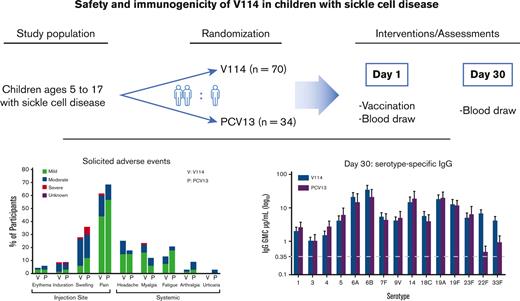
Key Points
V114, a 15-valent pneumococcal conjugate vaccine, was evaluated for safety and immunogenicity in children with sickle cell disease.
V114 had a safety profile comparable to that of PCV13 and demonstrated immunogenicity against all 15 serotypes after a single vaccination.
Abstract
Sickle cell disease (SCD) is an inherited red blood cell disease that results in a multitude of medical complications, including an increased risk of invasive disease caused by encapsulated bacteria, such as Streptococcus pneumoniae. Pneumococcal vaccines have contributed to a significant reduction in pneumococcal disease (PD) in children and adults, including those with SCD. This phase 3 study evaluated the safety and immunogenicity of V114, a 15-valent pneumococcal conjugate vaccine (PCV), in children with SCD. A total of 103 children aged 5 to 17 years with SCD were randomized and received a single dose of V114 or Prevnar 13 (PCV13). Safety was evaluated as the proportion of participants with adverse events (AEs). Serotype-specific immunoglobulin G (IgG) levels and opsonophagocytic activity (OPA) were measured immediately before vaccination and 30 days after vaccination. Overall, the rates of injection-site and systemic AEs reported after vaccination were similar between the vaccination groups. Up to 6 months after vaccination, serious AEs were those expected for patients with SCD, and none were assessed to be vaccine related. IgG geometric mean concentrations (GMCs) and OPA geometric mean titers (GMTs) for the 13 shared serotypes were generally comparable between recipients of V114 and PCV13. Additionally, V114 induced immune responses to serotypes 22F and 33F, which are not included in PCV13. The safety and tolerability profiles of V114 were consistent with those reported for PCV13. Immune responses following vaccination with V114 were generally comparable to PCV13 for the shared serotypes and higher for unique serotypes 22F and 33F. These results support the use of V114 in children with SCD. This trial was registered at www.clinicaltrials.gov as #NCT03731182.
Introduction
Sickle cell disease (SCD) is a monogenic red blood cell disease caused by a mutation in the β-hemoglobin gene, resulting in hemoglobin polymerization, erythrocyte rigidity, and vaso-occlusion. SCD is highly prevalent among individuals of African descent and is associated with anemia, progressive organ damage, and increased susceptibility to infection.1 Individuals with SCD have an increased risk of disease caused by encapsulated bacteria, such as Streptococcus pneumoniae, secondary to splenic dysfunction beginning in the first several months of life, and abnormalities of innate immunity. S. pneumoniae is a Gram-positive bacterium that causes 2 types of disease in humans, invasive pneumococcal diseases (IPD) such as sepsis and meningitis, and noninvasive PD such as otitis media, nonbacteremic pneumonia, and sinusitis.
Infants and young children with SCD are particularly vulnerable to PD.2-6 Although the burden of PD in children with SCD is lower in older children between 6 and 18 years, they remain at much higher risk of IPD than non-SCD children.7 Along with acute chest syndrome, pneumonia is a leading cause of hospitalization and death in infants and children with SCD.8 Pneumococcal vaccination has been shown to be immunogenic in individuals with SCD and effective at reducing overall pneumococcal infection rates and IPD.5,9-12 As a result, a combination of penicillin prophylaxis, beginning after identification by newborn screening and continued until at least 5 years of age, and pneumococcal vaccinations beginning in infancy are recommended for individuals with SCD to lower the risk of infection.7,13 Specifically, infants and children with SCD are recommended to follow the standard pneumococcal conjugate vaccine (PCV) infant vaccination schedule along with additional vaccinations with the 23-valent pneumococcal polysaccharide vaccine (PPSV23) after 2 years of age.7,13 If the infant PCV schedule was missed, PCV vaccination is recommended for children with SCD between 6 and 18 years of age.7 The challenge of increased infection rates from nonvaccine serotypes over time14 requires the ongoing development of safe and effective PCVs with broader serotype coverage. Moreover, individuals with asplenia or hyposplenism, as occurs in SCD, may have suboptimal antibody responses to some infections and vaccinations; therefore, specific studies in this population are needed.
