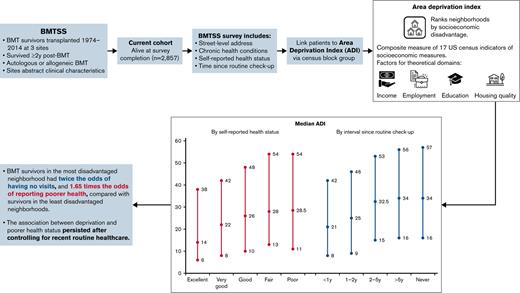
Key Points
BMT survivors living in disadvantaged neighborhoods reported a longer time since routine health care, all other things equal.
BMT survivors living in disadvantaged neighborhoods also reported poorer health status, even controlling for time since recent check-up.
Abstract
Living in a disadvantaged neighborhood is associated with poor health outcomes. Blood or Marrow Transplant (BMT) survivors remain at risk of chronic health conditions requiring anticipatory management. We hypothesized that among BMT survivors, neighborhood disadvantage was associated with poor self-reported routine health care utilization and health. We leveraged data from BMTSS – a retrospective cohort study examining long-term outcomes among individuals surviving ≥2 y following BMT at three institutions between 1974 and 2014. Participants in this analysis completed the BMTSS survey (sociodemographics; chronic health conditions; time since routine check-up; self-reported health). The Area Deprivation Index (ADI) represented neighborhood disadvantage; this composite indicator of 17 census measures is a percentile rank (0 = least deprived to 100 = most deprived). Multivariable ordered logit regression adjusted for clinical factors and individual-level sociodemographics, modeling associations between ADI, time since routine check-up, and self-reported health. Among 2,857 survivors, median ADI was 24 (interquartile range: 10-46). Adjusting for self-reported individual-level socioeconomic indicators and chronic health conditions, patients in more disadvantaged neighborhoods had higher odds of reporting longer intervals since routine check-up (ORADI_continuous = 1.007, P < .001) and poorer health status (controlling for time since check-up; ORADI_continuous = 1.005, P = .003). Compared with patients living in the least disadvantaged neighborhood (ADI = 1), patients in the most disadvantaged neighborhood (ADI = 100), had twice the odds (ORADI = 1.007^99 = 2.06) of reporting no routine visits and 1.65-times the odds of reporting poor health (ORADI = 1.005^99 = 1.65). In BMT survivors, access to health care and health status are associated with area disadvantage. These findings may inform strategies to address long-term care coordination and retention for vulnerable survivors.
Introduction
Residing in a neighborhood characterized by socioeconomic disadvantage has been associated with poorer health outcomes, such as increased comorbidity burden and premature mortality.1-4 For example, neighborhood disadvantage has been associated with general surgical5 and medical6 outcomes, as well as outcomes among cancer patients with solid tumors.7
Blood or marrow transplantation (BMT) is used with curative intent for hematologic malignancies and other life-threatening illnesses. Despite technical advances and the associated improvement in survival, BMT recipients are at a high risk of long-term and late-occurring chronic health conditions (such as subsequent neoplasms, heart failure, and endocrinopathies)8-12 that require anticipatory management. Prevention, early detection, and treatment of these chronic health conditions often requires specialty health care (cardiology, etc.) as well as preventive care (lifestyle modifications, surveillance for early detection). The complexity of BMT treatment and follow-up also requires the expertise of designated transplant centers.13
Social risk factors such as community characteristics impact health care utilization and outcomes.14 Indeed, health interventions that do not account for neighborhood disadvantage and social factors are at risk of being ineffective. However, the relationship among neighborhood socioeconomic disadvantage and health care utilization and outcomes in long-term BMT survivors remains unstudied.
In order to investigate this relationship and understand what domain may be targeted for patient-level or system-level interventions, we leveraged the Blood or Marrow Transplant Survivor Study (BMTSS), a retrospective cohort study designed to examine long-term outcomes of BMT.10,12,15-17 We hypothesized that in the context of clinical factors, socioeconomic disadvantage would be associated with poorer health status and decreased utilization of routine health care, as reported by BMT survivors.
Methods
Data source
The BMTSS cohort includes individuals who have lived at least 2 years following allogeneic or autologous BMT performed at the City of Hope National Medical Center, the University of Minnesota, or the University of Alabama at Birmingham between 1 January 1974 and 31 December 2014. Participants completed the BMTSS survey, self-reporting chronic health conditions, cancer relapse or development of a subsequent malignant neoplasm, health care utilization, and sociodemographic details. The analyses presented here included patients who had received a single BMT and were alive at the time of completing the BMTSS survey (supplemental Figure 1). The University of Alabama at Birmingham served as the single institutional review board of record; written informed consent was provided according to the Declaration of Helsinki.
