Key Points
TMI combined with fludarabine-melphalan is a feasible conditioning regimen for second or greater SCT.
The recommended tolerable dose of TMI is 12 Gy; 9 Gy may be considered for older or underweight patients.
Abstract
Relapse after allogeneic stem cell transplantation (allo-SCT) remains the primary cause of treatment failure. A second SCT can result in long-term survival in a subset of patients, but the relapse rate remains high. We conducted a single-center, phase 1, modified 3 + 3 dose-escalation study of the feasibility of combining intensity-modulated total marrow irradiation (IM-TMI) with fludarabine and melphalan for conditioning. Between December 2015 and May 2020, 21 patients with relapsed hematologic disease undergoing second or greater allo-SCT were treated with IM-TMI doses of 6 Gy, 9 Gy, or 12 Gy. Dose-limiting toxicity was defined as a grade 3 or higher treatment-related adverse event; mucositis was the primary dose-limiting toxicity. The median times to neutrophil and platelet engraftment were 10 and 18 days, respectively. The 1-year cumulative incidence of graft-versus-host disease was 65% (95% confidence interval CI, 38-83). The nonrelapse mortality at 2 years was 17% (95% CI, 4-39). Cumulative incidence of relapse at 2 years was 35% (95% CI, 13-58). Two-year progression-free survival and overall survival were 48% and 50%. We conclude that combining IM-TMI with fludarabine-melphalan is feasible. We recommend 12 Gy of IM-TMI with fludarabine-melphalan for second SCT, although 9 Gy may be used for older or underweight patients.
Introduction
Allogeneic stem cell transplantation (allo-SCT) is currently the only curative treatment for those at high risk for relapse with hematologic malignancies. In the past few decades, the number of allo-SCTs has steadily increased worldwide, while treatment-related mortality has decreased due to reductions in transplant toxicity, infections, and severe graft-versus-host disease (GVHD).1,2 As a consequence of these improvements, relapse after allo-SCT has emerged as the primary cause of treatment failure, and prognosis at the time of relapse is generally poor.
Treatment options after allo-SCT relapse are limited and come with varying efficacy and toxicity profiles. These options include withdrawal of immune suppression, donor lymphocyte infusion, chemotherapy, and second allogeneic transplant, along with cytokine and adoptive cell therapy.3 Previous reports show that a second allo-SCT after failure of first myeloablative SCT has limited efficacy in patients with acute myeloid leukemia (AML) due to high treatment-related mortality and relapse rate. However, a second SCT can result in long-term survival in a subset of patients, including those with remission durations >12 months and those who achieve a complete remission (CR) before the second SCT.4-7 A retrospective registry study by the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation evaluated treatment options, risk factors, and outcomes of adults with relapsed AML after reduced-intensity conditioning (RIC) allo-SCT; they noted an estimated 2-year overall survival (OS) from relapse after RIC allo-SCT of 14%.8 This study was able to identify 3 variables at the time of posttransplant relapse associated with improved survival: remission after SCT of >5 months, bone marrow blasts <27%, and absence of acute GVHD after SCT. Notably, long-term survival was seen only in patients who achieved a CR to cyto-reductive therapy followed either by donor lymphocyte infusion or second SCT as consolidation. Thus, a second transplant is a viable option for a select group of patients who relapse after RIC allo-SCT.
The challenges of a second allo-SCT are similar to those of a first SCT but are further amplified by prior regimen-related toxicities with or without the presence of GVHD. The relapse rate after second allo-SCT remains high, and disease control before allo-SCT has repeatedly been shown to correlate with allo-SCT outcomes. Thus, conditioning regimens that have tolerable toxicities and provide good disease control are needed. A spectrum of conditioning regimens exists for first allo-SCT, from high-intensity myeloablation to very-low-intensity nonablative approaches. Our center has adopted an intermediate-intensity regimen of fludarabine-melphalan, which provides good disease control, excellent engraftment, and tolerable toxicities.9,10
In addition to this standard conditioning regimen, total body irradiation (TBI) can play a potentially important role in conditioning before SCT. TBI causes immune suppression, leads to tumor cell killing, and augments chemotherapy, especially in cases of chemotherapy-resistant disease. Determining the ideal dose of TBI is important. Clift et al11 reported a decreased rate of relapse in patients in first CR undergoing allo-SCT when treated with 15.75 Gy compared with those in the 12 Gy group. However, treatment-related mortality remained high in the 15.75 Gy group, resulting in no difference in long-term OS.
