to develop any SM (HR, 0.63; 95% CI, 0.46-0.86; P < .01) (Table 2) and solid SM (HR, 0.66; 95% CI, 0.45-0.96; P = .03) when compared with patients diagnosed with MPNs at 66 to 69 years. Patients with disability were also less likely to develop any SM (HR, 0.57; 95% CI, 0.39-0.83; P < .01) and solid SM (HR, 0.60; 95% CI, 0.39-0.91; P = .02) than those without disability. However, males had a higher risk of any SM (HR, 1.23; 95% CI, 1.01-1.48; P = .04), lymphoid SM (HR, 1.90; 95% CI, 1.18-3.07; P < .01), and myeloid SM (HR, 1.95; 95% CI, 1.15-3.29; P = .01). When compared with patients residing in the highest socioeconomic status areas, patients in the fourth and third quintiles were less likely to develop solid SM (HR, 0.71; 95% CI, 0.52-0.97; P = .03) and lymphoid SM (HR, 0.40; 95% CI, 0.17-0.91; P = .03), respectively. Patients in the Midwest (HR, 3.68; 95% CI, 1.86-7.30; P < .01) and the West (HR, 2.22; 95% CI, 1.20-4.13; P < .01) were more likely to develop a myeloid SM when compared with patients in the Northeast. In addition, patients with MF were more likely to develop a myeloid SM when compared with patients with PV (HR, 3.48; 95% CI, 1.85-6.55; P < .01). Race, marital status, comorbidity score, and state buy-in did not appear to influence the development of solid, lymphoid, or myeloid SM (data not shown).
Sensitivity analysis
Among 3636 patients who had been followed for ≥9 months, 2412 patients used HU for >3 months. A total of 10.8% (n = 260) of HU users and 13.0% (n = 159) of nonusers developed an SM, respectively. When compared with nonusers, patients who used HU >3 months did not have a higher incidence of any SM or a specific SM subtype (data not shown). Among 3358 patients followed for ≥12 months, 222 out of 2225 (10.0%) HU users who received HU >6 months and 142 out of 1133 (12.5%) nonusers developed an SM ≥1 year after MPN diagnosis. There was no difference in the incidence of any SM or a specific SM subtype (solid or hematologic) among the 2 groups (data not shown).
Discussion
This large, comprehensive United States registry study followed 4023 older adults with MPN for up to 10 years, with 490 patients developing SM. The cumulative incidence probability of SM was 19.88% (95% CI, 17.16%-22.75%) among 2683 HU users and 22.31% (95% CI, 17.51%-27.47%) among 1340 nonusers. The use of HU did not impact the risk of developing SM overall, as well as solid and hematologic SM and AML/MDS in particular. In the multivariable competing risk model, the risk of myeloid hematologic SM did not differ by HU status (P = .09) and was based on every 10% increase in HU PDC (P = .07). The sensitivity analyses with more stringent observation length and HU exposure requirements, using patients followed for ≥9 months who received HU for >3 months and patients followed for ≥12 months who received HU for >6 months, also showed no difference in the incidence of any SM or a specific SM subtype (including myeloid hematologic SM) among HU users and nonusers with HU as a binary variable and as PDC.
In this study, 346 (8.6%) and 144 (3.6%) patients developed solid SM and hematologic SM, respectively, after a median follow-up of 3.25 years. These numbers are generally comparable to other studies, which have reported that 6.6% to 12.9% and 2.1% to 4.1% of patients develop a solid and hematologic SM after MPN diagnosis at a median follow-up ranging from 3.0 to 7.7 years.11,13-15 The median follow-up time was longer among HU users (median, 3.58 years; IQR, 2.42-5.33) than that among HU nonusers (median, 2.58 years; IQR, 1.38-4.42) as HU use was a time-dependent variable in our analysis to avoid bias.