V114 (VAXNEUVANCE, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ) is an approved 15-valent PCV containing all 13 serotypes in Prevnar 13 (PCV13, Wyeth LLC, marketed by Pfizer, New York, NY) and epidemiologically important serotypes 22F and 33F15-18 for expanded coverage. Disease caused by serotypes 22F and 33F is estimated to account for 16% of residual IPD in individuals with SCD.19 Several studies have demonstrated acceptable safety and immunogenicity profiles of V114 in adults and children without SCD.20-22 This study was designed to evaluate the safety, tolerability, and immunogenicity of V114 in children with SCD.
Methods
Study design
This was a phase 3, multicenter, randomized, active-controlled, parallel-group, double-blind study to evaluate the safety, tolerability, and immunogenicity of V114 in children 5 to 17 years of age with SCD (protocol V114-023). This study was conducted at 19 centers in 7 countries (Brazil, Colombia, Dominican Republic, Greece, Italy, Panama, and the United States) (supplemental Table 1) from January 2019 to June 2020 (clinicaltrials.govNCT03731182 and European Union at EudraCT 2018-001152-35).
The study was designed to randomize 100 participants in a 2:1 ratio to receive a single dose of V114 or PCV13, respectively. Treatment allocation/randomization was performed centrally using an interactive response technology system. The study vaccines were managed, prepared, and administered by an unblinded pharmacist and/or other qualified study site personnel who were not involved in any participant assessments or other study procedures. All safety and immunogenicity assessments were conducted by blinded personnel, and the participant and/or the participant’s parent/legal representative were also blinded to the study vaccine received by the participant.
The study was conducted in accordance with the principles of Good Clinical Practice and approved by the appropriate institutional review boards and regulatory agencies. An external data monitoring committee conducted periodic reviews of the safety and tolerability data for the study. A scientific advisory committee comprised of external and Merck & Co., Inc, Rahway, NJ scientists contributed to the development of the protocols, formulation of the statistical analysis plan, data analysis, interpretation of the data, and authoring of this manuscript.
Participants
The participants were males or females 5 to 17 years of age (inclusive) at the time of informed consent with a documented diagnosis of SCD in their medical records. Participants with the following SCD genotypes were eligible for inclusion in the study: (1) sickle cell anemia (HbSS), (2) hemoglobin SC disease (HbSC), or (3) sickle beta thalassemia (HbSβ+ or HbSβ0). Written informed consent was obtained from each participant or parent/legal representative in accordance with local regulations before any study procedure was performed.
The key exclusion criteria were: (1) history of IPD or other known culture-positive pneumococcal disease within 3 years of the first study visit, (2) known hypersensitivity to PCV components or any diphtheria toxoid-containing vaccine, (3) known/suspected impairment of immunological function (other than SCD-related splenic dysfunction) or congenital or acquired immunodeficiency, (4) documented human immunodeficiency virus infection, (5) history of autoimmune disease, (6) febrile illness or receipt of antibiotics within 72 hours of the study vaccine, and (7) receipt of any PCV or pneumococcal polysaccharide vaccine within 3 years before the first study visit.
