Key Points
Long-term romiplostim treatment is effective in children with primary ITP; serial bone marrow biopsy results showed no new safety concerns.
Romiplostim injections resulted in 8 AEs of neutralizing antibodies (romiplostim, n = 7 [transient n = 4]; TPO, n = 1 [transient n = 1]).
Abstract
Romiplostim is a thrombopoietin (TPO) receptor agonist approved for children and adults with immune thrombocytopenia (ITP) for ≥6 months, recommended as second-line treatment. This phase 3b, single-arm, multicenter study investigated long-term efficacy and safety of romiplostim in children ≥1 to <18 years old with ≥6 months’ ITP duration and platelet counts ≤30 × 109/L. Children received weekly subcutaneous romiplostim (1 μg/kg titrated to 10 μg/kg) to maintain platelets within 50 to 200 × 109/L. A subset underwent bone marrow examinations. The primary end point was percentage of time with platelet response during the first 6 months’ treatment (counts ≥50 × 109/L without rescue medication within the preceding 4 weeks). Overall, 203 patients (median age, 10.0 years) received ≥1 dose of romiplostim, median treatment duration was ∼3 years, and median average weekly dose was 6.9 μg/kg. Ninety-five (46.8%) discontinued (lack of efficacy, n = 43 [21.2%]). Platelet responses were achieved a median (interquartile range) of 50.0% (16.7%-83.3%) of the time during the first 6 months, increasing to 78.2% (26.7%-90.4%) during the overall 36-month treatment period. Eleven patients (5.4%) achieved sustained responses (consecutive counts ≥50 × 109/L without ITP medications for ≥24 weeks). Treatment-related adverse events (AEs) occurred in 56 patients (27.6%), with 8 (3.9%) experiencing serious treatment-related AEs; all of these led to discontinuation, including 4 cases of neutralizing antibodies (romiplostim, n = 3; TPO, n = 1). Bleeding occurred in 141 patients (69.5%), decreasing over time; grade ≥3 bleeding events occurred in 20 (9.9%). At year 2, eight of 63 evaluable patients (12.7%) had grade 2 reticulin. Long-term romiplostim resulted in sustained on-treatment platelet responses with an overall safety profile consistent with previous studies. This trial was registered at www.clinicaltrials.gov as #NCT02279173.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disease resulting from increased platelet destruction and decreased platelet production.1,2 Childhood ITP with a duration of >6 months has an estimated incidence of 0.46 per 100 000 children per year3 and occurs more frequently in adolescent girls.4-6 ITP can lead to severe bleeding, with ∼0.5% of children developing intracerebral hemorrhage.5,7,8
Most children with newly diagnosed ITP do not require treatment9-11; however, for those who do, corticosteroids and immunoglobulins (intravenous anti-D immunoglobulin and intravenous immunoglobulin) are recommended. For children who require continued treatment, thrombopoietin (TPO) receptor agonists (TPO-RAs) are recommended by the American Society of Hematology guidelines and International Consensus group.2,4 Although often effective, splenectomy should be avoided before 5 years of age and within 1 year of disease onset, and it should typically be considered after exhaustion of other available treatment options.4
Romiplostim (Nplate; Amgen Inc., Thousand Oaks, CA) is a TPO-RA indicated for adults with ITP and children ≥1 year of age with ITP for ≥6 months with insufficient response to corticosteroids, immunoglobulins, or splenectomy.12 In phase 1/2 and 3 studies, romiplostim was superior to placebo in children with ≥6 months’ duration of ITP and resulted in decreased rescue medication use and reduced bleeding.13,14 In long-term studies, stable platelet responses were observed, with no new safety signals identified.15,16
Here, we report final data from a 3-year open-label trial assessing long-term efficacy and safety of romiplostim in children with ITP (registered with clinicaltrials.gov as #NCT02279173).
Methods
Study design and population
This phase 3b, single-arm, open-label, multicenter study was conducted in 17 countries (locations and investigators are provided in supplemental Table 1). Enrollment began in December 2014, and the last patient visit was August 2019. Study procedures and informed consent and assent forms were approved by each site’s institutional review board. Written informed consent and assent (if applicable) were provided by patients and their parents or legal guardians. Eligible patients (aged ≥1 to <18 years) had the following: ITP diagnosed according to American Society of Hematology guidelines17 ≥6 months before screening, thrombocytopenia (platelet count ≤30 × 109/L) or bleeding that was uncontrolled with conventional therapy within 4 weeks of enrollment, hemoglobin concentrations >10.0 g/dL, serum creatinine and total serum bilirubin levels ≤1.5 times the upper limit of the normal range, and aspartate aminotransferase and alanine aminotransferase levels ≤3 times the upper limit of the normal range. Exclusion criteria included known history of a bone marrow (BM) stem cell disorder, prior hematopoietic stem cell transplant, active or previous malignancy (except non-melanoma skin cancers) within ≤5 years, congenital thrombocytopenia, venous thromboembolism, or thrombotic events. Patients were also excluded if they had previously received romiplostim, pegylated recombinant human megakaryocyte growth and development factor, or recombinant human TPO; rituximab or 6-mercaptopurine within 8 weeks or eltrombopag within 4 weeks of enrollment; or undergone splenectomy within 4 weeks of screening.