Independent variables
Primary exposure - Area Deprivation Index (ADI)
We measured neighborhood disadvantage using ADI, a validated composite indicator created by the Health Resources and Services Administration; 2015 ADI was used for patients completing surveys 2015 or earlier, while 2019 ADI was used for patients completing surveys 2016 and beyond.14,18 ADI has been linked to health care utilization19 and a wide range of health outcomes20,21 including cancer.22,23 ADI includes factors for theoretical domains of income, education, employment, and housing quality, using 17 measures derived from US census data (supplemental Table 1).21 Neighborhoods are then ranked by socioeconomic disadvantage based on Census Block Group using a percentile rank ranging from 0 (least deprived) to 100 (most deprived). ADI is validated at the census block group level, thus providing more granularity than county-level or zip-code (5-digit) measures; it is maintained with updated data,24 and is publicly available.25 BMT survivors were linked to ADI via census block group using the 12-digit Federal Information Processing Series code, derived from patient street-level addresses provided when completing the survey.
Self-reported sociodemographics
Survivors reported the following in the BMTSS Survey: age at study participation, sex, race/ethnicity (non-Hispanic white, African American, Hispanic, Asian, other), insurance (uninsured, Medicare, Medicaid, employer-based, direct purchase, military, Indian Health Service, other; not mutually exclusive), education (less than high school, high school diploma or general education diploma (GED), some college or post-high school training, college degree or higher, other), annual household income (<$20K, $20-$49K, $50K-$74K, $75K-$99K, > $100K), marital status (never married, married, divorced, separated, widowed, other).
Clinical characteristics
The following data were obtained from institutional transplant databases: primary diagnosis, age at BMT, donor relation (related, unrelated, autologous), stem cell source (peripheral blood stem cells, bone marrow, cord blood), intensity of conditioning regimen (myeloablative, non-myeloablative, reduced intensity), and history of chronic graft vs host disease (GvHD).
Chronic health conditions
Participants completing the BMTSS survey reported a diagnosis of chronic health conditions (endocrinopathies, central nervous system compromise, cardiopulmonary dysfunction, gastrointestinal and hepatic sequelae, musculoskeletal abnormalities, and subsequent malignancies) by answering the question “At any time after your BMT were you diagnosed with <chronic health condition>?” A positive response was followed by the questions “When was it diagnosed?” and “Where was it diagnosed?” The high sensitivity and specificity of the BMTSS survey confirms the accuracy of reports by BMT survivors regarding medical conditions.26 Scoring to determine the severity of chronic health conditions used the Common Terminology Criteria for Adverse Events (CTCAE v.5.0). We calculated a summative index, multiplying each CTCAE grade by the number of conditions present at that grade and then totaling them: 1∗(# grade 1) + 2∗(# grade 2) + 3∗(# grade 3) + 4∗(# grade 4). For instance, a patient with two CTCAE grade 1 conditions, one CTCAE grade 2 condition, no CTCAE grade 3 conditions, and two CTCAE grade 4 conditions would have a 12 on the summative index: 1∗(n = 2) + 2∗(n = 1) + 3∗(n = 0) + 4∗(n = 2) = 12. The aggregate measure accounted for the number and severity of the conditions, with a higher score indicating more/worse conditions.
Dependent variables
Outcomes - healthcare utilization and self-reported health
The BMTSS survey captures health care utilization (“When was your most recent routine check-up? <1 y ago, 1-2 y ago, 2-5 y ago, ≥5 y ago, never”) and self-reported health status (“In general would you say your health is: excellent, very good, good, fair, or poor?”). The patient’s routine check-up may have been with any type of provider (transplant center, primary care, etc.). The analyses modeled five outcome categories as below; neither outcome variable was dichotomized.
Statistical analysis
We modeled the association between ADI and the odds of a longer time since a routine health care visit or worse self-reported health through ordered logit models, which represent a series of cumulative logit models. The ordered logit models estimate a series of binary logit models, whereby the five ordered categories for each outcome (ie, health care utilization or self-reported health) are combined into different binary categories (ie, 1 vs 2, 3, 4, 5; 1, 2 vs 3, 4, 5; etc.). Each model was adjusted for available clinical factors (primary cancer diagnosis, donor source, conditioning intensity, chronic health conditions, chronic GvHD, time from BMT) and individual-level sociodemographic characteristics (age at survey, sex, payor, race/ethnicity, education, income and marital status). The regression modeling self-reported health status was also adjusted for interval since routine health care. The overall models did not violate the proportional odds assumption; therefore, the coefficients presented take the same values for each category of the outcome variable in our models.
Results
Patients
BMT survivors (n = 2857) completed surveys a median of 9 y (Interquartile range [IQR]: 5-16) following transplant. Table 1 summarizes patient characteristics. Median age at BMT was 47 y (IQR: 30-58). The majority of the cohort was male (n = 1535, 53.7%) and non-Hispanic white (n = 2176, 76.2%), with 12.2% Hispanic (n = 349), 4.8% Black/African American (n = 136), and 5.4% Asian/Pacific Islander (n = 153). Over half of the cohort reported employer-based coverage (n = 1531, 53.6%) and 41.3% reported Medicare (n = 1,180). The majority of the cohort had a college degree (n = 1553, 54.4%) and was married (n = 1780, 62.3%). The largest proportion of patients reported an annual household income of at least $100 000 (n = 781, 27.3%) or $20 000 to $49 000 (n = 559, 19.6%).