In conventional external beam radiotherapy, treatment is delivered with beams of uniform intensity, which results in a heterogeneous dose distribution due to differences in body thickness, contour, and tissue densities. This often leads to higher-than-intended doses to nearby critical organs. Intensity-modulated radiation therapy (IMRT) is an alternative radiation delivery technique commonly used for solid tumors, particularly for those with irregular shapes. With this method, treatment plans are generated based on a patient’s computed tomography images, allowing the higher doses to be conformed to the shape of the target while minimizing radiation dose to surrounding organs at risk (OARs). TMI can be delivered either by using a helical delivery technique (tomotherapy) or conventional C-arm linear accelerator (linac) technology. Tomotherapy allows the entire treatment to be delivered in a single session using continuous couch movement through the bore design. In contrast, multiple isocenters and sets of fields are required for treatment using conventional linacs, thus making TMI challenging. Nonetheless, both delivery methods provide comparable dose distributions, and their clinical feasibility has been previously reported.12
The first linac-based IMRT approach to selectively irradiate bone marrow was jointly developed at The University of Illinois Chicago and The University of Chicago. Aydogan et al13 reported considerable dose reduction to surrounding OARs, including the eyes, oral cavity, heart, liver, kidneys, brain, and lungs, with the linac-based intensity-modulated total marrow irradiation (IM-TMI) compared with conventional TBI. The dose reduction to organs underlying bony structures, particularly the lungs, was only modest. However, the median lung dose of 7.03 Gy was significantly less than the average TBI lung dose of 8.84 Gy obtained with a standard 50% partial lung block, a technique that is also subject to positioning errors and higher lung doses.14 This is particularly important as higher lung doses have been associated with inferior outcomes.15 In addition, Patel et al16 showed in a recent phase 1 study the feasibility and tolerability of adding linac-based IM-TMI to fludarabine and busulfan in high-risk patients undergoing first allo-SCT.
In the current study, we assessed the feasibility of combining linac-based IM-TMI with fludarabine-melphalan as the conditioning regimen for second allo-SCT.
Methods
Patients
We conducted a single-center, phase 1 dose-escalation study of the feasibility of combining IM-TMI with fludarabine-melphalan. Patients were eligible for the study if they were between ages 18 and 75 years, had relapsed hematologic malignancies and were undergoing a second or greater allo-SCT, had a Karnofsky performance status score ≥70, and demonstrated adequate cardiac, pulmonary, renal, and hepatic function. Patients were classified according to the revised Disease Risk Index.17
Treatment plan
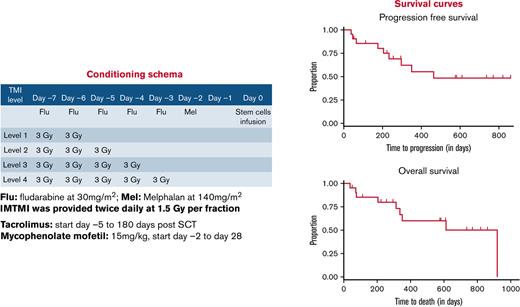
All patients received the following conditioning regimen: daily fludarabine 30 mg/m2 on days −7, −6, −5, −4, and −3; melphalan 140 mg/m2 on day −2; twice-daily 1.5 Gy IM-TMI on days −7 and −6 for a total dose of 6 Gy, on days −7 through −5 for 9 Gy, or on days −7 through −4 for 12 Gy; and stem cell infusion on day 0 (supplemental Figure 1). To determine the maximum tolerated dose (MTD), patients received twice-daily 1.5 Gy IM-TMI with total doses of 6 Gy, 9 Gy, or 12 Gy at dose escalation according to a modified “3 + 3” escalation schema. Tacrolimus (dosed to maintain levels of 8-12 ng/mL through day 180) and mycophenolate mofetil (15 mg/kg from day 0 to day 28) were used for GVHD prophylaxis.