Treatment and assessments
The study included a 4-week screening period, ≤3-year treatment period, and end-of-treatment (EOT) and end-of-study (EOS) visits (supplemental Figure 1). Patients received weekly subcutaneous romiplostim starting at 1 μg/kg. Initially, romiplostim was administered in the clinic by qualified health care providers. Patients provided weekly blood samples for platelet counts and underwent dose titrations by 1 μg/kg weekly up to a maximum dose of 10 μg/kg under the supervision of treating physicians to reach target platelet counts of 50 to 200 × 109/L. Patients who received their first 8 doses in the clinic with a stable dose of romiplostim for ≥4 weeks were given the option to self-administer or have treatment administered by a caregiver, and they were required to return to the site every 4 weeks for ongoing evaluations (including platelet counts). Patients who self-administered were required to keep diary cards to record the dose, dosing date, and any dosing or storage error, as well as return used vials at their next visit, for accountability. The need for dose adjustment was evaluated every 12 weeks to account for body weight changes. For patients with platelet counts >200 to 399 × 109/L for 2 consecutive weeks, romiplostim was reduced by 1 μg/kg. For counts >400 × 109/L, a dose was withheld and then reduced by 1 μg/kg on the next scheduled treatment when the count was <200 × 109/L. Patients with sustained platelet responses (consecutive counts ≥50 × 109/L) who were able to discontinue treatment with romiplostim or other ITP medications (concomitant or rescue) were monitored for ≥6 months beginning with the first off-treatment platelet count ≥50 × 109/L, then followed up every 12 weeks for the duration of the 36-month treatment period. If a subsequent reduction in platelet counts to <50 × 109/L occurred, romiplostim was resumed at 1 μg/kg.
Adverse events (AEs), assessed per the Common Terminology Criteria for Adverse Events version 3.0,18 and concomitant/rescue medications were assessed at weekly visits and at the EOT visit. Platelet counts were monitored every 4 weeks during the treatment period. Complete blood counts with differentials and peripheral blood smears were collected before the first dose, weeks 1 to 8, every 4 to 12 weeks thereafter, and at the EOT visit. For female patients of childbearing potential, urine or blood pregnancy test results were recorded before the first dose of romiplostim, every 12 weeks thereafter, and at the EOT visit. Vital signs, weight, serum chemistry, and physical examinations were done every 12 weeks and at the EOT visit. Blood samples to detect binding and neutralizing antibodies to romiplostim and endogenous TPO were collected on day 1 before the first dose, weeks 12 and 52, every 24 weeks thereafter, and at the EOT visit. Binding antibodies were assessed in a stepwise process for their ability to bind to romiplostim or the peptide portion of romiplostim, as well as their potential (if any) to cross-react with endogenous TPO (details on methodology are provided in the Supplement).19 Most patients positive for neutralizing antibodies to romiplostim or endogenous TPO during the study continued treatment and were asked to return for follow-up testing every 3 months for ≥1 year or until neutralizing antibodies were no longer detectable. Patients who tested positive for binding, nonneutralizing antibodies with clinical sequelae considered potentially related to an anti-romiplostim antibody response were also asked to return for follow-up testing.
As part of the Pediatric Investigation Plan review for participants from the European Union, United Kingdom, Switzerland, and Turkey (European cohort), BM biopsies were required at baseline (if the patient had not had BM analyzed in the preceding 12 months) and after 1 year (day 365 ± 4 weeks) or 2 years (day 730 ± 4 weeks) of treatment to assess the long-term safety of romiplostim. Patients who did not respond to treatment and withdrew were required to have a BM biopsy and aspirate at the EOT visit. These were also obtained for patients who discontinued without having a cohort-defined BM biopsy. In addition to routine BM biopsy analyses, reticulin (silver stain) and collagen (trichrome stain) were assessed by using the modified Bauermeister scale (0-4) by the central laboratory after years 1 and 2. Bauermeister grades 0 to 3 measure changes in reticulin, with grade 4 defined as a reticulin fiber network with areas of collagenization (positive trichrome staining) (supplemental Table 2).20,21 Changes were evaluated based on the incidence of BM reticulin (either increase in severity of ≥2 grades compared with baseline or increase to grade 3 or 4 based on reticulin silver staining using the modified Bauermeister grading scale after romiplostim exposure) and the incidence of BM abnormalities (based on cytogenetics and fluorescence in situ hybridization). BM biopsy specimens were assessed by a central laboratory and critically reviewed by an independent panel of pediatric hematopathologists, clinicians, and a biostatistician to determine whether any observed abnormalities were consistent with an underlying diagnosis of ITP. BM samples were not required for US participants.
