Key Points
Frontline therapies for iNHL should prioritize safety and convenience alongside activity.
For untreated iNHL, oral weekly ixazomib alone and with rituximab is effective, tolerated, and permits retention of immune competence.
Abstract
Patients with indolent B-cell non-Hodgkin lymphoma (iNHL) generally require treatment but experience normal survival, emphasizing the need for simpler, safer therapies. Proteasome inhibitors target aberrant signaling pathways within iNHL and have manageable toxicities. We evaluated the oral proteasome inhibitor ixazomib as initial monotherapy, and combined with rituximab, for first-line treatment of iNHL. Treatment-naïve patients with iNHL needing therapy received oral ixazomib 4 mg weekly until progressive disease or unacceptable adverse events. A 4-week course of rituximab was added during month 7. The primary end point was overall response rate (ORR) during the ixazomib monotherapy window. Correlations included gene expression profiling and response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination. Thirty-three patients with follicular lymphoma (FL) (n = 20), marginal zone lymphoma (n = 7), and other iNHL were treated with a median follow-up of 30.3 months. During the 6-month ixazomib window, the ORR was 24%, including 35% in FL. The best ORR over the entire study period was 52% overall and 65% in FL; complete response was achieved in 33% and 45%, respectively. The median duration of response was 25.8 months (range, 0-49.7), and the 24-month progression-free and overall survival rates were 51% (95% confidence interval [CI], 32-67) and 91% (95% CI, 74-97), respectively. Ixazomib was well tolerated. Baseline downregulation of proteasome genes, PSMB9 (P = .03) and PSMB8 (P = .007), were associated with response. All evaluated patients generated anti-S antibodies to SARS-CoV-2 vaccination, with a median of 254.9 binding arbitrary unit per mL. Ixazomib demonstrated efficacy alone and with short-course rituximab in untreated iNHL while exhibiting favorable toxicity, convenience, and retention of the B-cell immune response. This trial is registered at www.clinicaltrials.gov as NCT02339922.
Introduction
Most patients diagnosed with indolent B-cell non-Hodgkin lymphoma (iNHL) require treatment and achieve normal life expectancy.1,2 However, other than CD20-directed antibodies, no treatment has been shown to improve overall survival (OS), even if higher complete remission rates or prolonged progression-free survival (PFS) are achieved, and none are curative.1,3,4 Standard frontline therapies may include an anti-CD20 antibody alone for patients who are frail or in combination with cytotoxic chemotherapy for fit patients or those with a high-tumor burden.5-7 Though efficacious, such combination chemoimmunotherapy comes with logistical burdens of administration and monitoring and can result in toxicities, including prolonged cytopenias and late infections.1,8 Early and ongoing challenges from COVID-19 accelerated the recognition of value in cancer treatments conducive to telemedicine, remote management, and maintenance of immune function.9,10 Approaches to reduce the toxicity and logistical complexity of initial therapy while retaining efficacy sufficient to address lymphoma-related symptoms are thus being pursued.
The ubiquitin-proteasome system has fundamental roles in cell cycle progression, apoptosis, and cellular proliferation, including through nuclear factor-κB (NF-κB) signaling. Several reports have shown its inhibition by parenterally administered small molecules may be effective against iNHL in the relapsed or refractory settings,11-13 and its potential for additive efficacy with CD20 antibodies has been hypothesized in preclinical studies.14,15 Ixazomib is an orally bioavailable, selective, reversible, and potent inhibitor of the β5 subunit of the 20S proteasome. It was approved by the US Food and Drug Administration in 2015 for relapsed multiple myeloma and is the only approved oral proteasome inhibitor (PI). As such, it boasts an extensive track record of safety and tolerability, including in real-world settings.16,17 We hypothesized that weekly oral ixazomib would be an effective, convenient, and well-tolerated treatment of iNHL.
Frontline window-of-opportunity studies can efficiently evaluate therapies in treatment-naïve disease before the transition to or addition of standard agents. This design is ideally suited to low-toxicity therapies administered for diseases with an indolent natural history.18 We performed a prospective window-of-opportunity study of ixazomib monotherapy, followed by addition of rituximab, in patients with untreated iNHL. We formally assessed the impact of ixazomib on B-cell response and explored molecular predictors of efficacy.
Methods
Patients
Patients were accrued at the Seattle Cancer Care Alliance/University of Washington. Takeda Oncology (Cambridge, MA) provided the study drug and funding. All patients provided written informed consent. The trial was registered at www.clinicaltrials.gov (NCT02339922).
Eligibility included age ≥18 years with histologically confirmed iNHL, including follicular lymphoma (FL) of any grade, marginal zone lymphoma, mantle cell lymphoma, lymphoplasmacytic lymphoma, and small lymphocytic lymphoma. Additional requirements included performance status <3 by Eastern Cooperative Group criteria and radiographically measurable disease per standard criteria.19-21 In contrast to many studies of untreated iNHL, this trial mandated an indication for treatment according to standard criteria as detailed by the National Comprehensive Cancer Network (NCCN) guidelines.22 Previous antibiotic therapy was allowed for mucosa-associated marginal zone lymphoma. Full eligibility criteria are listed in supplemental Data.