Demographic and clinical characteristics of 2857 survivors of blood or marrow transplantation
| Variables . | n (%) . |
|---|---|
| Sex | |
| Male | 1535 (53.7%) |
| Age at study participation in years | |
| Median (interquartile range [IQR]) | 47 (30-58) |
| Time from transplant in years | |
| Median (IQR) | 9 (5-16) |
| Race/ethnicity, n (%) | |
| Non-Hispanic White | 2176 (76.2%) |
| Black or African American | 136 (4.8%) |
| Hispanic or Latino | 349 (12.2%) |
| Asian | 153 (5.4%) |
| Non-Hispanic other/unknown | 43 (1.5%) |
| Payor∗n (%) | |
| Uninsured | 66 (2.3%) |
| Medicare | 1180 (41.3%) |
| Medicaid | 267 (9.3%) |
| Employer | 1531 (53.6%) |
| Direct purchase | 383 (13.4%) |
| Military/Indian Health Service/other | 354 (12.4%) |
| Education, n (%) | |
| < High school | 131 (4.6%) |
| High school diploma or GED | 351 (12.3%) |
| Some college or post-high school training | 766 (26.8%) |
| College degree or higher | 1553 (54.4%) |
| Other/missing | 56 (2.0%) |
| Annual household income, n (%) | |
| <$20K | 286 (10.0%) |
| $20 to $49K | 559 (19.6%) |
| $50 to $74K | 449 (15.7%) |
| $75 to $99K | 372 (13.0%) |
| >$100K | 781 (27.3%) |
| Don’t know/ prefer not to answer/missing | 410 (14.4%) |
| Marital status, n (%) | |
| Never married | 487 (17.0%) |
| Married | 1780 (62.3%) |
| Divorced/separated/widowed/other/missing | 590 (20.7%) |
| ADI national ranking (percentile) | |
| Median (IQR) | 24 (10-46) |
| Donor relation, n (%) | |
| Related | 826 (28.9%) |
| Unrelated | 654 (22.9%) |
| Autologous | 1377 (48.2%) |
| Stem cell source, n (%) | |
| Peripheral blood stem cells | 1995 (69.8%) |
| Bone marrow | 669 (23.4%) |
| Cord blood | 193 (6.8%) |
| Conditioning regimen, n (%) | |
| Myeloablative | 2170 (76.0%) |
| Non-myeloablative or reduced intensity | 687 (24.0%) |
| Chronic graft-versus-host disease (among allogeneic BMT), n (%) | |
| Yes | 822 (55.5%) |
| Chronic conditions summative index | |
| Median (IQR) | 5 (2-8) |
| Diagnosis, n (%) | |
| Acute lymphoblastic leukemia | 231 (8.1%) |
| Acute myeloid leukemia or myelodysplastic syndrome | 716 (25.1%) |
| Chronic myelogenous leukemia | 235 (8.2%) |
| Hodgkin/non-Hodgkin lymphoma | 954 (33.4%) |
| Other leukemia/severe aplastic anemia/other | 241 (8.4%) |
| Plasma cell dyscrasias | 480 (16.8%) |
| Variables . | n (%) . |
|---|---|
| Sex | |
| Male | 1535 (53.7%) |
| Age at study participation in years | |
| Median (interquartile range [IQR]) | 47 (30-58) |
| Time from transplant in years | |
| Median (IQR) | 9 (5-16) |
| Race/ethnicity, n (%) | |
| Non-Hispanic White | 2176 (76.2%) |
| Black or African American | 136 (4.8%) |
| Hispanic or Latino | 349 (12.2%) |
| Asian | 153 (5.4%) |
| Non-Hispanic other/unknown | 43 (1.5%) |
| Payor∗n (%) | |
| Uninsured | 66 (2.3%) |
| Medicare | 1180 (41.3%) |
| Medicaid | 267 (9.3%) |
| Employer | 1531 (53.6%) |
| Direct purchase | 383 (13.4%) |
| Military/Indian Health Service/other | 354 (12.4%) |
| Education, n (%) | |
| < High school | 131 (4.6%) |
| High school diploma or GED | 351 (12.3%) |
| Some college or post-high school training | 766 (26.8%) |
| College degree or higher | 1553 (54.4%) |
| Other/missing | 56 (2.0%) |
| Annual household income, n (%) | |
| <$20K | 286 (10.0%) |
| $20 to $49K | 559 (19.6%) |
| $50 to $74K | 449 (15.7%) |
| $75 to $99K | 372 (13.0%) |
| >$100K | 781 (27.3%) |
| Don’t know/ prefer not to answer/missing | 410 (14.4%) |
| Marital status, n (%) | |
| Never married | 487 (17.0%) |
| Married | 1780 (62.3%) |
| Divorced/separated/widowed/other/missing | 590 (20.7%) |
| ADI national ranking (percentile) | |
| Median (IQR) | 24 (10-46) |
| Donor relation, n (%) | |
| Related | 826 (28.9%) |
| Unrelated | 654 (22.9%) |
| Autologous | 1377 (48.2%) |
| Stem cell source, n (%) | |
| Peripheral blood stem cells | 1995 (69.8%) |
| Bone marrow | 669 (23.4%) |
| Cord blood | 193 (6.8%) |
| Conditioning regimen, n (%) | |
| Myeloablative | 2170 (76.0%) |
| Non-myeloablative or reduced intensity | 687 (24.0%) |
| Chronic graft-versus-host disease (among allogeneic BMT), n (%) | |
| Yes | 822 (55.5%) |
| Chronic conditions summative index | |
| Median (IQR) | 5 (2-8) |
| Diagnosis, n (%) | |
| Acute lymphoblastic leukemia | 231 (8.1%) |
| Acute myeloid leukemia or myelodysplastic syndrome | 716 (25.1%) |
| Chronic myelogenous leukemia | 235 (8.2%) |
| Hodgkin/non-Hodgkin lymphoma | 954 (33.4%) |
| Other leukemia/severe aplastic anemia/other | 241 (8.4%) |
| Plasma cell dyscrasias | 480 (16.8%) |
GED, general education diploma; BMT, blood marrow transplantation.