Definitions
Dose-limiting toxicities (DLTs) were evaluated from time of conditioning until day 28 posttransplant. A DLT was defined as a grade 3 or higher treatment-related toxicity. The National Cancer Institute Common Toxicity Criteria for Adverse Events (version 4.0) was used to grade toxicities. Mucositis, an anticipated toxicity, was prospectively scored on days 7, 14, 21, and 28 by using the World Health Organization mucositis scale. Acute and chronic GVHD were diagnosed and graded according to the modified Glucksberg criteria and 2014 National Institutes of Health Consensus Conference criteria, respectively.18,19 Hematopoietic cell transplantation–specific comorbidity index (HCT-CI) was calculated by using a standard scoring system.20
IM-TMI planning and treatment delivery
The details of treatment planning, dosimetric accuracy, and clinical implementation of IM-TMI have been described previously.21,22 Briefly, an Alpha Cradle immobilization device (Smithers Medical Products, North Canton, OH) was used for both simulation and treatment to ensure accuracy of treatment delivery. Computed tomography simulations were performed and transferred to the Eclipse treatment planning system (Varian Medical Systems, Inc., Palo Alto, CA) for dose optimization and calculation. All bones from the head to the mid-femur, with the exception of those below the mid-humerus, were contoured and expanded by a 3-mm margin to generate planning target volume (PTV) and to account for possible uncertainties. OAR structures were contoured as avoidance structures to reduce radiation dose and included the eyes, oral cavity, brain, lungs, heart, kidneys, and liver. A 3-isocenter technique described by Wilkie et al21 was used for the treatment planning and involves creating separate treatment plans for the head and neck, chest, and pelvic regions. Each plan was generated with a minimum of 95% PTV coverage with the prescription dose. Delivery accuracy was verified before treatment using IMRT quality assessment measurements. On the day of treatment, simulation setup was reproduced using the same immobilization device, and accuracy was verified with imaging protocols before delivering the IM-TMI.
Donor chimerism monitoring
Chimerism in the bone marrow, along with monitoring for B-cell, T-cell, and immunoglobulin reconstitution, was analyzed at days 30, 100, 180, and 365 posttransplantation, according to methods previously described at our institution.23,24 Briefly, bone marrow mononuclear cells were analyzed by flow cytometry using fluorochrome-conjugated monoclonal antibodies specific to each lineage: T cells (CD3, CD4, and CD8) and B cells (CD19). Chimerism was analyzed by using short-tandem repeat analysis with the AmpFISTR Profiler Plus kit (Applied Biosystems, Foster City, CA); a result ≥95% was defined to be full donor chimerism. Immunoglobulin levels were measured by using the immunoturbidimetric assay.
Analysis
Descriptive statistics of the patient population and transplant characteristics were generated. Time to myeloid engraftment was defined as the first day in which the absolute neutrophil count (ANC) was >500/mm3 for 3 consecutive days. Time to platelet engraftment was defined as the first day in which the platelet count was >20000/mm3 without transfusion support for 7 consecutive days. Failure to engraft was defined as lack of evidence of hematopoietic recovery (ANC <500/mm3 and platelet count <20 000/mm3) by day 35 posttransplant, confirmed by a biopsy sample revealing a marrow cellularity <5%. Secondary graft failure was defined as initial myeloid engraftment by day 35 posttransplant and documented to be of donor origin, followed by a drop in the ANC to <500/mm3 for >3 days, independent of any myelosuppressive drugs, severe GVHD, cytomegalovirus, or other infection. Progression-free survival (PFS) and OS were calculated from the date of second allo-SCT and were analyzed by using Kaplan-Meier estimates. Cumulative incidences were calculated by using competing risks analysis; GVHD, nonrelapse death, and relapse were considered competing events. Statistical analyses were performed with Stata version 16.1 (StataCorp, College Station, TX) and RStudio version 4.0.3 (RStudio, Boston, MA).
Results
Patient demographic characteristics
Between December 2015 and May 2020, 22 patients provided written consent and enrolled in the trial, with 21 patients (18 with AML, 2 with myelodysplastic syndrome/myeloproliferative neoplasm, and 1 with acute lymphoblastic leukemia) eventually treated; 1 patient was consented but failed to start conditioning due to inadequate disease control. During this time period, all patients at our institution undergoing second or greater SCT consented to the trial except for 7 patients. This included 6 patients with haplo-cord donors, and 1 patient who was unable to receive TMI due to issues related to insurance coverage. Characteristics of the patient population undergoing second transplant are summarized in Table 1. The median age was 56 (22-72) years. Six patients were in CR2, 4 in CR3, and 11 had persistent disease before the second allo-SCT. Twenty patients underwent a second SCT, with 1 undergoing a third SCT. The median HCT-CI was 1 (0-6), and median Karnofsky performance status score was 90 (70-100). Seven patients had a matched related donor, 13 had a matched unrelated donor, and 1 had a 7/8 matched unrelated donor SCT. Based on the Disease Risk Index of the 21 patients in the study, 15 were high risk, and 6 were intermediate risk.