Vaccines and administration
V114 is a 15-valent PCV, and each dose contains 2 μg of capsular polysaccharide from serotypes 1, 3, 4, 5, 6A, 7F, 9V, 14, 18C, 19A, 19F, 23F, 22F, and 33F and 4 μg of serotype 6B individually conjugated to the CRM197 carrier protein and adjuvanted with 125 μg aluminum phosphate. PCV13 is a 13-valent PCV, and each dose contains 2.2 μg of capsular polysaccharide from serotypes 1, 3, 4, 5, 6A, 7F, 9V, 14, 18C, 19A, 19F, and 23F and 4.4 μg of serotype 6B individually conjugated to the CRM197 carrier protein and adjuvanted with 125 μg of aluminum phosphate. Both study vaccines were supplied as suspensions in prefilled syringes and stored at 2 to 8°C. A 0.5 mL dose of either V114 (lot #WL00068289 and WL00068572) or PCV13 (lot #T41416F; ×32864 and T94427) was administered intramuscularly by needle, and participants were observed for 30 minutes after vaccination for any immediate reactions.
Safety assessments
Participants were followed after vaccination for unsolicited, solicited injection-site (erythema, swelling, pain, and induration), and solicited systemic (headache, myalgia, fatigue, arthralgia, and urticaria) adverse events (AEs). All AEs were collected for 14 days after vaccination, and serious AEs and deaths were collected for 6 months after vaccination. The participant’s parent/legal representative recorded daily body temperatures for 7 days after vaccination and any complaints for 14 days after vaccination using an electronic Vaccination Report Card (eVRC). The complaints were subsequently reviewed by the study investigators to determine if they met the protocol-defined AE criteria and to assess their seriousness, intensity, and relatedness to the study vaccine. AE intensity (for solicited injection-site pain, headache, myalgia, fatigue, arthralgia, and urticaria) and maximum size (for solicited injection-site erythema, induration, and swelling) were recorded along with the AE duration in days. For injection-site erythema, injection-site induration, and injection-site swelling, intensity was assigned according to size as follows: mild events were those measuring 0 to ≤1 inch (2.5 cm), moderate events were those measuring >1 to ≤3 inches (7.6 cm), and severe events were those measuring >3 inches (7.6 cm). Safety assessments were completed on the “all participants as treated” population, consisting of all randomized participants who received the study vaccine.
Immunogenicity assessments
Blood was collected immediately before and 30 days after vaccination to measure the serotype-specific anti pneumococcal polysaccharide (PnPs) antibodies. Anti-PnPs serotype-specific immunoglobulin G (IgG) for the 15 serotypes (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F) contained in V114 and the 13 serotypes contained in PCV13 (all except 22F and 33F) were measured in sera using the pneumococcal electrochemiluminescence (PnECL) v2.0 assay, which was developed by Merck & Co, Inc, Rahway, NJ.23,24 The validated multiplex opsonophagocytic assay was used to assess serotype-specific antibody-mediated killing activity. Immunogenicity assessments were completed on the ‘per-protocol’ population, consisting of all randomized participants without protocol deviations that could have substantially affected immunogenicity measurements.
Study end points and statistical methods
Determination of study sample size
The primary safety and immunogenicity end points were descriptive; therefore, no formal statistical comparisons were performed between vaccination groups. The sample size was selected to obtain a reasonably sized safety database for V114 exposure in the study population. There was an estimated 80% chance of observing a serious AE with a 2.4% underlying incidence in the V114 group.
Safety end points and statistical methods
The primary safety end point was the evaluation of safety and tolerability of V114 with respect to the proportion of participants with AEs. The within-group 95% confidence intervals (CIs) were calculated based on the exact method by Clopper and Pearson.25
Immunogenicity end points and statistical methods
The primary immunogenicity end point was to evaluate the anti-PnPs serotype-specific IgG geometric mean concentrations (GMCs) at 30 days after vaccination for each vaccination group. The secondary objectives were to evaluate the anti-PnPs serotype-specific opsonophagocytic activity (OPA) geometric mean titers (GMTs) at 30 days after vaccination for each vaccination group, as well as the OPA and IgG geometric mean fold rises (GMFRs) from before vaccination to 30 days after vaccination for each group. Point estimates and within-group 95% CIs were calculated by exponentiating the CIs of the mean natural log values based on the t-distribution. For continuous end points, within-group 95% CIs were obtained by exponentiating the CIs of the mean of the natural log values based on the t-distribution. For dichotomous end points, the within-group 95% CIs were based on the exact method by Clopper and Pearson.