Patients may select multiple payors.
Over half of the cohort received allogeneic transplants (related donor: 28.9%, n = 826; unrelated donor: 22.9%, n = 654). The majority of patients underwent a myeloablative transplant (n = 2,170, 76.0%) with peripheral blood stem cells (n = 1995, 69.8%). The most common primary diagnoses included acute myeloid leukemia/myelodysplastic syndrome (n = 716, 25.1%) and Hodgkin or non-Hodgkin lymphoma (n = 954, 33.4%). Over half of the allogeneic BMT recipients had a history of chronic GvHD (n = 822, 55.5%). The median summative index of chronic health conditions for patients was 5 (IQR: 2-8).
ADI
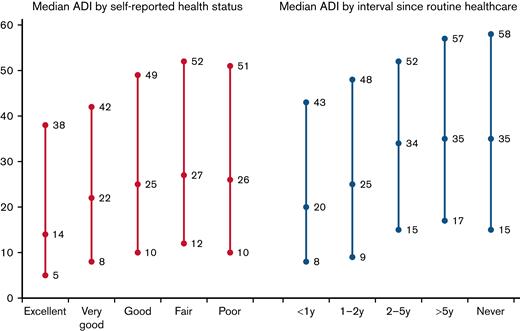
The median ADI was 24 (IQR: 10-46), with the sample ADI minimum and maximum 1 and 100, respectively. The median ADI ranged from 14 (IQR: 5-38) in patients rating their health as excellent to 26 (IQR: 10-51) in those rating their health as poor (see Figure 1). Similarly, median ADI ranged from 20 (IQR: 8-43) in patients with visits < 1 y ago to 35 (IQR: 15-58) in patients reporting no visits (see Figure 1).
Median Area Deprivation Index (ADI) by self-reported health status and by interval since routine healthcare (including 25th and 75th percentile).
Median Area Deprivation Index (ADI) by self-reported health status and by interval since routine healthcare (including 25th and 75th percentile).
Time since routine check-up
Supplemental Table 2 summarizes patient characteristics by interval since routine check-up. While 68% of the cohort (n = 1946) reported having a routine check-up less than a year prior to study completion, 9% (n = 259) reported a routine visit between 1 y and 2 y prior and 6% (n = 173) reported a routine visit within 2 y to 5 y. In addition, 10% (n = 289) reported it had been over 5 y since their last routine checkup and 7% (n = 190) reported never having had a routine checkup. There was a statistically significant association between ADI and the interval since routine checkup (Table 2). Thus, patients living in more disadvantaged neighborhoods (represented by a higher ADI) had higher odds of a longer time since routine checkup for each one-unit increase in ADI ranking (ORper_unit_higher_ADI = 1.007, 95%CI, 1.00-1.01, P < .001). Thus, a patient living in the most disadvantaged neighborhood had twice the odds (ORADI = 1.007^99 = 2.06) of reporting no visits compared with a patient living in the least disadvantaged neighborhood (representing a 99-unit increase).