A total of 79 (2.5%) patients developed AML/MDS in our study. This rate mirrors the previously reported 1% to 4% range of leukemic transformation rates among PV and ET patients at 10-year follow-up.5,11,13,14 Prior studies have offered conflicting data as to whether any specific MPN subtype is associated with a higher risk of solid SM, with some reporting similar risk between subtypes11,14 and others reporting a lower risk of solid SM for patients with primary MF.13,15 We also found, as expected, a higher risk of myeloid SM among patients with MF who are known for a higher risk of AML transformation when compared with other MPN subtypes.4,5
The most commonly observed solid SM in our study was lung and bronchus cancer, which is consistent with a prior SEER analysis.13 After excluding nonmelanoma skin cancers, lung cancer was also the most frequent SM in the Danish registry-based analysis by Frederiksen and colleagues.11 Prostate and breast cancer are consistently reported as the next most common SM in patients with MPN,14,15 as is the case in our study, which investigated older patients (median age of 77), when compared with other studies.
We found a similar cumulative incidence of SM, including solid and lymphoid SM, in HU-treated and untreated patients. This finding was consistent with the results from Barbui and colleagues and their recent nested case-control study of 1881 patients with MPN from the ELN database, which reported that patients treated with HU had a similar risk of SM as their counterparts treated without HU.15 However, in cancer-specific analysis, the study observed HU exposure was associated with a twofold higher risk of nonmelanoma skin cancers, which we were not able to study because of SEER limitations.15 This study did not report on the incidence of myeloid SM. We investigated hematologic SM and did not find any difference in cumulative incidence of AML/MDS among HU users and nonusers. Similarly, an analysis of the Swedish cancer registry showed that HU users did not have an increased risk of AML/MDS regardless of HU doses.24 Although the French Polycythemia Vera Study Group observed an increased risk of leukemia transformation among patients using 32P and HU maintenance compared with those using 32P alone,25 the Swedish study suggested that the risk of AML/MDS could be increased because of exposure to 32P rather than HU.24 To clarify the risk of SM in patients with MPN and evaluate the contribution of HU to this risk, our study excluded patients treated with alkylating agents (busulfan and chlorambucil) as well as ruxolitinib before the occurrence of SM.
While HU use was not found to be associated with SM, several covariates were strongly associated with the cadence of SM. Patients diagnosed with MPN at the age of ≥85 years, as well as patients with a disability, were less likely to develop any SM and solid SM when compared with those diagnosed at the age of 66 to 69 years and without disability, respectively, which may be because of an increase in the competing risk of death in these groups.
Among our study patients, 42% were diagnosed with PV. The recent approval of ropegIFN α-2b in Europe38 and the United States39 broadened the cytoreductive therapy options for patients with this disease. This prompted the development of the ELN 2021 expert opinion recommendations, which emphasized the preference for IFNs as cytoreductive therapy for younger patients with PV but affirmed that HU remains the preferred cytoreductive drug for individuals >60 years with PV.40 The recommendations specifically cite the observation that HU users are reported to have an increased risk of nonmelanoma skin cancers, which is not observed among IFN users, and favor IFN for younger patients because of overall lower SM risk but acknowledge that the quality of evidence behind this statement is low. Furthermore, ropegIFN α-2b at present is significantly more expensive than HU, and dedicated studies to evaluate the cost-effectiveness of one cytoreductive strategy vs another for patients with PV are needed.
This study has several strengths. We evaluated SM development among a large and current, real-world cohort of older US patients diagnosed with MPN between 2010 and 2017. The utilization of linked SEER–Medicare data allowed us to focus on HU use and evaluate any association with a higher risk of SM, including solid, lymphoid, and myeloid malignancies, which we ultimately did not detect. Limitations of our study include its applicability to only older patients with MPN, as the median age at MPN diagnosis in our cohort was 77 (IQR, 71-83) years. The French Polycythemia Study Group publications showed an increasing incidence of SM among HU users over time.25,41,42 Our study was subject to a relatively short duration of follow-up with a median follow-up time of 3.58 (IQR, 2.42-5.33) years among HU users, thus limiting the HU exposure period. However, Barbui and colleagues drew their conclusions based on a similar follow-up (median, 3.0 years).15 The Swedish registry data analysis had a longer duration of follow-up but reported a finding similar to ours: no significant increase in the risk of AML/MDS among HU users.24 Furthermore, the majority of patients who developed AML/MDS in that study did so within 5 years of MPN diagnosis.24 We conducted sensitivity analyses looking at the subgroups of patients with longer follow-up and HU exposure, including patients who had been followed for ≥9 months and used HU for >3 months, as well as patients who were followed for ≥12 months and received HU for >6 months. Both showed no difference in the incidence of any SM or a specific SM subtype (solid or hematologic) among HU users and nonusers.