End points
The primary end point was the percentage of time that patients maintained platelet counts ≥50 × 109/L (platelet response) from weeks 2 to 24 of treatment without rescue medication within the preceding 4 weeks. Secondary end points included the percentage of time patients maintained a platelet response from week 2 until EOT without rescue medication within the preceding 4 weeks; the percentage of time patients maintained platelet counts ≥20 × 109/L above baseline from week 2 until the EOT without rescue medication within the preceding 4 weeks; the incidence of rescue ITP medication use; the incidence of binding and neutralizing antibodies to romiplostim and TPO; and the incidence of treatment-emergent AEs (ie, all AEs reported during the treatment period) and treatment-related AEs (ie, AEs considered by the site investigator to be related to romiplostim), including clinically significant events and changes in laboratory values. Exploratory end points included the incidence of sustained platelet response for ≥6 months without use of any ITP medications (concomitant, rescue, or romiplostim), the incidence of splenectomy during the treatment period, the incidence of romiplostim self-administration, and BM changes after 1 and 2 years.
Statistical analysis
The planned sample size was 200 patients. The percentage of time that patients had a platelet response in the first 6 months of romiplostim treatment was based on previous romiplostim data and estimated to be 74% with a standard deviation of 30% to 36%. Using the planned sample size, the half-width of the 95% confidence interval for the percentage of time that patients had a platelet response was estimated as 4% to 5%.
Demographic and baseline characteristics were analyzed for all patients and were summarized by using descriptive statistics. Efficacy and safety end points were analyzed for patients who received ≥1 dose of romiplostim. Summary statistics of platelet counts and responses were determined at weeks 1 to 8 and every 4 weeks thereafter. The percentage of time patients had a platelet response from week 2 until EOT was summarized with 95% confidence intervals. Missing platelet counts at weeks 12 and 16, and every 4 weeks thereafter, were imputed by using the mean of the 2 closest nonmissing platelet counts within ±1 week before study discontinuation. Platelet counts were considered as nonresponse if they were missing after imputation. The proportion of patients who underwent splenectomy during the study was summarized. The exposure-adjusted incidence rates and patient incidence rates of AEs were summarized by system organ class and by preferred term according to the Medical Dictionary for Regulatory Activities, version 22.0. Exposure-adjusted rate was defined as total number of events divided by the duration that patients were observed. Summary statistics were provided for cumulative and average weekly romiplostim doses.
Results
Baseline characteristics and disposition
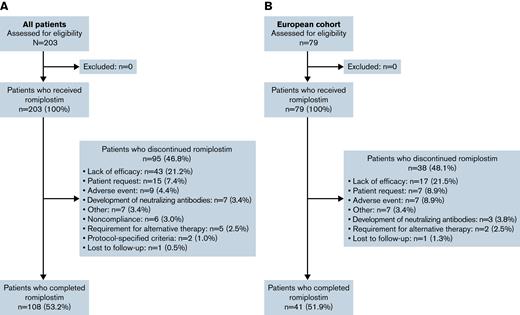
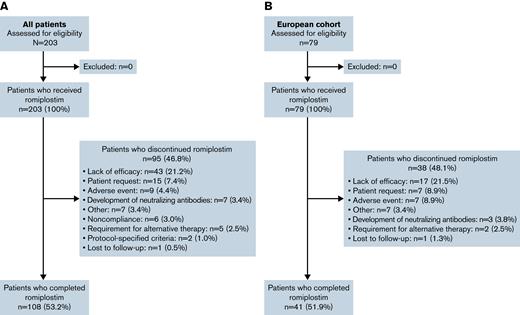
Overall, 203 patients from 17 countries were enrolled and received ≥1 dose of romiplostim (Figure 1; supplemental Table 3). Baseline patient and disease characteristics are summarized in Table 1. Most patients were white and ≥6 years of age, and approximately one-half were boys. At baseline, 54 patients (26.6%) had a disease duration of 6 to 12 months, 54 (26.6%) had a duration of 12 to 24 months, and 95 (46.8%) had a duration of >24 months. A total of 13 patients (6.4%) had platelet counts >30 × 109/L during screening. Overall, 108 patients (53.2%) completed the 36-month treatment period. Among the 95 patients (46.8%) who discontinued, the primary reasons were lack of efficacy (n = 43 [21.2%], 24 of whom had an initial response), patient request (n = 15 [7.4%]), and AE (n = 9 [4.4%]).
CONSORT flow diagram. Summary of the overall population (A) and European cohort (B).