Multivariable ordered logistic regression§
| Variables . | Longer Time since Routine Check-up (n = 2857) . | Worse Self-Reported Health (n = 2852) . | ||||
|---|---|---|---|---|---|---|
| OR (Ologit) . | P value . | 95% CI . | OR (Ologit) . | P value . | 95% CI . | |
| Area Deprivation Index (ADI) national ranking (percentile) | ||||||
| Per unit increase in ADI ranking | 1.01 | < 0.001 | 1.00–1.01 | 1.01 | 0.003 | 1.00–1.01 |
| Age at transplant (years) | ||||||
| Per year increase in age at Blood or marrow transplantation (BMT) | 1.00 | 0.67 | 0.99–1.01 | 0.99 | 0.001 | 0.97–1.0 |
| Time from transplant (years) | ||||||
| Per year increase in time from BMT | 1.11 | < 0.001 | 1.09–0.12 | 0.98 | 0.03 | 0.97–1.00 |
| Chronic conditions summative index | ||||||
| Per unit increase in summative index | 0.95 | < 0.001 | 0.94–0.97 | 1.14 | < 0.001 | 1.12–1.16 |
| Time since recent check-up (reference: never) | ||||||
| <1 y | – | – | – | 1.03 | 0.83 | 0.77–1.39 |
| 1-2 y | – | – | – | 0.95 | 0.78 | 0.67–1.36 |
| 2-5 y | – | – | – | 1.28 | 0.22 | 0.86–1.88 |
| >5 y | – | – | – | 1.21 | 0.28 | 0.86–1.72 |
| Sex (reference: female) | ||||||
| Male | 0.91 | 0.28 | 0.77–1.08 | 1.17 | 0.03 | 1.02–1.35 |
| Race/ethnicity (reference: Non-Hispanic White) | ||||||
| Black or African American | 1.17 | 0.46 | 0.77–1.80 | 1.22 | 0.24 | 0.87–1.72 |
| Hispanic or Latino | 1.12 | 0.41 | 0.85–1.48 | 1.16 | 0.19 | 0.92–1.45 |
| Asian | 1.52 | 0.03 | 1.03–2.23 | 1.76 | < 0.001 | 1.28–2.39 |
| Other/unknown | 1.37 | 0.31 | 0.73–2.53 | 1.11 | 0.73 | 0.63–1.95 |
| Payor (reference: uninsured) | ||||||
| Medicare | 0.95 | 0.68 | 0.76–1.20 | 1.28 | 0.01 | 1.06–1.55 |
| Medicaid | 0.73 | 0.07 | 0.52–1.02 | 1.39 | 0.02 | 1.05–1.83 |
| Employer | 0.93 | 0.55 | 0.72–1.19 | 1.05 | 0.64 | 0.86–1.29 |
| Direct purchase | 1.03 | 0.86 | 0.77–1.37 | 0.96 | 0.76 | 0.76–1.23 |
| Military/Indian Health Service/other | 1.09 | 0.56 | 0.82–1.43 | 1.12 | 0.33 | 0.89–1.42 |
| Education (reference: < high school) | ||||||
| High school diploma or GED | 1.02 | 0.92 | 0.67–1.57 | 0.57 | 0.004 | 0.39–0.84 |
| Some college or post-high school training | 1.17 | 0.44 | 0.78–1.74 | 0.57 | 0.002 | 0.40–0.82 |
| College degree or higher | 0.84 | 0.39 | 0.57–1.25 | 0.49 | <0.001 | 0.34–0.70 |
| Other/missing | 0.79 | 0.52 | 0.38–1.64 | 0.38 | 0.002 | 0.21–0.70 |
| Annual household income (reference: < $20K) | ||||||
| $20 to $49K | 0.82 | 0.24 | 0.59–1.14 | 0.96 | 0.79 | 0.73–1.28 |
| $50 to $74K | 0.80 | 0.22 | 0.56–1.14 | 0.79 | 0.12 | 0.58–1.07 |
| $75 to $99K | 0.82 | 0.33 | 0.56–1.21 | 0.69 | 0.03 | 0.50–0.96 |
| >$100K | 0.66 | 0.03 | 0.46–0.96 | 0.52 | <0.001 | 0.38–0.71 |
| Other‡ | 0.88 | 0.48 | 0.62–1.25 | 0.77 | 0.10 | 0.57–1.05 |
| Marital status (reference: never married) | ||||||
| Married | 1.16 | 0.30 | 0.87–1.53 | 1.25 | 0.06 | 0.99–1.59 |
| Other∗ | 1.17 | 0.29 | 0.87–1.59 | 1.38 | 0.02 | 1.06–1.78 |
| Donor relation (reference: related donor) | ||||||
| Unrelated | 0.85 | 0.24 | 0.64–1.12 | 0.87 | 0.21 | 0.70–1.09 |
| Autologous | 1.28 | 0.16 | 0.91–1.78 | 1.07 | 0.69 | 0.80–1.43 |
| Conditioning intensity (reference: myeloablative) | ||||||
| Non-myeloablative or reduced intensity | 0.91 | 0.48 | 0.72–1.17 | 1.04 | 0.71 | 0.85–1.28 |
| Stem cell source (reference: cord blood) | ||||||
| Peripheral blood stem cells | 0.62 | 0.03 | 0.40–0.97 | 1.25 | 0.24 | 0.87–1.79 |
| Bone marrow | 0.78 | 0.25 | 0.51–1.19 | 0.83 | 0.33 | 0.58–1.20 |
| Chronic graft vs host disease (reference: no) | ||||||
| Yes | 0.80 | 0.09 | 0.61–1.04 | 1.24 | 0.06 | 0.99–1.55 |
| Diagnosis (reference: acute lymphoblastic leukemia) | ||||||
| Acute myeloid leukemia or myelodysplastic syndrome | 1.22 | 0.27 | 0.86–1.73 | 1.22 | 0.18 | 0.91–1.62 |
| Chronic myelogenous leukemia | 1.18 | 0.44 | 0.78–1.77 | 1.91 | < 0.001 | 1.34–2.74 |
| Hodgkin/non-Hodgkin lymphoma | 1.31 | 0.19 | 0.88–1.95 | 1.24 | 0.20 | 0.89–1.72 |
| Other leukemia/severe aplastic anemia/other | 1.08 | 0.70 | 0.73–1.62 | 1.23 | 0.25 | 0.86–1.75 |