Patient and transplant characteristics
| Characteristic . | Value . |
|---|---|
| Total no. of patients | 21 |
| Age, median (range), y | 56 (22-72) |
| Sex | |
| Male | 12 (57%) |
| Female | 9 (43%) |
| Diagnosis | |
| AML | 18 (86%) |
| MDS/MPN | 2 (9%) |
| ALL | 1 (5%) |
| Donor status | |
| MRD | 7 (33%) |
| MUD | 13 (62%) |
| 7/8 MUD | 1 (5%) |
| IM-TMI regimen | |
| RIC (Flu-mel-TMI), 6 Gy | 5 (24%) |
| RIC (Flu-mel-TMI), 9 Gy | 10 (48%) |
| RIC (Flu-mel-TMI), 12 Gy | 6 (28%) |
| Disease status before SCT | |
| CR2 | 6 (29%) |
| CR3 | 4 (19%) |
| Active disease | 11 (52%) |
| Duration of remission of first SCT, median (range), d | 355 (55-1494) |
| Time from last relapse to protocol therapy, median (range), d | 206 (85-2534) |
| HCT-CI, median (range) | 1 (0-6) |
| Karnofsky performance status score, median (range) | 90 (70-100) |
| Characteristic . | Value . |
|---|---|
| Total no. of patients | 21 |
| Age, median (range), y | 56 (22-72) |
| Sex | |
| Male | 12 (57%) |
| Female | 9 (43%) |
| Diagnosis | |
| AML | 18 (86%) |
| MDS/MPN | 2 (9%) |
| ALL | 1 (5%) |
| Donor status | |
| MRD | 7 (33%) |
| MUD | 13 (62%) |
| 7/8 MUD | 1 (5%) |
| IM-TMI regimen | |
| RIC (Flu-mel-TMI), 6 Gy | 5 (24%) |
| RIC (Flu-mel-TMI), 9 Gy | 10 (48%) |
| RIC (Flu-mel-TMI), 12 Gy | 6 (28%) |
| Disease status before SCT | |
| CR2 | 6 (29%) |
| CR3 | 4 (19%) |
| Active disease | 11 (52%) |
| Duration of remission of first SCT, median (range), d | 355 (55-1494) |
| Time from last relapse to protocol therapy, median (range), d | 206 (85-2534) |
| HCT-CI, median (range) | 1 (0-6) |
| Karnofsky performance status score, median (range) | 90 (70-100) |
ALL, acute lymphoblastic leukemia; Flu-mel, fludarabine-melphalan; MDS/MPN, myelodysplastic syndrome/myeloproliferative neoplasm; MRD, matched related donor; MUD, matched unrelated donor.
Total marrow irradiation
PTV coverage of >95%, on average, was achieved. All plans passed our standard plan quality assessment, including dose measurement and γ analysis test before the delivery. Doses to the OARs for the patients in this study are listed in Table 2. Depending on the specific organ, the average dose to the surrounding OARs ranged from 27% to 68% of the dose prescribed to the PTV and is comparable to previously described work.16
Average dose to specific organs
| Organ . | Average organ dose ± SD (relative to the prescription dose) . |
|---|---|
| Brain | 63.9 ± 3.7 |
| Heart | 57.1 ± 3.4 |
| Lungs | 68.3 ± 4.9 |
| Bowel | 53.4 ± 2.9 |
| Liver | 59.8 ± 3.4 |
| Kidneys | 52.5 ± 4.7 |
| Eyes | 40.6 ± 4.1 |
| Oral cavity | 33.5 ± 2.8 |
| Lenses | 27.5 ± 4.2 |
| Whole body | 62.1 ± 6.6 |
| Organ . | Average organ dose ± SD (relative to the prescription dose) . |
|---|---|
| Brain | 63.9 ± 3.7 |
| Heart | 57.1 ± 3.4 |
| Lungs | 68.3 ± 4.9 |
| Bowel | 53.4 ± 2.9 |
| Liver | 59.8 ± 3.4 |
| Kidneys | 52.5 ± 4.7 |
| Eyes | 40.6 ± 4.1 |
| Oral cavity | 33.5 ± 2.8 |
| Lenses | 27.5 ± 4.2 |
| Whole body | 62.1 ± 6.6 |
The average doses to each organ, as well as the associated standard deviation (SD), are listed as a percentage of the dose prescribed to the PTV.