Study design
This investigator-initiated, open-label, single-center, single-arm study was conducted in accordance with the general ethical principles in the Declaration of Helsinki and approved by the institutional review board at the Fred Hutchinson Cancer Research Center. Enrolled patients received ixazomib 4 mg orally once weekly on consecutive 28-day cycles until disease progression or unacceptable toxicity. The 4 mg dose was based on the established maximum tolerated dose of 3 mg/m2 weekly for 3 of 4 weeks in the relapsed/refractory myeloma space.23 Patients without disease progression or unacceptable toxicity during the initial 6 cycles of ixazomib monotherapy were additionally administered 4 weekly doses of 375 mg/m2 rituximab during the seventh cycle (supplemental Data). This window-of-opportunity design both efficiently estimated the efficacy ceiling of ixazomib monotherapy in iNHL before treatment resistance and ensured that patients remaining on protocol received a standard therapy. Prophylaxis against reactivation of herpes viruses was recommended while taking ixazomib.
The primary end point was investigator-assessed overall response rate (ORR; complete response [CR] plus partial response [PR]) after radiology review (performed by radiologists separate from the clinical study team) during the ixazomib monotherapy window. Required imaging included computerized tomography (CT) of the chest, abdomen, and pelvis, +/− neck depending on clinically evident or previously described disease. Fluorodeoxyglucose (FDG)-positron emission tomography imaging was required for all cases of suspected FDG-avid disease (FL, mantle cell lymphoma) during screening and to confirm metabolic CR in patients with stable PR on 2 consecutive CT staging exams or in patients with a CR based on CT staging and baseline FDG-avid disease. Response assessment occurred every 2 cycles using standard criteria,19-21 including bone marrow exams to confirm CR in patients with unknown bone marrow status or bone marrow positive for iNHL at screening. Secondary end points included ORR to ixazomib combined with rituximab, assessments of safety, duration of response, PFS, and OS. Any reduction in tumor burden, including that not meeting criteria for PR, was assessed in post hoc analysis as a measure of ixazomib’s potential for disease control because a full assessment was confounded by the addition of rituximab at month 7.
Statistics
The study was designed for a preliminary assessment of the efficacy of ixazomib in untreated iNHL compared with a historical ORR of 40% to 70% to frontline rituximab in low-tumor-burden iNHL without an indication for treatment.7 This historical comparator was chosen because the regimen's tolerability aligns with the relatively modest toxicities associated with single-agent ixazomib. In this single-stage phase 2 study, the null hypothesis that the true response rate is ≤40% was evaluated against the alternative hypothesis that the true response rate is ≥60%, with a type I error rate of 8% and a power of 85%. An ORR of at least 19 out of a planned cohort of 36 patients was required to conclude promising efficacy. The Kaplan-Meier method was used to estimate time-to-event in months with a corresponding 95% confidence interval (CI). Clinical variables known to correlate with outcomes of iNHL, including bulky disease, extranodal disease, follicular lymphoma international prognostic index (FLIPI), and lactate dehydrogenase, were evaluated for association with efficacy in exploratory univariate analyses. Statistical software R version 3.4.1 was used for analyses.
Correlative studies
Available pretreatment formalin-fixed, paraffin-embedded tissue biopsies were subjected to RNA extraction by standard methods and gene expression profiling (GEP) using the NanoString PanCancer IO 360 panel (Seattle, WA) to query pathways in proteasomal degradation and lymphomagenesis. Standard GEP quality control and data processing were performed with the ROSALIND platform (San Diego, CA). In a post hoc analysis, patients vaccinated per standard of care for COVID-19 while actively receiving ixazomib and not before at least 6 months after completing rituximab were evaluated for serologic response ≥2 weeks after the final dose of vaccine using the Roche Elecsys anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) S assay (Indianapolis, IN), a semiquantitative total antibody assay against the spike protein receptor-binding domain (anti-S), and converted to WHO International Standard binding arbitrary units per mL) by multiplying by 1.0288.24 As vaccinations were typically administered in the local community setting, precise timing relative to rituximab course were not available. The reference interval for a negative result was <0.8 AU/mL. Individuals were also assessed for previous COVID-19 infection using the Roche Elecsys Anti-SARS-CoV-2 assay to test for antinucleocapsid antibodies.
Results
Patients
A total of 33 patients enrolled, and all began treatment on study within the 3 years ending January 2020. Baseline characteristics are displayed in Table 1, including specific data on the 20 with FL. All patients had a specific indication for initiation of treatment, with the most common being bulky disease defined as involvement of ≥3 nodal sites, each ≥3 cm and/or any nodal or extranodal tumor ≥7 cm (N = 13, 39%). High-tumor burden per Groupe d'Etude des Lymphomes Folliculaires (GELF) criteria25 was present in 82% of the entire study population, including 80% of cases of FL, reflecting the protocol-defined selection of a high-risk population. The remaining patients met criteria for treatment based on the rate of disease progression. Patient disposition is summarized in supplemental Data.