| Plasma cell dyscrasia | 0.70 | 0.15 | 0.43–1.14 | 2.42 | < 0.001 | 1.65–3.56 |
| Variables . | Longer Time since Routine Check-up (n = 2857) . | Worse Self-Reported Health (n = 2852) . | ||||
|---|---|---|---|---|---|---|
| OR (Ologit) . | P value . | 95% CI . | OR (Ologit) . | P value . | 95% CI . | |
| Area Deprivation Index (ADI) national ranking (percentile) | ||||||
| Per unit increase in ADI ranking | 1.01 | < 0.001 | 1.00–1.01 | 1.01 | 0.003 | 1.00–1.01 |
| Age at transplant (years) | ||||||
| Per year increase in age at Blood or marrow transplantation (BMT) | 1.00 | 0.67 | 0.99–1.01 | 0.99 | 0.001 | 0.97–1.0 |
| Time from transplant (years) | ||||||
| Per year increase in time from BMT | 1.11 | < 0.001 | 1.09–0.12 | 0.98 | 0.03 | 0.97–1.00 |
| Chronic conditions summative index | ||||||
| Per unit increase in summative index | 0.95 | < 0.001 | 0.94–0.97 | 1.14 | < 0.001 | 1.12–1.16 |
| Time since recent check-up (reference: never) | ||||||
| <1 y | – | – | – | 1.03 | 0.83 | 0.77–1.39 |
| 1-2 y | – | – | – | 0.95 | 0.78 | 0.67–1.36 |
| 2-5 y | – | – | – | 1.28 | 0.22 | 0.86–1.88 |
| >5 y | – | – | – | 1.21 | 0.28 | 0.86–1.72 |
| Sex (reference: female) | ||||||
| Male | 0.91 | 0.28 | 0.77–1.08 | 1.17 | 0.03 | 1.02–1.35 |
| Race/ethnicity (reference: Non-Hispanic White) | ||||||
| Black or African American | 1.17 | 0.46 | 0.77–1.80 | 1.22 | 0.24 | 0.87–1.72 |
| Hispanic or Latino | 1.12 | 0.41 | 0.85–1.48 | 1.16 | 0.19 | 0.92–1.45 |
| Asian | 1.52 | 0.03 | 1.03–2.23 | 1.76 | < 0.001 | 1.28–2.39 |
| Other/unknown | 1.37 | 0.31 | 0.73–2.53 | 1.11 | 0.73 | 0.63–1.95 |
| Payor (reference: uninsured) | ||||||
| Medicare | 0.95 | 0.68 | 0.76–1.20 | 1.28 | 0.01 | 1.06–1.55 |
| Medicaid | 0.73 | 0.07 | 0.52–1.02 | 1.39 | 0.02 | 1.05–1.83 |
| Employer | 0.93 | 0.55 | 0.72–1.19 | 1.05 | 0.64 | 0.86–1.29 |
| Direct purchase | 1.03 | 0.86 | 0.77–1.37 | 0.96 | 0.76 | 0.76–1.23 |
| Military/Indian Health Service/other | 1.09 | 0.56 | 0.82–1.43 | 1.12 | 0.33 | 0.89–1.42 |
| Education (reference: < high school) | ||||||
| High school diploma or GED | 1.02 | 0.92 | 0.67–1.57 | 0.57 | 0.004 | 0.39–0.84 |
| Some college or post-high school training | 1.17 | 0.44 | 0.78–1.74 | 0.57 | 0.002 | 0.40–0.82 |
| College degree or higher | 0.84 | 0.39 | 0.57–1.25 | 0.49 | <0.001 | 0.34–0.70 |
| Other/missing | 0.79 | 0.52 | 0.38–1.64 | 0.38 | 0.002 | 0.21–0.70 |
| Annual household income (reference: < $20K) | ||||||
| $20 to $49K | 0.82 | 0.24 | 0.59–1.14 | 0.96 | 0.79 | 0.73–1.28 |
| $50 to $74K | 0.80 | 0.22 | 0.56–1.14 | 0.79 | 0.12 | 0.58–1.07 |
| $75 to $99K | 0.82 | 0.33 | 0.56–1.21 | 0.69 | 0.03 | 0.50–0.96 |
| >$100K | 0.66 | 0.03 | 0.46–0.96 | 0.52 | <0.001 | 0.38–0.71 |
| Other‡ | 0.88 | 0.48 | 0.62–1.25 | 0.77 | 0.10 | 0.57–1.05 |
| Marital status (reference: never married) | ||||||
| Married | 1.16 | 0.30 | 0.87–1.53 | 1.25 | 0.06 | 0.99–1.59 |
| Other∗ | 1.17 | 0.29 | 0.87–1.59 | 1.38 | 0.02 | 1.06–1.78 |
| Donor relation (reference: related donor) | ||||||
| Unrelated | 0.85 | 0.24 | 0.64–1.12 | 0.87 | 0.21 | 0.70–1.09 |
| Autologous | 1.28 | 0.16 | 0.91–1.78 | 1.07 | 0.69 | 0.80–1.43 |
| Conditioning intensity (reference: myeloablative) | ||||||
| Non-myeloablative or reduced intensity | 0.91 | 0.48 | 0.72–1.17 | 1.04 | 0.71 | 0.85–1.28 |
| Stem cell source (reference: cord blood) | ||||||
| Peripheral blood stem cells | 0.62 | 0.03 | 0.40–0.97 | 1.25 | 0.24 | 0.87–1.79 |
| Bone marrow | 0.78 | 0.25 | 0.51–1.19 | 0.83 | 0.33 | 0.58–1.20 |
| Chronic graft vs host disease (reference: no) | ||||||
| Yes | 0.80 | 0.09 | 0.61–1.04 | 1.24 | 0.06 | 0.99–1.55 |
| Diagnosis (reference: acute lymphoblastic leukemia) | ||||||
| Acute myeloid leukemia or myelodysplastic syndrome | 1.22 | 0.27 | 0.86–1.73 | 1.22 | 0.18 | 0.91–1.62 |
| Chronic myelogenous leukemia | 1.18 | 0.44 | 0.78–1.77 | 1.91 | < 0.001 | 1.34–2.74 |
| Hodgkin/non-Hodgkin lymphoma | 1.31 | 0.19 | 0.88–1.95 | 1.24 | 0.20 | 0.89–1.72 |