Dose escalation
Patients were enrolled according to a modified “3 + 3” dose escalation schema. Three patients were initially enrolled at the level 1 dose (6 Gy), with no patients experiencing a DLT. The dose was subsequently escalated, and 3 patients were enrolled in dose level 2 (9 Gy). Of these patients, only 1 patient developed a DLT (grade 3 mucositis on day 7 that resolved by day 21). An additional 3 patients were treated with the 9 Gy dose, and 5 of the 6 total patients in this cohort did not experience DLTs. At dose level 3 (12 Gy), one patient developed grade 3 mucositis that resolved by day 14; thus, 3 additional patients were treated at a dose of 12 Gy. One patient in this group had disease refractory to chemotherapy and had received a 5-day course of clofarabine bridging therapy before fludarabine-melphalan-TMI conditioning and subsequently developed grade 4 mucositis (Table 3). Because there were more patients eligible to undergo a second SCT, we allowed expansion of the level 1 and 2 cohorts while patients enrolled in dose level 3 were completing their DLT window. In total, 5 patients were enrolled in dose level 1 dose (6 Gy), 10 patients in dose level 2 (9 Gy), and 6 patients in dose level 3 (12 Gy). The MTD for IM-TMI was determined to be 12 Gy. A dose of 9 Gy was used, at the treating physician’s discretion, for some patients who were underweight (<100 pounds) or older (aged >65 years).
Mucositis during dose escalation
| Dose level . | Patient . | Day 7 . | Day 14 . | Day 21 . | Day 28 . |
|---|---|---|---|---|---|
| Level 1, 6 Gy | 1–1 | Grade 0 | Grade 0 | Grade 0 | Grade 0 |
| 1–2 | Grade 0 | Grade 0 | Grade 0 | Grade 0 | |
| 1–3 | Grade 1 | Grade 0 | Grade 0 | Grade 0 | |
| 1–4 | Grade 1 | Grade 0 | Grade 1 | Grade 0 | |
| 1–5 | Grade 0 | Grade 0 | Grade 0 | Grade 0 | |
| Level 2, 9 Gy | 2–1 | Grade 3 | Grade 2 | Grade 0 | Grade 0 |
| 2–2 | Grade 1 | Grade1 | Grade0 | Grade 0 | |
| 2–3 | Grade 1 | Grade 0 | Grade 0 | Grade 0 | |
| 2–4 | Grade 0 | Grade 0 | Grade 0 | Grade 0 | |
| 2–5 | Grade 1 | Grade 0 | Grade 0 | Grade 0 | |
| 2–6 | Grade 0 | Grade 0 | Grade 0 | Grade 0 | |
| 2–7 | Grade 1 | Grade 1 | Grade 1 | Grade 1 | |
| 2–8 | Grade: 0 | Grade: 0 | Grade 0 | Grade 0 | |
| 2–9 | Grade 0 | Grade 0 | Grade 0 | Grade 0 | |
| 2–10 | Grade: 3 | Grade 2 | Grade: 0 | Grade 0 | |
| Level 3, 12 Gy | 3–1 | Grade 0 | Grade 0 | Grade 0 | Grade 0 |
| 3–2 | Grade 1 | Grade 0 | Grade 0 | Grade 0 | |
| 3–3 | Grade 3 | Grade 0 | Grade 0 | Grade 0 | |
| 3–4 | Grade 0 | Grade 0 | Grade 1 | Grade 0 | |
| 3–5 | Grade 0 | Grade 0 | Grade 0 | Grade 0 | |
| 3–6 | Grade: 2 | Grade: 3 | Grade: 4 | Grade: 0 |
| Dose level . | Patient . | Day 7 . | Day 14 . | Day 21 . | Day 28 . |
|---|---|---|---|---|---|
| Level 1, 6 Gy | 1–1 | Grade 0 | Grade 0 | Grade 0 | Grade 0 |
| 1–2 | Grade 0 | Grade 0 | Grade 0 | Grade 0 | |
| 1–3 | Grade 1 | Grade 0 | Grade 0 | Grade 0 | |
| 1–4 | Grade 1 | Grade 0 | Grade 1 | Grade 0 | |
| 1–5 | Grade 0 | Grade 0 | Grade 0 | Grade 0 | |
| Level 2, 9 Gy | 2–1 | Grade 3 | Grade 2 | Grade 0 | Grade 0 |
| 2–2 | Grade 1 | Grade1 | Grade0 | Grade 0 | |
| 2–3 | Grade 1 | Grade 0 | Grade 0 | Grade 0 | |