CONSORT flow diagram. Summary of the overall population (A) and European cohort (B).
Exposure to romiplostim
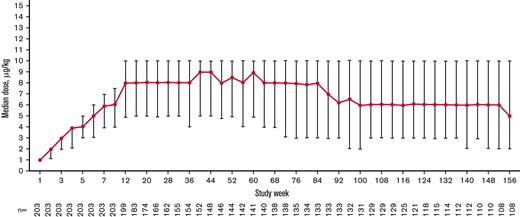
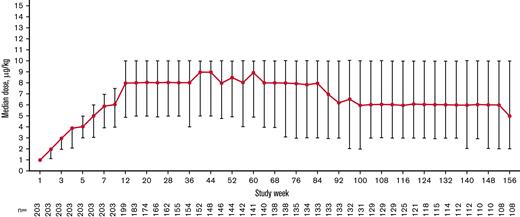
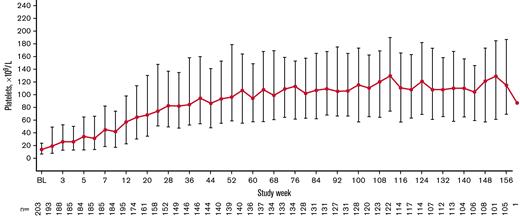
The total exposure was 435.6 patient-years (2.15 years per patient), and the median (interquartile range [IQR]) treatment duration was 155.9 (39.1-156.0) weeks per patient. The median (IQR) average weekly romiplostim dose was 6.9 (4.6-8.9) μg/kg overall, 8.0 (4.9-10.0) μg/kg at week 12, 8.0 (4.9-10.0) μg/kg at week 24, 8.5 (5.0-10.0) μg/kg at week 52, and 6.0 (3.0-10.0) μg/kg at week 104 after starting romiplostim. The median weekly dose increased during the first 12 weeks of treatment, remained steady until week 84, decreased from weeks 84 to 92, and remained stable until week 156 (Figure 2). Overall, 138 (68.0%) patients received ≥1 dose of romiplostim, either by self-administration or home administration by a caregiver.
Median (IQR) weekly romiplostim dose over time stabilized after 12 weeks and decreased slightly after 100 weeks during the treatment period.
Median (IQR) weekly romiplostim dose over time stabilized after 12 weeks and decreased slightly after 100 weeks during the treatment period.
Among patients who discontinued treatment, the median (IQR) treatment duration was 31.0 (17.0-99.0) weeks, and the median average weekly dose was 7.0 (5.3-8.3) μg/kg. For those who discontinued for lack of efficacy (n = 43 [21.2%]), the median duration of exposure was 20.1 (14.9-41.1) weeks. Among the 24 of 43 discontinuing patients (55.8%) who had an initial response, median duration of exposure was 38.6 (20.6-50.6) weeks and was 15.0 (14.0-18.0) weeks for the 19 of 43 patients (44.2%) who did not respond before discontinuation. Only 2 (4.7%) of 43 patients discontinued before reaching the maximum dose of romiplostim.
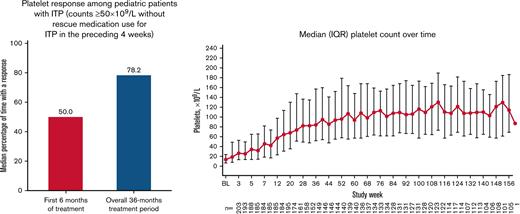
Platelet response
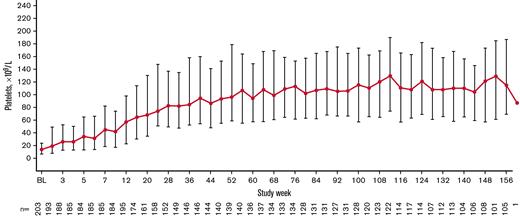
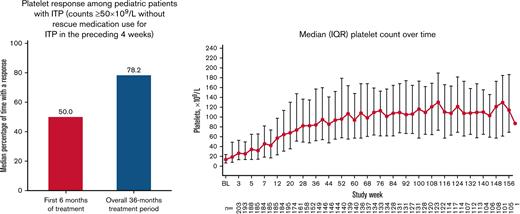
Overall, 179 patients (88.2%) had a platelet response at any time during treatment. Responses were achieved a median (IQR) of 50.0% (16.7%-83.3%) of the time during the first 6 months of treatment (primary end point). Responses were achieved 78.2% (26.7%-90.4%) of the time during the overall treatment period (Table 2). Platelet counts ≥20 × 109/L above baseline were achieved 80.1% (39.1%-92.3%) of the time during the treatment period. Median platelet counts increased steadily over the first 56 weeks of treatment and then stabilized, remaining ≥50 × 109/L from week 12 until the EOT (Figure 3). For patients who administered romiplostim at home (n = 138 of 203 [68.0%]), the median percentage of time with a platelet response was 85.6% (70.5%-93.2%). Among patients who initially responded but discontinued due to lack of efficacy (n = 24 of 43 [55.8%]), platelet responses were achieved a median of 18.0% (8.9%-31.0%) of the time during the overall treatment period. Eleven (5.4%) of 203 patients had a sustained platelet response (consecutive platelet counts ≥50 × 109/L in the absence of any ITP medications for ≥24 weeks), with a mean ± standard deviation time to onset of sustained response of 57.1 ± 36.0 weeks.