Baseline characteristics of treated patients (entire cohort and separately those with FL)
| Characteristic . | All (N = 33) . | FL (N = 20) . | ||
|---|---|---|---|---|
| N . | % . | N . | % . | |
| Median age, y (range) | 62 (38-84) | 57 (38-67) | ||
| Sex | ||||
| Female | 11 | 33 | 8 | 40 |
| Male | 22 | 67 | 12 | 60 |
| ECOG performance status | ||||
| 0 | 27 | 82 | 17 | 85 |
| 1 | 5 | 15 | 2 | 10 |
| 2 | 1 | 3 | 1 | 5 |
| Lymphoma histology | ||||
| FL | 20 | 61 | ||
| MZL | 7 | 21 | ||
| MCL | 4 | 12 | ||
| SLL | 2 | 6 | ||
| Ann Arbor stage | ||||
| I/II | 4 | 12 | 2 | 10 |
| III/IV | 29 | 88 | 18 | 90 |
| FLIPI (FL, MZL) | ||||
| 0-1 | 13 | 48 | 12 | 60 |
| 2 | 8 | 30 | 4 | 20 |
| ≥3 | 6 | 22 | 4 | 20 |
| MIPI (MCL only) | ||||
| <5.7 | 0 | 0 | ||
| 5.7-6.5 | 2 | 50 | ||
| ≥6.5 | 2 | 50 | ||
| TP53 aberration (SLL only) | 0 | 0 | ||
| FL grade (FL only) | ||||
| 1/2 | 20 | 100 | ||
| 3A | 0 | 0 | ||
| Indication for treatment∗ | ||||
| Bulky disease† | 13 | 39 | 11 | 55 |
| Symptoms | 9 | 27 | 5 | 25 |
| Threatened end-organ | 4 | 12 | 2 | 10 |
| Cytopenia(s) | 4 | 12 | 2 | 10 |
| Disease progression only | 6 | 18 | 4 | 20 |
| Tumor mass ≥7 cm | 9 | 27 | 6 | 30 |
| Extranodal disease | 21 | 64 | 13 | 65 |
| Characteristic . | All (N = 33) . | FL (N = 20) . | ||
|---|---|---|---|---|
| N . | % . | N . | % . | |
| Median age, y (range) | 62 (38-84) | 57 (38-67) | ||
| Sex | ||||
| Female | 11 | 33 | 8 | 40 |
| Male | 22 | 67 | 12 | 60 |
| ECOG performance status | ||||
| 0 | 27 | 82 | 17 | 85 |
| 1 | 5 | 15 | 2 | 10 |
| 2 | 1 | 3 | 1 | 5 |
| Lymphoma histology | ||||
| FL | 20 | 61 | ||
| MZL | 7 | 21 | ||
| MCL | 4 | 12 | ||
| SLL | 2 | 6 | ||
| Ann Arbor stage | ||||
| I/II | 4 | 12 | 2 | 10 |
| III/IV | 29 | 88 | 18 | 90 |
| FLIPI (FL, MZL) | ||||
| 0-1 | 13 | 48 | 12 | 60 |
| 2 | 8 | 30 | 4 | 20 |
| ≥3 | 6 | 22 | 4 | 20 |
| MIPI (MCL only) | ||||
| <5.7 | 0 | 0 | ||
| 5.7-6.5 | 2 | 50 | ||
| ≥6.5 | 2 | 50 | ||
| TP53 aberration (SLL only) | 0 | 0 | ||
| FL grade (FL only) | ||||
| 1/2 | 20 | 100 | ||
| 3A | 0 | 0 | ||
| Indication for treatment∗ | ||||
| Bulky disease† | 13 | 39 | 11 | 55 |
| Symptoms | 9 | 27 | 5 | 25 |
| Threatened end-organ | 4 | 12 | 2 | 10 |
| Cytopenia(s) | 4 | 12 | 2 | 10 |
| Disease progression only | 6 | 18 | 4 | 20 |
| Tumor mass ≥7 cm | 9 | 27 | 6 | 30 |
| Extranodal disease | 21 | 64 | 13 | 65 |
ECOG, Eastern Cooperative Oncology Group; MCL, mantle cell lymphoma; MIPI, Mantle Cell Lymphoma International Prognostic Index; MZL, marginal zone lymphoma; SLL, small lymphocytic lymphoma.
More than 1 indication possible unless indicated.
Bulky disease defined as ≥3 nodal sites, each ≥3 cm and/or any nodal or extranodal tumor ≥7 cm.
Clinical efficacy
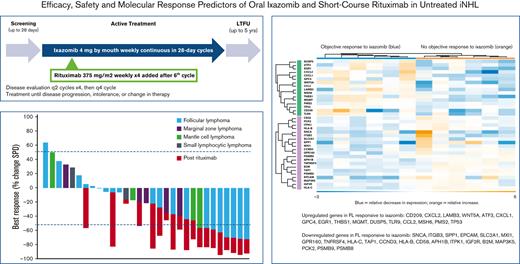
Primary and secondary end points were analyzed for all 33 enrolled patients on an intention-to-treat basis. Of them, 23 (70%) patients had reduction in tumor burden over the 6-month ixazomib monotherapy window period, including 8 responses (24%; 95% CI, 0.09-0.39), and a CR in 2 (6%; 95% CI, 0-0.14). Two patients were not evaluable for response but were included as nonresponders. The median time to objective response (TTR) to ixazomib monotherapy was 4.0 months (range 1.8-5.5). The best objective responses pre- and post-rituximab treatment are shown for all histologies and for FL only (Figure 1).