| 2–4 | Grade 0 | Grade 0 | Grade 0 | Grade 0 | |
| 2–5 | Grade 1 | Grade 0 | Grade 0 | Grade 0 | |
| 2–6 | Grade 0 | Grade 0 | Grade 0 | Grade 0 | |
| 2–7 | Grade 1 | Grade 1 | Grade 1 | Grade 1 | |
| 2–8 | Grade: 0 | Grade: 0 | Grade 0 | Grade 0 | |
| 2–9 | Grade 0 | Grade 0 | Grade 0 | Grade 0 | |
| 2–10 | Grade: 3 | Grade 2 | Grade: 0 | Grade 0 | |
| Level 3, 12 Gy | 3–1 | Grade 0 | Grade 0 | Grade 0 | Grade 0 |
| 3–2 | Grade 1 | Grade 0 | Grade 0 | Grade 0 | |
| 3–3 | Grade 3 | Grade 0 | Grade 0 | Grade 0 | |
| 3–4 | Grade 0 | Grade 0 | Grade 1 | Grade 0 | |
| 3–5 | Grade 0 | Grade 0 | Grade 0 | Grade 0 | |
| 3–6 | Grade: 2 | Grade: 3 | Grade: 4 | Grade: 0 |
Mucositis, 1 of the primary DLT, was graded on the World Health Organization Mucositis Scale at days 7, 14, 21, and 28 posttransplant.
allo-SCT outcomes
After SCT, engraftment occurred in 19 (91%) of 21 patients. Graft failure occurred in 2 patients due to infection and early disease relapse. One of these patients had cytomegalovirus viremia while the second had a complicated posttransplant course with human herpesvirus 6 viremia, BK viruria, bacteremia, and fungemia. The median times to neutrophil and platelet engraftment were 10 days (8-20 days) and 18 days (13-40 days), respectively (Table 4). Median CD3+ donor chimerism at day 30 posttransplantation was 100% (75%-100%) and increased to 100% in all patients by day 180 (supplemental Table 2); 2 patients who experienced graft failure were excluded.
Transplant outcomes
| Outcomes . | . |
|---|---|
| Engraftment | |
| Neutrophil engraftment, median (range), d | 10 (8-20) |
| Platelet engraftment, median (range), d | 18 (13-40) |
| Cumulative incidence of any GVHD at 1 y | 65% (95% CI, 38-83) |
| PFS | |
| PFS from second SCT, median (range), d | 350 (38-859) |
| OS from second SCT, median (range), d | 608 (38-919) |
| 1-y PFS | 55% |
| 2-y PFS | 48% |
| OS | |
| 1-y OS | 60% |
| 2-y OS | 50% |
| Relapse | |
| 1-y cumulative incidence of relapse | 28% (CI, 9-50) |
| 2-y cumulative incidence of relapse | 35% (CI, 13-58) |
| 1-y NRM | 17% (95% CI 4-39%) |
| 2-y NRM | 17% (95% CI 4-39%) |
| No. of patients with acute GVHD | |
| Grade 1 | 0 |
| Grade 2 | 8 |
| Grade 3 | 3 |
| Grade 4 | 0 |
| No. of patients with chronic GVHD (mild/moderate/severe) | |
| Mild | 1 |
| Moderate | 1 |
| Severe | 2 |
| Outcomes . | . |
|---|---|
| Engraftment | |
| Neutrophil engraftment, median (range), d | 10 (8-20) |
| Platelet engraftment, median (range), d | 18 (13-40) |
| Cumulative incidence of any GVHD at 1 y | 65% (95% CI, 38-83) |
| PFS | |
| PFS from second SCT, median (range), d | 350 (38-859) |
| OS from second SCT, median (range), d | 608 (38-919) |
| 1-y PFS | 55% |
| 2-y PFS | 48% |
| OS | |
| 1-y OS | 60% |
| 2-y OS | 50% |
| Relapse | |
| 1-y cumulative incidence of relapse | 28% (CI, 9-50) |
| 2-y cumulative incidence of relapse | 35% (CI, 13-58) |
| 1-y NRM | 17% (95% CI 4-39%) |
| 2-y NRM | 17% (95% CI 4-39%) |
| No. of patients with acute GVHD | |
| Grade 1 | 0 |
| Grade 2 | 8 |
| Grade 3 | 3 |
| Grade 4 | 0 |
| No. of patients with chronic GVHD (mild/moderate/severe) | |
| Mild | 1 |
| Moderate | 1 |
| Severe | 2 |
CI, confidence interval.