Median (IQR) platelet count increased over time. Only platelet counts for which rescue medication was not administered <28 days before evaluation were included.
Median (IQR) platelet count increased over time. Only platelet counts for which rescue medication was not administered <28 days before evaluation were included.
Use of rescue therapy and splenectomy
Sixty patients (29.6%) received rescue medication during the treatment period. Use during sequential 12-week periods decreased up to approximately week 60, remaining generally stable at <12% thereafter (supplemental Figure 2). Rescue medications included corticosteroids (53 of 60 [88.3%]) followed by immunoglobulins (44 of 60 [73.3%]) and other (7 of 60 [11.7%]). Use tended to be higher among younger patients: 46.9% (23 of 49) in patients aged ≥1 to <6 years, 28.4% (23 of 81) in those aged ≥6 to <12 years, and 19.2% (14 of 73) in those aged ≥12 to <18 years.
Of 193 patients who entered the study not having undergone splenectomy, 3 patients (1.6%) aged 9 years (female), 10 years (female), and 13 years (male) underwent splenectomy during the study period (all between the EOT and EOS visits) after having remained on treatment for 28.0, 13.1, and 33.0 weeks, respectively. The 13-year-old achieved platelet counts ≥50 × 109/L (platelet response) without rescue medication use during the 4 weeks before the count 27.3% of the time during the overall treatment period, and achieved counts ≥20 × 109/L above baseline without rescue medication in the 4 weeks before the count 57.6% of the time. The other 2 patients did not achieve platelet responses or counts ≥20 × 109/L above baseline without rescue medication in the preceding 4 weeks.
Safety
Treatment-emergent AEs occurred in 193 patients (95.1%) and serious AEs in 60 patients (29.6%) (Table 3). The most common AEs (>20% of patients) listed in order were epistaxis, headache, nasopharyngitis, pyrexia, cough, petechiae, vomiting, and hematoma. Treatment-emergent AEs resulted in discontinuation in 15 patients (7.4%). Treatment-related AEs occurred in 56 patients (27.6%) and were serious in 8 (3.9%), all of whom discontinued (neutralizing antibodies, n = 4 [2.0%]; headache and abdominal pain, each n = 2 [1.0%]; presyncope, n = 1 [0.5%]; all events occurred in 1 patient each, except 1 patient who experienced presyncope and headache). No fatal AEs occurred.
The overall population included 79 patients (38.9%) in the European cohort who were required per protocol to have a BM aspirate and biopsy at baseline or in the 12 months preceding study enrollment and another BM biopsy again at either year 1 or 2; baseline patient and disease characteristics of this cohort are provided in supplemental Table 4. Among evaluable patients in this cohort, 22 patients had increased reticulin (year 1, 5 of 27 [18.5%]; year 2, 17 of 36 [47.2%]) (supplemental Table 5) after receiving a median average weekly dose of 7.45 μg/kg. The 22 patients achieved a platelet response a median (IQR) of 79.4% (67.3%-90.4%) of the time and 72.7% experienced treatment-emergent bleeding events. At baseline, no patients had Bauermeister scale grade 3 or 4, and none went on to develop grade 3 or 4. Only one patient (3.7%) increased by two Bauermeister grades (from 0 to 2; year 1), and this patient achieved a platelet response 7% of the time. After 1 year, 4 (14.8%) of 27 patients increased one grade (1 of 27 [3.7%] from grade 0 to 1; 3 of 27 [11.1%] from grade 1 to 2). In addition, 4 (14.8%) of 27 patients had decreasing reticulin, including 3 (11.1%) of 27 who decreased one grade from baseline (from 1 to 0) and 1 of 27 (3.7%) who decreased two grades (from 2 to 0). After 2 years, 15 (41.7%) of 36 patients increased one grade, and none increased two grades. Also, 3 (8.3%) of 36 patients decreased one grade from baseline (1 of 36 [2.8%] from grade 1 to 0; 2 of 36 [5.6%] from grade 2 to 1). Patients without increasing reticulin had platelet responses a median of 84.0% (53.6%-92.2%) of the time. Among patients with evaluable BM biopsy specimens, platelet responses were achieved among 25 (92.6%) of 27 patients in year 1 and 36 (100%) of 36 in year 2. Only one patient increased by two Bauermeister grades (year 1; same patient as mentioned earlier); this patient achieved a platelet response.