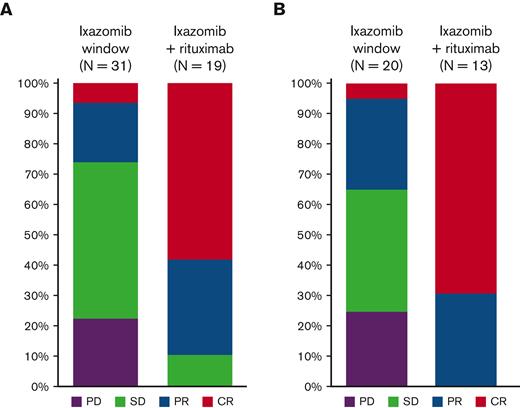
Objective response rates. Best objective response in evaluable patients (N = 31) during initial ixazomib window and after addition of rituximab, overall (A) and for patients only with FL (B). Note, 2 patients administered ixazomib stopped therapy on study before first scheduled restaging evaluation.
Objective response rates. Best objective response in evaluable patients (N = 31) during initial ixazomib window and after addition of rituximab, overall (A) and for patients only with FL (B). Note, 2 patients administered ixazomib stopped therapy on study before first scheduled restaging evaluation.
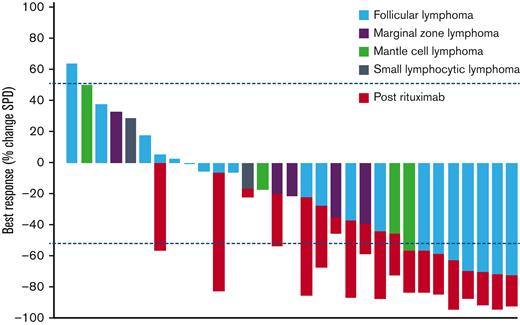
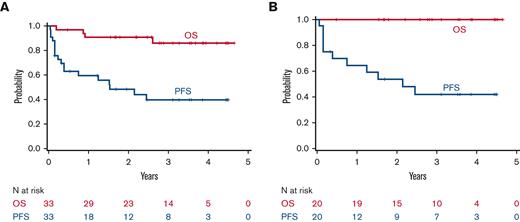
The median follow-up was 30.3 months (range, 2.2-55.9). Rituximab was administered to all 8 (24%) patients with an objective response plus an additional 11 (33%) patients who had stable disease at the end of the 6-cycle ixazomib window, 9 (27%) with a reduction in tumor size, and 2 (6%) with an increase in the sum of product of diameters on ixazomib (but not meeting criteria for PD). Of the whole study population, 14 patients (42%) did not receive rituximab because of one of the following: progression on ixazomib monotherapy (10 patients, 30%), toxicity resulting in discontinuation (2 patients, 6%), or patient preference to stop treatment on study (2 patients, 6%). Addition of rituximab resulted in increased depth of response in all cases (median % change in sum of product of diameters −27, range −6 to −76), with ORR increasing to 17 of 33 overall (52%; 95% CI, 0.35-0.69), including a CR in 11 (33%; 95% CI, 0.17-0.49) (Figure 1). In those that received ixazomib plus rituximab, the ORR increased from 8 of 19 (42%; 95% CI, 0.25-0.59) pre-rituximab to 17 of 19 (89%; 95% CI, 0.75-1.00) post-rituximab; the CR rate increased from 2 of 19 (11%; 95% CI, 0-0.25) pre-rituximab to 11 of 19 (58%; 95% CI, 0.36-0.80) pre-rituximab (Figure 2). The median duration of response was 25.8 months (range 0-49.7). The PFS and OS rates at 24 months were 51% (95% CI, 32-67) and 91% (95% CI, 74-97), respectively (Figure 3). Progression of disease (POD) within 24 months of the start of therapy occurred in 14 patients (42%).
Outcomes of patients evaluable for response (N = 31). Best response as measured by % change in SPD of index lesions. Color corresponds to histology of iNHL. Change in SPD after course of rituximab indicated by red bars. SPD, sum of product of diameters.
Outcomes of patients evaluable for response (N = 31). Best response as measured by % change in SPD of index lesions. Color corresponds to histology of iNHL. Change in SPD after course of rituximab indicated by red bars. SPD, sum of product of diameters.
Survival outcomes. Kaplan-Meier plots of PFS and OS in all patients (N = 33) treated (A) and for patients only with FL (N = 20) (B).
Survival outcomes. Kaplan-Meier plots of PFS and OS in all patients (N = 33) treated (A) and for patients only with FL (N = 20) (B).