Nonmelanoma skin cancers are the most common SM in patients with MPN, with increased incidence linked to hydroxyurea use.15,27-29 We could not identify these patients as SEER does not reliably record such neoplasms. Another limitation of this database is that it does not contain information on laboratory results, such as blood counts, pathology, or molecular test, including JAK2 V617F, CALR, and MPL mutation status, and does not allow us to confirm the SEER-reported MPN diagnosis, which is based on ICD codes. In addition, our findings may not be generalizable to other populations as we only included Medicare patients not participating in HMO plans. Finally, the observational nature of our study raises the possibility of confounding because of unobserved factors for which we could not account.
Although patients with MPNs are known to have a higher risk of SM when compared with the general population, our study demonstrated that HU use in older patients with MPN was not associated with an increased incidence of SM overall or AML/MDS, specifically, supporting HU as the preferred cytoreductive option for this patient population. However, a longer follow-up may be necessary to confirm these findings.
Acknowledgments
The authors acknowledge the efforts of the National Cancer Institute, the Office of Research, Development, and Information, Centers for Medicare and Medicaid Services, Information Management Services (IMS), Inc., and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
The study was supported by Frederick A. Deluca Foundation.
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885, the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute, and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement #U58DP003862-01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the author(s), and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
Authorship
Contribution: R.W., X.M., and N.A.P. conceived the study, with all other authors contributing to the study design; R.W. conducted statistical analyses with input from R.M.S., X.M., and N.A.P; R.W. and R.M.S. wrote the first draft of the manuscript, which was critically revised by all other authors; and all authors contributed to the interpretation of results.
Conflict-of-interest disclosure: R.M.S. participated in advisory boards for Bristol Myers Squibb and Gilead Sciences, Inc.; reports divested equity in a private or publicly traded company in the past 24 months at Curis. S.F.H. reports consultancy from Janssen, Pharmacyclics, AbbVie, AstraZeneca, Flatiron Health Inc., Novartis, SeaGen, Genetech, Merck, TG Therapeutics, ADC Therapeutics, Epizyme, Servier, Thyme Inc.; research funding from Celgene, DTRM Biopharm, TG Therapeutics; and honoraria form Pharmacyclics, AstraZeneca, and Bayer. A.M.Z. reports consultancy and research funding from AbbView, Acceleron, Amgen, Aprea, Boehringer Ingelheim, Cardiff Oncology, BMS, Incyte, and Novartis; research funding from ADC Therapeutics, Astex, and Pfizer; consultancy from Agios, Astellas, BeyondSpring, Daiichi Sankyo, Epizyme, Genentech, Gilead, Kura, Ionis, Loxo Oncology, Janssen, AstraZeneca, Jasper, and Jazz; serving on clinical trial committees at AbbVie, BioCryst, BMS, Geron, Gilead, Kura, Loxo Oncology, and Novartis; and travel support from Cardiff Oncology, Novartis, and Pfizer. X.M. received research funding (institutional) from Celgene/Bristol Myers Squibb and consults for Bristol Myers Squibb. N.A.P. received consulting fees from Pfizer, Agios Pharmaceuticals, Blueprint Medicines, Incyte, Novartis, Celgene/Bristol Myers Squibb, CTI BioPharma, PharmaEssentia, Constellation Pharmaceuticals, and AbbVie; and other financial support for serving on an Independent Data Review Committee for Cogent Biosciences. The remaining authors declare no competing financial interests.
The current affiliation for S.D.G. is Investigational Drug Branch, Cancer Therapy Evaluation Program, National Cancer Institute, Shady Grove, MD.
Correspondence: Nikolai A. Podoltsev, Department of Internal Medicine, Section of Hematology, Yale School of Medicine, 37 College St, New Haven, CT 06510; e-mail: nikolai.podoltsev@yale.edu.
References
Author notes
The data used in this manuscript was provided through a collaboration between the National Cancer Institute and the Centers for Medicare and Medicaid Services. The Data Use Agreement precludes the authors from sharing the data.