Bleeding events
Bleeding events occurred in 141 patients (69.5%) (male, n = 72 [72.0%]; female, n = 69 [67.0%]). The most common bleeding-related AEs (occurring in >10% of patients) were epistaxis (n = 80 [39.4%]), petechiae (n = 48 [23.6%]), hematoma (n = 42 [20.7%]), contusion (n = 40 [19.7%]), and gingival bleeding (n = 21 [10.3%]). Grade ≥2 bleeding events occurred in 62 patients (30.5%), and grade ≥3 events occurred in 20 patients (9.9%) (supplemental Figure 3). Of 20 patients with grade ≥3 bleeding events, 16 (80.0%) experienced events within the first 6 months of treatment and 3 (15.0%) between 6 and 12 months. The overall number of bleeding events, as well as the grade 2 and 3 bleeding events, per period decreased during the first 24 months of treatment.
Binding and neutralizing antibodies
Binding antibodies to romiplostim and TPO were detected in 17 patients (8.4%) and 7 patients (3.4%), respectively. No baseline neutralizing antibodies were detected. Two patients (1.0%) had preexisting nonneutralizing binding antibodies to romiplostim and 2 (1.0%) to TPO. One patient with preexisting romiplostim antibodies achieved a platelet response at 5% of visits, whereas the other patient achieved a response at 93% of visits. Both patients with preexisting TPO antibodies had platelet responses at >90% of visits. Fifteen patients (7.7%) developed binding antibodies to romiplostim (transient, n = 7), and 5 developed binding antibodies to TPO (transient, n = 4) (supplemental Table 6).
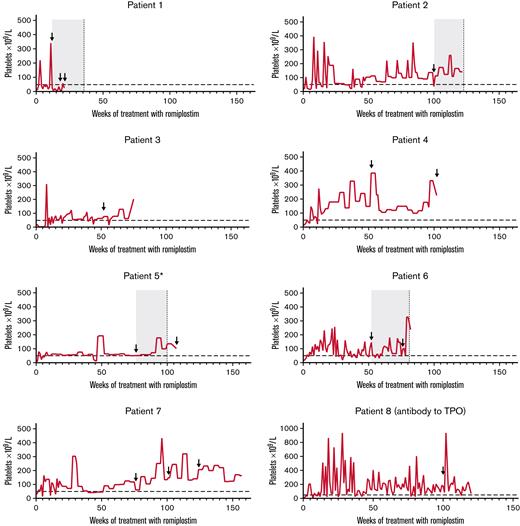
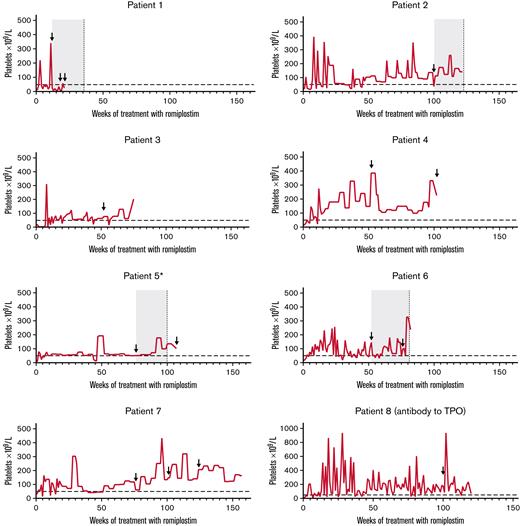
Neutralizing antibodies to romiplostim developed in 7 patients (3.4%; four transient), and transient neutralizing antibodies to TPO developed in 1 patient (supplemental Table 6). In one patient (patient 1 in Figure 4), neutralizing antibodies to romiplostim were first observed 12 weeks after starting treatment; platelet counts decreased, and rescue medication was initiated. The patient discontinued treatment after week 20. In the other 6 patients, romiplostim-neutralizing antibodies were first detected ≥1 year after starting treatment; no reduced therapeutic effect was evident. Six of 7 patients with romiplostim-neutralizing antibodies discontinued treatment (patients 1-6 in Figure 4). Transient TPO-neutralizing antibodies were reported in a 6-year-old boy who did not have romiplostim-neutralizing antibodies (patient 8 in Figure 4). Five samples were taken for antibody measurements (weeks 12, 52, 76, 100, and EOT). The TPO-neutralizing antibodies were first detected 100 weeks after starting treatment. The platelet count was 176 × 109/L at the time of detection, and the patient continued treatment for ∼4 months, maintaining platelet counts ≥50 × 109/L for all but one assessment (94% of visits; week 113; platelet count, 37 × 109/L). The romiplostim dose at detection was 3.0 μg/kg.