A post hoc immunogenicity analysis was performed to evaluate the proportion of participants with a ≥4-fold increase in IgG and OPA responses from before vaccination to 30 days after vaccination.
Analysis software
All analyses were performed using the SAS software, version 9.4, of the SAS System for Unix. Copyright 2012 SAS Institute Inc. SAS and all other SAS Institute Inc product or service names are registered trademarks or trademarks of SAS Institute Inc (Cary, NC).
Results
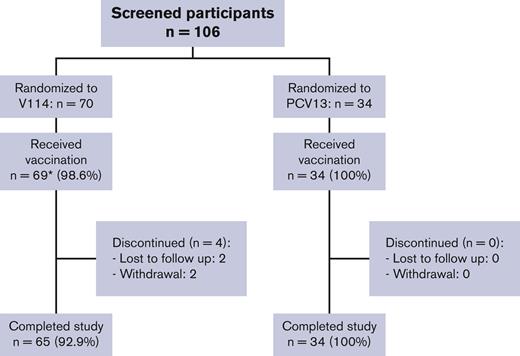
One hundred four participants with SCD were randomized, and 103 received a single vaccination with V114 (n = 69) or PCV13 (n = 34). Of the vaccinated participants, 99 (96.1%) completed the study (Figure 1). Because of the history of SCD in the study population, most participants reported current medications (97.1%) and medical history conditions other than SCD (85.4%) at the time of study enrollment. The study population included participants from Black or African American (60.2%), American Indian or Alaska Native (11.7%), White (11.7%), and multiple (16.5%) self-reported racial identities as well as participants of Hispanic or Latino ethnicity (66.0%). Participant age at enrollment, gender, race, and ethnicity were generally comparable between vaccination groups (Table 1).
Participant disposition. ∗One participant in the V114 group was randomized but not vaccinated because of the physician’s decision.
Participant disposition. ∗One participant in the V114 group was randomized but not vaccinated because of the physician’s decision.
Participant demographics
| . | V114 (N = 69) . | PCV13 (N = 34) . | Total (N = 103) . |
|---|---|---|---|
| n (%) . | n (%) . | n (%) . | |
| Gender | |||
| Male | 36 (52.2) | 20 (58.8) | 56 (54.4) |
| Female | 33 (47.8) | 14 (41.2) | 47 (45.6) |
| Age (mean [y] ± SD) | 10.8 ± 3.5 | 10.8 ± 3.3 | 10.8 ± 3.4 |
| Race | |||
| Black or African American | 37 (53.6) | 25 (73.5) | 62 (60.2) |
| Multiple∗ | 13 (18.8) | 4 (11.8) | 17 (16.5) |
| American Indian or Alaska Native | 9 (13.0) | 3 (8.8) | 12 (11.7) |
| White | 10 (14.5) | 2 (5.9) | 12 (11.7) |
| . | V114 (N = 69) . | PCV13 (N = 34) . | Total (N = 103) . |
|---|---|---|---|
| n (%) . | n (%) . | n (%) . | |
| Gender | |||
| Male | 36 (52.2) | 20 (58.8) | 56 (54.4) |
| Female | 33 (47.8) | 14 (41.2) | 47 (45.6) |
| Age (mean [y] ± SD) | 10.8 ± 3.5 | 10.8 ± 3.3 | 10.8 ± 3.4 |
| Race | |||
| Black or African American | 37 (53.6) | 25 (73.5) | 62 (60.2) |
| Multiple∗ | 13 (18.8) | 4 (11.8) | 17 (16.5) |
| American Indian or Alaska Native | 9 (13.0) | 3 (8.8) | 12 (11.7) |
| White | 10 (14.5) | 2 (5.9) | 12 (11.7) |
SD, standard deviation.
Multiple includes all reports of more than one race category for a given participant.