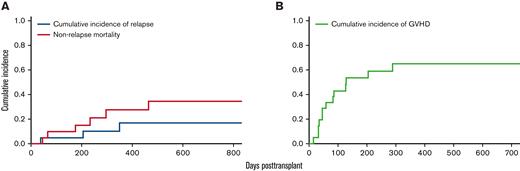
Eleven patients developed acute GVHD (grade 2, n = 8; grade 3, n = 3) at a median time of 45 days (14-204 days). Four patients developed chronic GVHD (mild, n = 1; moderate, n = 1; severe, n = 2) at a median time of 247 days (83-383 days) (Table 5). The cumulative incidence of any GVHD at 1 year was 65% (95% CI, 38-83) (Figure 1). At a median follow-up of 11 months, 6 patients had relapsed at a median of 205 days (46-464 days), and 10 patients had died (5 of disease progression, 2 of infection, 1 of GVHD, and 2 of secondary malignancies). Cumulative incidence of relapse at 1 year and 2 years were 28% (CI, 9-50) and 35% (CI, 13-58), respectively. The nonrelapse mortality (NRM) at 1 year and 2 years were both 17% (95% CI, 4-39). Deaths not due to disease relapse were attributed to acute GVHD (n = 1), infection (n = 1), organ failure (n = 1), and secondary malignancies (n = 2, one from donor-derived malignancy and another from posttransplant lymphoproliferative disease).
AEs and SAEs
| AE and SAE . | No. of patients, all grades . | No. of patients, grade ≥3 . |
|---|---|---|
| AEs | ||
| Infection | 1 | 1 |
| Gastrointestinal disorders | ||
| Esophagitis | 1 | 1 |
| Mucositis | 11 | 4 |
| Elevated transaminases | 1 | |
| Musculoskeletal disorders | 1 | |
| Nutritional disorders | 2 | |
| SAEs | ||
| Renal failure | 1 | |
| Infections requiring hospitalization | 2 | |
| Death | 10 | |
| AE and SAE . | No. of patients, all grades . | No. of patients, grade ≥3 . |
|---|---|---|
| AEs | ||
| Infection | 1 | 1 |
| Gastrointestinal disorders | ||
| Esophagitis | 1 | 1 |
| Mucositis | 11 | 4 |
| Elevated transaminases | 1 | |
| Musculoskeletal disorders | 1 | |
| Nutritional disorders | 2 | |
| SAEs | ||
| Renal failure | 1 | |
| Infections requiring hospitalization | 2 | |
| Death | 10 | |
Adverse events (AEs) and serious AEs (SAEs) directly related to IM-TMI fludarabine-melphalan conditioning are shown.
Cumulative incidences of relapse, NRM, and GVHD. (A) Cumulative incidences of relapse at 1 year and 2 years were 28% (95% CI, 9-50) and 35% (95% CI, 13-58%), respectively. The NRM at 1 year and 2 years were both 17% (95% CI, 4-39). (B) The cumulative incidence of any GVHD at 1 year was 65% (95% CI, 38-83).
Cumulative incidences of relapse, NRM, and GVHD. (A) Cumulative incidences of relapse at 1 year and 2 years were 28% (95% CI, 9-50) and 35% (95% CI, 13-58%), respectively. The NRM at 1 year and 2 years were both 17% (95% CI, 4-39). (B) The cumulative incidence of any GVHD at 1 year was 65% (95% CI, 38-83).
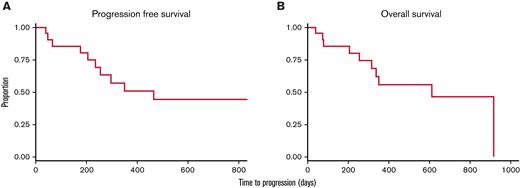
PFS was 55% at 1 year and 48% at 2 years for all patients. One and 2-year OS were 60% and 50%, respectively (Figure 2). For the 10 patients in remission, 1- and 2-year PFS and OS were both 66% and 63% (supplemental Figure 1). Median PFS and OS were not reached after 28.6 months of observation (supplemental Table 3). For patients with refractory disease, 1- and 2-year PFS were 41% and 31%; 1- and 2-year OS were 51% and 38%. Median PFS was 10 months and median OS was 19 months for these patients with refractory disease.
PFS and OS. Estimated 2-year PFS (A) and OS (B) were 48% and 50%, respectively, with IM-TMI.