Platelet counts over the course of treatment for those who developed neutralizing antibodies. Horizontal dashed line indicates threshold for platelet response (50 × 109/L). Arrows indicate visits at which the patient tested positive for neutralizing antibodies. For patients who had transient neutralizing antibodies (ie, patients 1, 2, 5, and 6), the gray boxes denote indicative periods for which the patients were positive for neutralizing antibodies, and vertical dashed lines indicate visits when the patient no longer tested positive. ∗Patient 5 had multiple positive-to-negative and negative-to-positive visits; only the duration of the first positive-to-negative result is shown.
Platelet counts over the course of treatment for those who developed neutralizing antibodies. Horizontal dashed line indicates threshold for platelet response (50 × 109/L). Arrows indicate visits at which the patient tested positive for neutralizing antibodies. For patients who had transient neutralizing antibodies (ie, patients 1, 2, 5, and 6), the gray boxes denote indicative periods for which the patients were positive for neutralizing antibodies, and vertical dashed lines indicate visits when the patient no longer tested positive. ∗Patient 5 had multiple positive-to-negative and negative-to-positive visits; only the duration of the first positive-to-negative result is shown.
Discussion
In this open-label trial in children with ITP, up to 3 years of romiplostim treatment was associated with maintenance of increased platelet counts in responders. Platelet responses were achieved about one-half of the time during the first 6 months of treatment, increasing to three-quarters of the time during the overall treatment period.
The substantial platelet response rates described here are similar to those in other long-term romiplostim studies. Here, the median percentage of time patients had a platelet response (platelet count ≥50 × 109/L) was 50% during months 1 to 6 and 78% during the overall treatment period; this level was achieved ≥1 time in 88% of children. Among 21 pediatric patients from a phase 1/2 extension study who received romiplostim (median combined treatment duration, 167 weeks; total treatment duration, 63 patient-years), the median percentage of time with a response was 84%.15 In an extension study with 65 patients from a phase 1/2 and a phase 3 study, 94% of patients who received romiplostim for a median of 2.7 years achieved ≥1 platelet response and 72% achieved a response ≥75% of the time. Fifteen patients (23%) achieved a sustained platelet response in the absence of any ITP medications for ≥24 weeks with only one subsequent relapse. Among patients with a treatment-free response, platelet counts were >100 × 109/L at a median of 46 (25-109) weeks.16
In our study, rescue medication use decreased during the first several weeks, remaining stable thereafter. Rescue medication use was low throughout the treatment period in the phase 1/2 extension study15 and, similar to our study, was highest during the first months of the combined extension study.16 The incidence of bleeding AEs in our study was similar to that of the long-term studies in which 77% and 88% of patients experienced bleeding-related AEs.15,16 In the current study, because the median weekly dose of romiplostim increased during the first 12 weeks of treatment before remaining relatively stable, it would be expected that the use of rescue medicine and bleeding-related AEs would decrease over this initial treatment period while the dose of romiplostim was being optimized.
Similar to previous reports in children, romiplostim was well tolerated, and the majority of AEs were not treatment related. In the phase 1/2 extension in 21 patients, 7 (32%) experienced serious AEs, and none resulted in discontinuation.15
Binding or neutralizing antibodies may affect treatment outcomes,22 and their development to romiplostim or TPO led to treatment discontinuation in a small number of patients. Patients who tested positive for antibodies, but were reported as having a platelet response to romiplostim, remained in the study at the investigator’s discretion. Differences in development of neutralizing antibodies may be due to differences in study design, population size, or patient characteristics (eg, ethnicity); similar methods of detection were used among the studies (respective study reports13-16). Antibodies were assessed more frequently in the current study than in previous long-term pediatric reports, as we believed that additional time points would provide a more complete assessment of antidrug antibody development. Importantly, neutralizing antibodies seemed to affect platelet counts in only 1 patient, and almost one-half of the detected antibodies were transient. In the phase 1/2 extension (n = 21), no neutralizing antibodies to romiplostim or TPO were reported.15 In the combined phase 1/2 and phase 3 extension study (n = 65), 1 patient developed neutralizing anti-romiplostim antibodies detected at the EOS visit, and no patients developed neutralizing antibodies to TPO.16 The median treatment doses of the phase 1/2 extension study (5.4 μg/kg) and phase 1/2 and phase 3 extension study (4.8 μg/kg)15,16 were lower than what is reported here (6.9 μg/kg); it is not clear if there is an effect of dose on antibody development.