| Other leukemia/severe aplastic anemia/other | 1.08 | 0.70 | 0.73–1.62 | 1.23 | 0.25 | 0.86–1.75 |
| Plasma cell dyscrasia | 0.70 | 0.15 | 0.43–1.14 | 2.42 | < 0.001 | 1.65–3.56 |
CI, confidence interval; OR, odds ratio. Bolded values represent statistically significant findings (P < 0.05).
Divorced/separated/widowed/other/missing.
Ordered logit models using five outcome categories, also adjusted for BMT center and age at BMT.
Don’t know/prefer not to answer/missing.
Self-reported health
Supplemental Table 3 summarizes patient characteristics by self-reported health status. Patients reported their health to be excellent (n = 259, 9%), very good (n = 823, 29%), good (n = 1118, 39%), fair (n = 539, 19%), and poor (n = 113, 4%). In multivariable analyses adjusting for time since routine health care along with clinical and sociodemographic characteristics and chronic health conditions, there was a statistically significant association between ADI and self-reported health (Table 2). A one-unit increase in ADI ranking was associated with a statistically significant increased odds of worse self-reported health (ORper_unit_higher_ADI = 1.005, 95%CI, 1.00-1.01, P = .003). Thus, for our cohort, a patient living in the most disadvantaged neighborhood (ADI = 100) had 1.65 times the odds of poorer self-reported health (ORADI = 1.005^99 = 1.65) as compared with a patient living in the least disadvantaged neighborhood (ADI = 1).
Discussion
Among long-term BMT survivors, living in a disadvantaged neighborhood was associated with a longer time interval since a routine health care visit and poorer self-reported health, after adjusting for self-reported individual socioeconomic indicators and chronic health conditions. The significant association between area deprivation and poorer self-reported health persisted after controlling for the interval since routine health care.
Discrete characteristics of the communities in which patients live have been associated with health outcomes among both the general population2,27 and cancer patients with solid tumors.4 Taking this a step further, the Health Resources and Services Administration linked census data to health outcomes21 to create the ADI, and this composite measure of 17 US Census indicators of socioeconomic deprivation made it feasible to examine numerous facets of socioeconomic deprivation simultaneously. ADI also serves as a proxy for individual social risk factors such as economic stability and the built environment of the neighborhood, which are social determinants of health14 and have the potential for implications for value-based reimbursement.28 In the general population, ADI has been associated with surgical and medical outcomes,6 health care utilization,19 and hospital readmission,29 while in solid tumor cancer patients it has been associated with survival7 and anxiety.30 Our findings suggest that among long-term survivors of BMT, ADI is associated not only with self-reported routine health care utilization, but also with self-reported health, even after accounting for chronic health conditions, individual-level sociodemographics, and time since routine check-up.
Given the significant improvements in post-BMT survival and increased utilization of BMT,13 an increased population of BMT survivors remain at risk for significant morbidity following BMT.8,9,11,12 Fifteen years following allogeneic or autologous BMT, the overwhelming majority (71%) of patients experience a chronic health condition, with 41% experiencing severe/life-threatening conditions12; furthermore, survivors report severe/life-threatening health conditions significantly more frequently than controls.9,11,12 While chronic health conditions are associated with health-related quality of life in the general population,31 our findings support the premise that BMT survivors living in a disadvantaged neighborhood experience poorer self-reported health after accounting for chronic health conditions.