FL comprised the largest disease cohort (N = 20, 61%) and included 40% with intermediate (20%) or high-risk (20%) disease by FLIPI (Table 1). The ORR of FL was 35% (95% CI, 0.15-0.59), including CR in 5% (95% CI, 0-0.25) to ixazomib alone, and 65% (95% CI, 0.41-0.85), including CR in 45% (95% CI, 0.23-0.68) overall (Figure 2). The PFS and OS rates at 24 months were 54% (95% CI, 30-73) and 100%, respectively (Figure 3). Univariate analyses found that a higher FLIPI score at treatment was associated with FL response to ixazomib monotherapy; bulky disease, extranodal disease, and increased lactate dehydrogenase had no association with ixazomib efficacy (Table 2).
Univariate associations of clinical factors with odds for FL response to ixazomib monotherapy
| Variable . | OR (95% CI) . | P value . |
|---|---|---|
| Bulky disease (GELF) | 1.1 (0.2-7.3) | .89 |
| Extranodal disease | 0.2 (0.03-1.6) | .14 |
| FLIPI∗ | 3.9 (1.1-14.1) | .04 |
| Largest tumor ≥7 cm | 0.9 (0.1-6.8) | .92 |
| LDH∗ | 1.0 (1.0-1.0) | .20 |
| Variable . | OR (95% CI) . | P value . |
|---|---|---|
| Bulky disease (GELF) | 1.1 (0.2-7.3) | .89 |
| Extranodal disease | 0.2 (0.03-1.6) | .14 |
| FLIPI∗ | 3.9 (1.1-14.1) | .04 |
| Largest tumor ≥7 cm | 0.9 (0.1-6.8) | .92 |
| LDH∗ | 1.0 (1.0-1.0) | .20 |
LDH, lactate dehydrogenase.
Modeled as continuous linear variables; OR represents increase in odds associated with each 1-unit increase in variable.
Treatment exposure and safety
Adverse events (AEs) were collected for all patients (N = 33) that received at least 1 dose of ixazomib. The median duration of treatment with ixazomib was 14.6 months (range, 0.5-54) for all patients and 19.3 months (range, 0.6-54) for those with FL. Rituximab was administered to 19 patients (58%). Treatment-emergent AEs were identified in 31 patients (94%) and the most common are summarized (Table 3). Grade 3 or higher AEs occurred in 11 patients (33%; Table 4). Ixazomib was discontinued because of toxicity in 3 patients (9%), including in 1 patient because of grade 2 confusion, in 1 patient with grade 3 hyponatremia, and in 1 patient, who was 85 years old at enrollment, and died from influenza after 35 days on treatment. Ixazomib was temporarily interrupted because of toxicity in 10 patients (30%) and dose reduced to 3 mg weekly in 4 patients (12%). After 6 months on ixazomib dose, holds due to toxicity were uncommon, occurring in 7 patients (21%), and no dose reductions were needed. Serious AEs occurred in 4 patients (12%) and were considered at least possibly related to treatment in 1 patient (3%): these included a disease-related pleural effusion in 1 patient, syncope in 1 patient, hyponatremia in 1 patient, and 1 case of fatal influenza. Peripheral neuropathy (PN), an AE of special interest, occurred in 5 patients (15%) and included 2 cases of grade 1 sensory PN, 2 cases of grade 1 motor PN, and 1 case of grade 2 motor PN. The median time to onset of PN was 4.4 months (range, 1.6-6.3).
Most common (>10%) treatment-emergent AEs, regardless of attribution
| AE . | Grades 1 and 2 . | Grades 3 and 4 . | Grade 5 . |
|---|---|---|---|
| Nausea | 64 | 0 | |
| Diarrhea | 42 | 5 (18) | |
| Rash | 39 | 2 (6) | |
| Dizziness | 33 | 0 | |
| Headache | 30 | 0 | |
| Vomiting | 30 | 0 | |
| Infection | 27 | 0 | 1 (3) |
| Fatigue | 24 | 0 | |
| Fall | 24 | 1 (3) | |
| Anorexia/decreased appetite | 24 | 0 | |
| Thrombocytopenia | 21 | 0 | |
| Constipation | 21 | 0 | |
| Cough | 21 | 0 | |
| Fever | 15 | 0 | |
| Extremity pain | 15 | 0 | |
| PN | 15 | 0 | |
| Arthralgia | 15 | 0 | |
| Chills | 15 | 0 | |
| Myalgia | 15 | 0 | |
| Pruritis | 15 | 0 | |
| Abdominal pain | 12 | 0 | |
| AST increase | 12 | 1 (3) | |
| Flu-like symptoms | 12 | 0 | |
| Edema | 12 | 0 |
| AE . | Grades 1 and 2 . | Grades 3 and 4 . | Grade 5 . |
|---|---|---|---|
| Nausea | 64 | 0 | |
| Diarrhea | 42 | 5 (18) | |
| Rash | 39 | 2 (6) | |
| Dizziness | 33 | 0 | |
| Headache | 30 | 0 | |
| Vomiting | 30 | 0 | |
| Infection | 27 | 0 | 1 (3) |