During the study period, most participants (80.6%) reported one or more AEs. The overall proportions of participants with AEs, injection-site AEs, and serious AEs were generally comparable across vaccination groups (Table 2). A higher proportion of vaccine-related systemic AEs were reported after V114 (40.6%) than after PCV13 (20.6%) (Table 2). Although 20.4% of the participants reported one or more serious AEs during the study, none were assessed by the investigators to be related to the study vaccine. The most common serious AE was sickle cell anemia with crisis (supplemental Table 2). No participant deaths occurred during the study period.
Adverse event summary
| . | V114 (N = 69) . | PCV13 (N = 34) . |
|---|---|---|
| n (%) . | n (%) . | |
| Participants with: | ||
| One or more AEs | 56 (81.2) | 27 (79.4) |
| Injection-site AEs | 48 (69.6) | 26 (76.5) |
| Systemic AEs | 42 (60.9) | 19 (55.9) |
| Vaccine-related AEs∗ | 51 (73.9) | 26 (76.5) |
| Injection-site AEs | 48 (69.6) | 26 (76.5) |
| Systemic AEs | 28 (40.6) | 7 (20.6) |
| Serious AEs | 13 (18.8) | 8 (23.5) |
| Serious vaccine-related AEs | 0 (0.0) | 0 (0.0) |
| Deaths | 0 (0.0) | 0 (0.0) |
| . | V114 (N = 69) . | PCV13 (N = 34) . |
|---|---|---|
| n (%) . | n (%) . | |
| Participants with: | ||
| One or more AEs | 56 (81.2) | 27 (79.4) |
| Injection-site AEs | 48 (69.6) | 26 (76.5) |
| Systemic AEs | 42 (60.9) | 19 (55.9) |
| Vaccine-related AEs∗ | 51 (73.9) | 26 (76.5) |
| Injection-site AEs | 48 (69.6) | 26 (76.5) |
| Systemic AEs | 28 (40.6) | 7 (20.6) |
| Serious AEs | 13 (18.8) | 8 (23.5) |
| Serious vaccine-related AEs | 0 (0.0) | 0 (0.0) |
| Deaths | 0 (0.0) | 0 (0.0) |
Reported AEs include nonserious AEs within 14 days following vaccination and serious AEs within 6 months following vaccination.
Determined by the investigator to be related to the vaccine.
The most commonly reported AEs were those solicited in the trial and included injection-site and systemic AEs graded by intensity (arthralgia, fatigue, headache, myalgia, urticaria, and injection-site pain) or size (injection-site erythema, injection-site induration, and injection-site swelling). The proportions of participants with solicited AEs were generally comparable across vaccination groups, except for myalgia, which was more frequent in the V114 group (Figure 2). Most participants experienced solicited AEs with a maximum intensity of mild or moderate in both vaccination groups (Figure 2). In addition, the proportion of participants in each maximum intensity category was generally comparable across the vaccination groups, and the majority of AEs were of short duration (≤3 days) (supplemental Table 3). The distribution of the maximum body temperature measurements after vaccination was also similar between the vaccination groups (supplemental Table 4).
Assessment of solicited AEs. The proportion of participants reporting solicited AEs within 14 days of vaccination is shown by the intensity. ∗For injection-site erythema, induration, and swelling, 0 to ≤1 in. was considered mild, >1 to ≤3 in. was considered moderate, and >3 in. was considered severe. V=V114, P=PCV13.
Assessment of solicited AEs. The proportion of participants reporting solicited AEs within 14 days of vaccination is shown by the intensity. ∗For injection-site erythema, induration, and swelling, 0 to ≤1 in. was considered mild, >1 to ≤3 in. was considered moderate, and >3 in. was considered severe. V=V114, P=PCV13.