Healthcare utilization evaluated here in the context of socioeconomic disadvantage, accounting for individual factors. While previous studies have reported that specialized health care among BMT survivors is underutilized and that primary care utilization increased over time,32 we show that BMT survivors living in disadvantaged neighborhoods are less likely to have recently engaged in routine health care. Thus our findings indicate that the previously identified trend of increasing utilization of routine health care over time32 may not be consistent across patient populations based on disadvantage.
Although we hypothesized that health care access and utilization would relate to poorer self-reported health, utilization of routine health care did not mitigate poor self-reported health among survivors living in disadvantaged neighborhoods among BMT survivors in our study. Several frameworks propose how characteristics of the social and built environment provide context33 for individual patient outcome and behavior,34-39 each highlighting the need to understand the impact of factors at more than one level. Many variables and studies (such as ours) reflect the poverty paradigm40 with a focus on disadvantage33 using census measures of poverty (household income, unemployment, overcrowded housing, access to car, etc.),33 although ours is able to uniquely include detailed clinical and sociodemographic data. One consideration is that a patient’s environment also influences health behaviors41 (dietary patterns and obesity,42 smoking,43 reduced physical activity,44 and alcohol intake45); while these were previously perceived as lifestyle choices,46,47 they are greatly influenced by the built and social environment and remain relevant to both general populations and BMT survivors.10,48 Use of the ADI serves as a proxy for social risk factors in these findings,14,18 but further work is needed to better understand the complex interplay of the built and social environment with health behaviors and their effect on self-reported health.
This study needs to be interpreted within the context of its limitations. The cohort included patients who were alive at study participation, thus it is conceivable that they may have differed significantly from deceased patients with respect to socioeconomic deprivation. In addition, health care utilization was not formally validated with documentation at a facility or claims level, and relied on patient self-report. Nevertheless, these limitations are overcome by the robust access to patients having been treated in three geographically diverse regions across four decades and providing self-report of individual-level sociodemographic information and long-term morbidity data. Furthermore, with access to patient addresses we were able to evaluate deprivation at the level of census block group rather than a more general geographic unit such as 5-digit zip code or county. Further, chronic health conditions were included from the time of transplant and beyond, but did not include comorbidities prior to transplant. In addition, we did not consider late relapses as a covariate when examining the association between ADI and health care utilization or health status. Finally, while the summative index used for chronic conditions could not differentiate between various permutations that would result in the same score (eg, two grade-1 conditions versus one grade-2 condition), it was able to account for both the quantity and severity of conditions with more nuance than choosing one or more representative conditions.
In summary, we find that BMT survivors living in a disadvantaged neighborhood reported a longer time since a routine health care visit and poorer health status, after adjusting for chronic health conditions and self-reported individual-level socioeconomic characteristics. Even after controlling for health care utilization, the significant association between area deprivation and poorer self-reported health remained. These findings suggest that access to health care and health status are associated with area disadvantage in BMT survivors. In that the ADI serves as a proxy for social risk and place-based determinants of health, these findings may have implications for how providers incorporate strategies to address long-term care coordination and retention for vulnerable BMT survivors. Future research is needed to understand the mechanisms underlying these results, such as the facilitators and barriers to health care access and to attrition from long-term follow-up treatment. Depending on the mechanism underlying these findings, potential interventions could be at the patient level (screening survivors for concrete resource deprivation and/or health literacy and providing targeted education or support such as transportation) or at the system level (policy-level intervention regarding payor support for identified health care services). In this way, these findings can set the stage to better serve these patients and inform implications for survivorship care and multi-level interventions that include policy.
Acknowledgments
This work was supported by the National Institutes of Health (NCI; S.B.), (U01 CA213140; S.B.), (R01CA078938; S.B.), and Leukemia Lymphoma Society (S.B.).
Authorship
Contribution: J.A.W., S.B., and M.S.A. conceptualized and designed the study; L.H., E.S.R., N.B., A.B., H.S.T., L.F., E.F., J.H., W.L., J.W., A.S., S.L., F.L.W., M.A., and S.H.A. were responsible for data acquisition; M.S.A. analyzed the data; J.A.W., S.B., and M.S.A. interpreted the data; J.A.W. drafted the manuscript; S.B. and M.S.A. revised the manuscript; L.H., E.S.R., N.B., A.B., H.S.T., L.F., E.F., J.H., W.L., J.W., A.S., S.L., F.L.W., M.A., and S.H.A. critically reviewed the manuscript; and all authors have approved the manuscript as submitted, and agree to be accountable for all aspects of the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Julie A. Wolfson, Division of Pediatric Hematology-Oncology, Institute for Cancer Outcomes and Survivorship, University of Alabama at Birmingham, 1600 7th Ave South, Lowder 500, Birmingham, AL 35233; e-mail: jwolfson@uabmc.edu.
References
Author notes
Requests for data sharing should be sent to Julie A. Wolfson (jwolfson@uabmc.edu).
These findings were presented from the podium at the Annual Meeting of the American Society of Hematology (12 December 2021, Atlanta, GA).
The full-text version of this article contains a data supplement.