In the current study, pretreatment and posttreatment BM biopsy specimens were assessed in the European cohort. The median average weekly dose of romiplostim for the patients with increasing reticulin was only slightly higher (7.5 μg/kg) than in the overall study population (6.9 μg/kg). In the European cohort, patients with and without increasing reticulin experienced similar percentages of time with platelet responses and a similar incidence of treatment-related bleeding events, suggesting that increasing reticulin (no patient reached grade 3) did not affect platelet responses or bleeding. Our BM findings confirm the results of the only previous study to assess BM biopsy specimens in pediatric patients with ITP treated with TPOs.23 The failure to identify any trichrome (grade 4) in the BM samples, the lack of grade 3 fibrosis, and identification of some patients with reduced reticulin suggest that clinically important fibrosis occurs rarely, if at all, after prolonged treatment with romiplostim in children with ITP, consistent with previous studies in adults.24-27
A limitation of our study is the open-label, single-arm design. However, our study was strengthened by a large sample size drawn from several geographic regions, comparisons of baseline vs on-treatment results, and the >2-year duration of treatment in most patients. Previous studies were smaller and less geographically diverse.13,16 Although this is the largest study of pediatric patients treated with a TPO-RA who underwent BM evaluation, examinations were not performed in all participants and were only conducted at years 1 and 2. Despite detection of antibodies (including neutralizing antibodies) to romiplostim and TPO, it was encouraging that an effect on platelet response occurred in only 1 patient.
In conclusion, the findings reported here suggest that long-term romiplostim use in children with ITP is efficacious and has a good overall safety profile, confirming previous studies showing that romiplostim is an effective treatment for children with ITP for ≥6 months. The median romiplostim dose required to achieve a platelet response in this study was 6.9 μg/kg. Further research is required to determine if some pediatric patients may benefit from an initial higher starting dose and how to determine who those patients are. We had expected that most patients would achieve a response within the first 6 months of treatment. However, the continued improvements in platelet responses and bleeding events seen after 6 months on treatment suggest that certain patients benefit from longer treatment (if administered at the discretion of the treating health care professional). Future clinical trials could explore the use of romiplostim in patients with ITP for <6 months, an analysis of romiplostim responders and nonresponders including investigation of any correlation between BM findings and platelet responses, why anti-romiplostim and anti-TPO antibodies do not have more clinical effect, and how romiplostim might be used in children with other thrombocytopenic conditions.
Acknowledgments
This work was supported by Amgen Inc. Medical writing support was provided by Erin P. O’Keefe, and Gerard P. Johnson (ICON), whose work was funded by Amgen Inc. Kathryn J. Boorer (KB Scientific Communications, LLC) provided editorial support with funding from Amgen Inc.
Authorship
Contribution: J.G. analyzed and interpreted the data, drafted the manuscript, and critically revised the manuscript for intellectual content; J.B., M.T., N.C., D.B., and J.D. analyzed and interpreted the data, and critically revised the manuscript for intellectual content; A.M. analyzed and interpreted the data; K.W. analyzed and interpreted the data, performed the statistical analysis, and critically revised the manuscript for intellectual content; M.E. contributed to study design and critically revised the manuscript for intellectual content; C.B. acquired data, analyzed and interpreted the data, drafted the manuscript, and critically revised the manuscript for intellectual content; and all authors approved the final manuscript for submission.
Conflict-of-interest disclosure: J.G. has received consulting fees from Novartis, Amgen Inc., Ono, Alexion, Biotest, Dova, and Octapharma; has received speakers bureau fees from Novartis and Amgen; and is an ITP Support Association medical advisor. J.B. has participated in advisory boards and received consultancy fees from Amgen Inc., Novartis, Dova, Rigel, UCB, Argenx, Momenta, Regeneron, Kezar, Principia, and CSL-Behring; has participated in speakers bureaus with Novartis and 3S Bio; and has received honoraria from UpToDate. M.T. has received funding for clinical trials from Amgen Inc., Grifols, Novo Nordisk, and Principia; has participated in advisory boards supported by Amgen Inc., Dova, Genentech, Grifols, Novo Nordisk, and Shire/Takeda; and received speakers bureau fees from Novo Nordisk and Shire/Takeda. N.C. has received honoraria for speaking engagements and participated in advisory boards with Novartis, Amgen Inc., Rigel, and Principia; and has received support for clinical trials from Amgen Inc., Novartis, Rigel, Principia, and UCB. J.D. has received research grants from Novartis and consulting fees from Dova and Novartis. A.M. has received lecturer fees from Novartis and Amgen Inc. K.W., M.E., and C.B. are employees of and may hold stock options in Amgen Inc. The remaining author declares no competing financial interests.
Correspondence: John Grainger, Ward 84, Second Floor, Royal Manchester Children’s Hospital, Oxford Road, Manchester, M13 9WL, United Kingdom; e-mail: john.grainger@mft.nhs.uk.
References
Author notes
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: http://www.amgen.com/datasharing.
The full-text version of this article contains a data supplement.