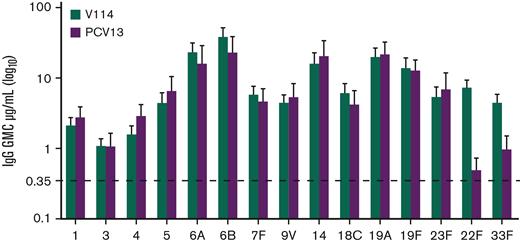
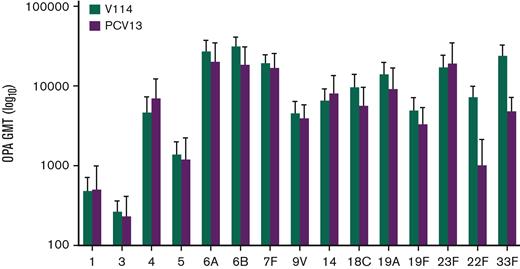
For the evaluation of immunogenicity, serotype-specific IgG were measured 30 days after vaccination. IgG GMCs in recipients of V114 at 30 days after vaccination were generally comparable to PCV13 for the 13 shared serotypes, and higher than PCV13 for V114 serotypes 22F and 33F (Figure 3). As observed with IgG GMCs, serotype-specific OPA GMTs for V114 were generally comparable to PCV13 for the 13 shared serotypes and higher than PCV13 for V114 serotypes 22F and 33F (Figure 4). Both V114 (for all 15 serotypes) and PCV13 (for all 13 serotypes) were immunogenic, as assessed by the geometric mean fold rise in serotype-specific IgG GMCs and OPA GMTs from before vaccination to 30 days after vaccination (supplemental Tables 5-6). There was generally no change in the IgG GMCs and OPA GMTs for serotypes 22F and 33F in the PCV13 group from before to after vaccination.
Serotype-specific IgG GMCs at 30 days after vaccination. The serotype-specific IgG GMCs and 95% confidence intervals on day 30 are shown for each vaccination group.
Serotype-specific IgG GMCs at 30 days after vaccination. The serotype-specific IgG GMCs and 95% confidence intervals on day 30 are shown for each vaccination group.
Serotype-specific OPA GMTs at 30 days after vaccination. The serotype-specific OPA GMTs and 95% confidence intervals on day 30 are shown for each vaccination group.
Serotype-specific OPA GMTs at 30 days after vaccination. The serotype-specific OPA GMTs and 95% confidence intervals on day 30 are shown for each vaccination group.
Discussion
This study evaluated the safety, tolerability, and immunogenicity of V114 in children with SCD. V114 was well tolerated, with a safety profile comparable to that of PCV13. Although no formal statistical analyses were performed on the safety data because of the relatively small number of participants, vaccine-related systemic AEs were higher in the V114 group. Most AEs were of mild or moderate intensity in this pediatric population with SCD, which was similarly observed following vaccination with V114 in healthy adults and children.20-22 No serious AEs were assessed by the investigator to be related to the study vaccine. Overall, these study results are consistent with prior studies evaluating the safety of PCV in infants and children with SCD.10,26,27
After a single dose of V114, an immune response to all 15 serotypes could be detected by an increase in serotype-specific IgG from before vaccination to 30 days after vaccination. Antibodies were functional as measured by OPA, and IgG GMCs and OPA GMTs to the 13 shared serotypes were generally comparable to those elicited by PCV13. Additionally, increases in IgG and OPA responses to serotypes 22F and 33F were observed after vaccination with V114 (>fourfold rise for GMCs and >3 fold rise for OPA), but not with PCV13. However, some nonspecific antibodies were observed in the OPA assay for serotype 33F at baseline among the recipients of both vaccination groups. This could be related to natural exposure but more likely to the nature of the reagents used for this serotype in the OPA assay. This phenomenon has been observed in previously published studies of V114 for serotype 33F,28 as well as in studies comparing PCV7 and PCV13.29 Although responses varied across serotypes, immune measurements for serotypes 22F and 33F were well within the range of the other 13 serotypes. Overall, these data support catch-up vaccination with V114 for broader serotype coverage in children with SCD.