| Fatigue | 24 | 0 | |
| Fall | 24 | 1 (3) | |
| Anorexia/decreased appetite | 24 | 0 | |
| Thrombocytopenia | 21 | 0 | |
| Constipation | 21 | 0 | |
| Cough | 21 | 0 | |
| Fever | 15 | 0 | |
| Extremity pain | 15 | 0 | |
| PN | 15 | 0 | |
| Arthralgia | 15 | 0 | |
| Chills | 15 | 0 | |
| Myalgia | 15 | 0 | |
| Pruritis | 15 | 0 | |
| Abdominal pain | 12 | 0 | |
| AST increase | 12 | 1 (3) | |
| Flu-like symptoms | 12 | 0 | |
| Edema | 12 | 0 |
All grade ≥3 TEAEs, regardless of attribution
| Any grade ≥3 TEAE . | Events . | Patients affected . | % patients . |
|---|---|---|---|
| Diarrhea | 6 | 6 | 18 |
| Syncope | 7 | 5 | 15 |
| Neutropenia | 4 | 4 | 12 |
| Hyponatremia | 2 | 2 | 6 |
| Anemia | 2 | 2 | 6 |
| Rash | 2 | 2 | 6 |
| Infection | 1 | 1 | 3 |
| Transaminitis | 1 | 1 | 3 |
| Thromboembolic event | 1 | 1 | 3 |
| Vasovagal reaction | 1 | 1 | 3 |
| Heart failure | 1 | 1 | 3 |
| Fall | 1 | 1 | 3 |
| Hypoxia | 1 | 1 | 3 |
| Hypertension | 1 | 1 | 3 |
| Noncardiac chest pain | 1 | 1 | 3 |
| Any grade ≥3 TEAE . | Events . | Patients affected . | % patients . |
|---|---|---|---|
| Diarrhea | 6 | 6 | 18 |
| Syncope | 7 | 5 | 15 |
| Neutropenia | 4 | 4 | 12 |
| Hyponatremia | 2 | 2 | 6 |
| Anemia | 2 | 2 | 6 |
| Rash | 2 | 2 | 6 |
| Infection | 1 | 1 | 3 |
| Transaminitis | 1 | 1 | 3 |
| Thromboembolic event | 1 | 1 | 3 |
| Vasovagal reaction | 1 | 1 | 3 |
| Heart failure | 1 | 1 | 3 |
| Fall | 1 | 1 | 3 |
| Hypoxia | 1 | 1 | 3 |
| Hypertension | 1 | 1 | 3 |
| Noncardiac chest pain | 1 | 1 | 3 |
TEAE, treatment-emergent AEs.
Correlative studies
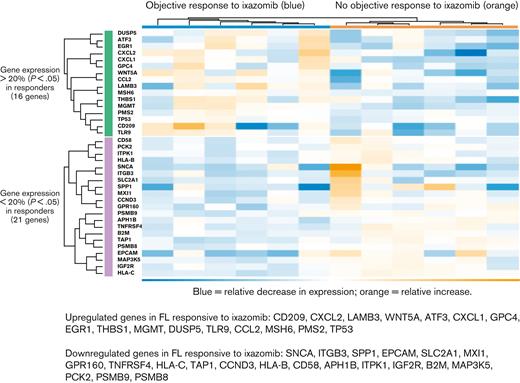
Pretreatment biopsy specimens from 12 patients, all with FL, were available for GEP analysis. Six of these patients had an objective response to ixazomib monotherapy. Using cutoffs of 20% change of expression and P < .05, hierarchical cluster analysis identified 37 genes that differentiated between responding and nonresponding FL (Figure 4). Expression of genes involved in proteasomal degradation was downregulated in patients with FL responsive to ixazomib, including PSMB9 (−1.28-fold change, P = .03) and PSMB8 (−1.26-fold change, P = .007). Two other proteasome genes included in the PanCancer IO 360 panel, PSMB5 (fold change −1.14, P = .21) and PSMB10 (fold change −1.07, P = .31), were also relatively decreased but did not meet statistical significance. Of the 16 upregulated genes, 4 have a described role in chemokine signaling, including CXCL2 (fold change 3.21, P = .02), CXCL1 (fold change 2.5, P = .008), TLR9 (fold change 1.89, P = .03), and CCL2 (fold change 1.64, P = .01). Proteasomal degradation of IκB is thought to liberate NF-κB and promote lymphomagenesis through proliferation and antiapoptotic signaling.26,27 We thus queried GEP of members of the NF-κB complex and found that there was a consistent though statistically nonsignificant increase in expression in cases of FL responsive to ixazomib, including NF-κB1 (fold change 1.22, P = .08), NF-κB2 (fold change 1.13, P = .34), and NF-κBIE (fold change 1.07, P = .56). Together, these data suggest that the GEP of untreated FL may predict for sensitivity to PI.
Hierarchical cluster analysis of GEP among patients with FL with vs without objective response to ixazomib monotherapy. Heat map shows normalized RNA expression levels.
Hierarchical cluster analysis of GEP among patients with FL with vs without objective response to ixazomib monotherapy. Heat map shows normalized RNA expression levels.
The serologic response to SARS-CoV-2 mRNA vaccination was evaluable in 9 patients, who were vaccinated while on therapy with ixazomib and at least 6 months after last rituximab (median, 33.4 months; range, 10.1-45.3). The median anti-S antibody concentration was 254.9 binding arbitrary unit per mL (range, 13.7-1172), and the seroconversion rate was 100%. Anti-N antibody, an indicator of exposure to SARS-CoV-2, was negative in all cases (median, 0.06 ISU/mL; range, 0.06-0.08) (supplemental Data).
Discussion
This single-arm, investigator-initiated, window-of-opportunity study evaluated ixazomib alone and the subsequent addition of rituximab in patients with iNHL and an indication for therapy. The study design demonstrates the feasibility and efficiency of evaluating appropriate novel agents in the frontline iNHL treatment space.
Ixazomib monotherapy fell short of the prespecified threshold for efficacy across disease cohorts, and 42% of patients stopped treatment, the majority of these because of progression, before the close of the ixazomib monotherapy window. Of all, 70% of patients demonstrated reduction in disease burden after only 6 months of weekly dosing. The median time to response of 4 months may imply that a longer single-agent window period may have led to higher response rates, particularly in FL with a 35% initial ORR. Indeed, a more adaptable treatment scheme permitting the addition of rituximab sooner or later according to clinical factors and patient preference may optimally balance the efficacy and convenience of this regimen. Notably, 80% of patients with FL were classified as having high tumor burden by GELF criteria, the presence of which has been associated with worse treatment sensitivity and clinical outcomes, and an NCCN-defined indication for therapy was required for eligibility.28
Contemporary options for frontline treatment of iNHL with high tumor burden include combination chemoimmunotherapy and, though not approved by the US Food and Drug Administration, lenalidomide plus an anti-CD20 antibody. The efficacy of such regimens, for example, as published in the GALLIUM and RELEVANCE trials, features ORRs of up to 65% to 90% and 24-month PFS rates of approximately 80% to 90%.3,6 These regimens are associated with generally manageable but still substantial AE rates, including grade ≥3 events in 65% to 75% of patients. In addition to the cyclical toxicities characteristic of infusional cytotoxic chemotherapy, induction chemoimmunotherapy for iNHL risks long-term immune suppression, especially in older or frail patients, because of extended or maintenance courses of anti-CD20 antibody and/or protracted recovery from bendamustine-induced T-cell lymphopenia.29 Ixazomib plus 4 doses of rituximab resulted in the expected safety signals, with the most common being low-grade gastrointestinal symptoms amenable to supportive care and without dose interruption or modification, with patients able to safely remain on therapy beyond 4 years. High-grade cytopenias were uncommon (grade ≥3 neutropenia in 12% and no cases of grade ≥3 anemia or thrombocytopenia), and PN, an important toxicity of other members of the PI class, occurred in 15% (0% grade ≥3). Thus, compared with chemoimmunotherapy or lenalidomide plus an anti-CD20 antibody, ixazomib plus a short course of rituximab appears safe and potentially requires less monitoring and clinic-based supportive care.
The major challenge with these efficacy data is to define the appropriate comparator group. Other studies that evaluated upfront rituximab with or without low-intensity / oral therapy in iNHL did not require GELF criteria or an NCCN-defined indication for therapy for eligibility, likely resulting in improved outcomes.30-32 In the case of RESORT, rituximab alone was given frontline therapy for FL expressly not meeting GELF criteria, and the PFS estimates for the primary end point of this study did not consider the 30% of patients that did not respond to the initial rituximab induction.7 Given these discrepancies in trial design, our data suggest that future ixazomib-rituximab combinations could be comparatively evaluated in FL with an indication for therapy.
Correlative GEP analyses generated interesting findings regarding potential predictive markers and mechanisms-of-action for PI use in iNHL. In addition to the correlation of ixazomib efficacy with gene expression of proteasomal components, the suggestion that FL reliant on NF-κB signaling may be more susceptible to PIs aligns with previously published preclinical data in lymphoma14,33 and raises the question of possibly harnessing synergistic benefit with other agents active in this pathway.34 An Eastern Cooperative Oncology Group-ACRIN cooperative group randomized study evaluated the independent additions of bortezomib and the immunomodulatory drug lenalidomide to standard chemoimmunotherapy regimens for FL, but the combination of PI and immunomodulatory drugs has yet to be explored in depth in untreated FL.35 Several chemokines are implicated in B-cell development, migration, and lymphomagenesis.36 The novel finding of chemokine expression level as a potential marker for FL behavior and PI activity requires further investigation, particularly in the context of recent data identifying increased chemokine receptor expression in transformed FL specimens.37
Early POD, generally defined as within 24 months after intensive frontline treatment of iNHL, is strongly correlated with worse long-term outcomes, including OS.38-40 Here, the rate of 42% of patients with 24-month POD is substantially higher than what has been observed with conventional chemoimmunotherapy induction regimens, including rates of 19% after R-CHOP (rituximab-cyclophosphamide-hydroxydaunorubicin-oncovin-prednisone) and 16% after bendamustine-rituximab, respectively.39,41 However, recent data have shown early POD may be more common after low-intensity frontline therapy and be associated with a smaller impact on long-term outcomes.42 Further follow-up is required to assess any risk associated with early POD to ixazomib +/− rituximab. Of note, a single patient (3%) experienced confirmed histologic transformation of iNHL during treatment or long-term follow-up.