A strength of this study is its focus on an important, historically marginalized, and vulnerable pediatric population. Although penicillin prophylaxis is recommended to reduce clinically significant bacterial infections in children under 5 years of age,13,30 breakthrough infections can occur. Theoretically, continuous antibiotic exposure may contribute to the development of antibiotic-resistant pathogens and negatively affect gut microbiome development. As free polysaccharide vaccines have shown limited effectiveness against PD in children, the significant impact of licensed PCVs in the reduction of PD burden in children with SCD supports the continued development of PCVs to ensure protection against disease caused by serotypes not included in licensed PCVs, as well as disease caused by vaccine serotypes for which licensed PCVs have been shown to have limited effectiveness. Although relatively understudied compared to healthy populations, the safety and immunogenicity of PCVs have been demonstrated in children with SCD.10,26,27
This study has several limitations. This study was descriptive in nature and not powered to statistically test between-group differences in safety and immunogenicity. Because of the small number of participants, it is unlikely that rare AEs were identified during the study. Although V114 induced robust immune responses to all 15 serotypes included in the vaccine, we did not evaluate its effectiveness against pneumococcal disease in this study. The study did not evaluate the safety and immunogenicity of the recommended pneumococcal vaccination strategy in patients with SCD, as study volunteers were not administered PPSV23 approximately 6 to 8 weeks after V114 or PCV13. However, the safety of sequential administration of V114 followed by PPSV23 was evaluated in adults living with HIV,31 and no unexpected safety events were noted following the receipt of PPSV23. After study completion, a 20-valent PCV was approved for use in adults32 and therefore was not available for use as an active comparator in this study.
In conclusion, these data suggest that V114 has a generally comparable safety, tolerability, and immunogenicity profile to PCV13 and further induces immunogenicity to serotypes 22F and 33F, serotypes not contained in PCV13, after a single vaccination in children with SCD. These results support the use of V114 in the pediatric SCD population for protection against PD.
Acknowledgments
The authors would like to thank the participants and their families, study staff, and investigators in the V114-023 study group for their contribution. Medical writing support was provided by Timothy J. Chapman and editorial support was provided by Karyn Davis (both of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ).
The funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ.
Authorship
Contribution: L.M., K.B., G.B., R.D., R.L., M.P., C.T.Q., G.T., R.T.W., and R.M. conceived and designed the study; L.M., H.R., E.L.-M., M.P., C.T.Q., and R.T.W. acquired of the data; L.M., K.B., R.L., and Q.S. analyzed the data; L.M., K.B., R.D., K.F., D.J., R.L., C.T.Q., P.R., Q.S., and R.T.W. performed interpretation of the data; L.M. and R.T.W. drafted the manuscript. All authors critically reviewed or revised the manuscript for important intellectual content and approved the final version, except for R.M., who reviewed the manuscript but did not approve the final version because he died.
Richard McFetridge died on 24 March 2022.
A complete list of the members of the PNEU-SICKLE Study Group appears in the supplemental Appendix.
Conflict-of-interest disclosure: R.T.W., M.P., G.B., Q.S., K.F., R.M., G.T., R.L., and L.M. are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, and may hold stock in Merck & Co, Inc, Rahway, NJ. K.B. was an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ at the time of the study, may hold stock in Merck & Co, Inc, Rahway, NJ, and is currently employed by Affinivax Inc. D.J. has received i) speaker honoraria from Pfizer, GlaxoSmithKline, Janssen, Sanofi, Abbott, and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, ii) honoraria as a clinical trial investigator from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, iii) honoraria as a clinical trial investigator from GlaxoSmithKline, Takeda, and Janssen outside the submitted work, and iv) an institutional research grant from Pfizer outside the submitted work. R.D. reports grants or contracts from Pfizer, Medimmune, and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ; consulting fees from Pfizer, Biondvax, MeMed, and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ; serving on advisory boards of Pfizer, Biondvax, and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ; providing expert testimony for Pfizer; and participating on an advisory board for Pfizer, Biondvax, and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ. P.R. has served on vaccine advisory boards for Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, Pfizer, and GlaxoSmithKline, and received institutional grant funding from GlaxoSmithKline and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Richard T. Wiedmann, Merck & Co, Inc, 126 E. Lincoln Ave, Rahway, NJ 07065; e-mail: richard.wiedmann@merck.com.
References
Author notes
The data sharing policy, including restrictions, of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ is available at http://engagezone.msd.com/ds_documentation.php.
Requests for access to the clinical study data can be submitted through the Engage Zone site or via email (dataaccess@merck.com).
The full-text version of this article contains a data supplement.