Key observations from the COVID-19 pandemic included the need for safe, simple, preferably oral, therapies that may be monitored via telehealth, and the significant infection risks posed by many of the agents used in the treatment of hematologic malignancies.9,10 Especially, patients with B-cell malignancies are at substantially higher risk of morbidity and mortality from SARS-CoV-2 infection and have attenuated, if any, response to vaccination.43-46 The ease of weekly oral ixazomib administration, the comparatively favorable AE profile, and the consistently positive serologic response to SARS-CoV-2 vaccination in 9 of 9 evaluable patients on active therapy support its consideration for further evaluation in frontline FL management; certain circumstances, including rural and resource-limited populations, may be especially well suited to this strategy.
The approach for managing iNHL continues to rapidly evolve with the recognition that most patients will do well over the long term regardless of the initial therapy, with more intensive regimens that may yield dramatically longer PFS not clearly improving survival. This window study of ixazomib reflects an alternative strategy by designing initial treatments that focus on minimized toxicity, retained immune competence, and ease of outpatient weekly oral dosing while maintaining sufficient efficacy and the potential for long-term disease control. Future efforts in this setting could continue to focus on further improving patient stratification and predictive molecular biomarkers to identify the simplest, least-toxic, and most cost-effective interventions that can resolve the specific indication for treatment of iNHL.
Acknowledgments
The authors thank the patients who participated in this study. They also thank Kristi Matsumura, Laura Naylor, Peter Wilcox, and Sabrina Wilcox for study coordination and database support. S.A.G. is supported by a Lymphoma Research Foundation Mentorship Grant.
In addition to primary funding of this study from Takeda Pharmaceutical Company, A.K.G. is supported by National Institutes of Health K24CA184039, a Clinical Scholar Award by the Leukemia and Lymphoma Society, and philanthropic gifts from Frank and Betty Vandermeer, Sonya and Tom Campion, Dean and Gwen Polik, The Holbrook Family Foundation, and other generous donors.
Authorship
Contribution: S.A.G. and A.K.G. designed the trial and collected and assembled all data; S.A.G., R.C.L., C.S.U., D.G.C., A.J.C., S.D.S., M.S., E.H.W., E.N.L., and A.K.G. were involved in data collection; S.A.G., C.S.U., T.A.G., A.L.G., J.R.F., and A.K.G. were involved in data analysis and interpretation; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: S.A.G. received research funding from TG Therapeutics, AstraZeneca, BeiGene, GSK, and MorphoSys. R.C.L. serves as a consultant for MorphoSys; received research funding from TG Therapeutics, SeaGen, RAPT, Juno Therapeutics, Incyte, Bayer, Cyteir, and Genentech. C.S.U. serves as a consultant for Atara, AstraZeneca, AbbVie, ACDT, Epizyme, TG Therapeutics, Pharmacyclics, and BeiGene. A.J.C. received research funding from Bristol Myers Squibb, Harpoon, and Nektar; serves as a consultant for Secura Bio, GSK, and Cellectar; and serves as a consultant and received research funding from Sanofi Aventis, AbbVie, and Janssen. S.D.S. serves as a consultant for Millenium/Takeda, Karyopharm, KITE pharm, Incyte, and ADC Therapeutics; received research funding from Portola Pharmaceuticals, Incyte Corporation, Merck Sharp & Dohme Corp, Genetech, Acerta Pharma BV, De Novo Biopharma, and Bayer; received research funding from Ignyta (spouse), Bristol Myers Squibb (spouse), Ayala (spouse); and serves as a consultant and received research funding from BeiGene and AstraZeneca. M.S. serves as a consultant for AbbVie, Genentech, AstraZeneca, Sound Biologics, Pharmacyclics, BeiGene, Bristol Myers Squibb, MorphoSys, TG Therapeutics, Innate Pharma, Kite Pharma, Adaptive Biotechnologies, Epizyme, Eli Lilly, Adaptimmune, Mustang Bio, and Atara Biotherapeutics: Consultancy; and received research funding from Mustang Bio, Celgene, Bristol Myers Squibb, Pharmacyclics, Gilead, Genentech, AbbVie, TG Therapeutics, BeiGene, AstraZeneca, Sunesis, Atara Biotherapeutics, and GenMab. E.N.L. serves as a consultant and received research funding from Janssen; and received research funding from BMS, Genentech, and GSK. A.K.G. received research funding from Agios, Takeda, IGM Biosciences, AstraZeneca, Bristol Myers Squibb, and Teva; serves as a consultant for and received Honoraria from BeiGene, Servier, ADC Therapeutics, Kite, Acrotech, Karyopharm, Epizyme, Nurix Inc, and Cellectar; received Honoraria from MorphoSys and Incyte; serves as a consultant for, received research funding and Honoraria from Merck, SeaGen, Genetech, Gilead, I-Mab Biopharma, and Janssen.
Correspondence: Ajay K. Gopal, 825 Eastlake Ave E, Seattle, WA 98109; e-mail: agopal@uw.edu.
References
Author notes
Data are available on request from the corresponding author, Ajay K. Gopal (agopal@uw.edu).
The full-text version of this article contains a data supplement.