PFS and OS. Estimated 2-year PFS (A) and OS (B) were 48% and 50%, respectively, with IM-TMI.
Discussion
In this single-center, phase 1 dose-escalation study, we report that fludarabine-melphalan in combination with 12 Gy of IM-TMI is feasible and well tolerated for second allo-SCTs. Mucositis was the primary DLT, and in the majority of cases it resolved by day 21 post-SCT. The MTD of IM-TMI with fludarabine-melphalan is 12 Gy, but 9 Gy can be used in patients who are underweight (<100 lb) or older (aged >65 years). Importantly, we report a 2-year PFS and OS of 48% and 50% with IM-TMI, respectively, which compares favorably to our institutional 2-year PFS and OS of 18% and 23% in patients receiving a second allo-SCT without IM-TMI.25
Our study builds on the current literature of using TMI as part of the conditioning regimen. An initial study from the City of Hope reported the first successful use of IM-TMI with tomotherapy in a patient undergoing first autologous SCT without concomitant chemotherapy. The investigators reported an MTD of 16 Gy, reduced toxicity, and rapid stem cell engraftment.26 A subsequent phase 1/2 study showed that the addition of TMI to RIC is feasible and safe and could be offered to patients with advanced hematologic malignancies who might not otherwise be candidates for RIC.27 The same group found that TMI dose escalation to 15 Gy combined with cyclophosphamide and etoposide therapy was associated with acceptable toxicities and encouraging outcomes in patients with advanced acute leukemia undergoing first HCT. The novel contribution of our current study to this literature is to establish the feasibility of adding IM-TMI in patients undergoing second or greater allo-SCT.
To our knowledge, this study is the first that prospectively examines outcomes after a second allo-SCT in hematologic malignancies. Retrospective studies evaluating outcomes in patients with relapsed hematologic malignancies undergoing second allo-SCT have reported PFS and OS rates ranging from 17% to 27% for PFS and 21% to 36% for OS (supplemental Table 4).28-30 Notably, among these retrospective studies is a recent analysis by Choi et al29 examining outcomes of 80 patients with relapsed AML or acute lymphoblastic leukemia undergoing second allo-SCT. Similar to our trial, the majority of the patients in the analysis (n = 67 [84%]) received an RIC regimen; the 2-year NRM was 19%, and 2-year cumulative incidence of relapse was 60%. Direct comparisons with the data in the current literature are beyond the scope of this phase 1 clinical trial; however, we use these historical data as benchmarks for future studies. Our results suggest that the addition of IM-TMI to an intermediate-intensity conditioning regimen allows for a higher dose of radiation to be given to the marrow without increasing NRM. This platform results in lower rates of disease relapse and improvements in OS and PFS.
There are several limitations to the current study, including the small sample size (N = 21), heterogeneous patient population with multiple disease histologies, the varying duration of remission from first allo-SCT (1.8-49.8 months; median, 11.8 months), and varying time from last relapse to protocol therapy (2.8-84.4 months; median, 6.7 months). In addition, TBI is known to cause late-onset pulmonary toxicities, while fludarabine-melphalan along with radiation can cause cardiac toxicities. These late-onset toxicities may not have been adequately captured at the follow-up time point in this phase 1 study and may be better assessed in future phase 2 studies. Lastly, IM-TMI is a novel application of image-guided radiation treatment to the bone marrow and therefore requires specific capability and expertise, which may not be widely available at all institutions.
Second allo-SCT can provide durable remission and long-term survival in a subset of patients. In this phase 1 clinical trial, we show that combining 12 Gy of IM-TMI with daily fludarabine 30 mg/m2 at days −7 to −3 and melphalan 140 mg/m2 on day −2 is a feasible and well-tolerated conditioning regimen in patients undergoing second or greater allo-SCT. This regimen also resulted in improved 2-year PFS and OS at 48% and 50%, respectively, compared with our institutional experience in patients undergoing second allo-SCT without IM-TMI.
Acknowledgments
The authors thank all the research and clinical staff whose work supported this trial and all the patients and their families who participated in the trial.
H.L., and B.A. were supported by the University of Chicago Medicine Comprehensive Cancer Center pilot grant and The University of Chicago Cancer Center Support Grant (CA014599). H.L. was supported by an Ullman Scholar Award.
Authorship
Contribution: H.L., as the Principal Investigator of the study, designed and wrote the protocol and supervised the clinical trial; B.A., as the Co–Principal Investigator of the study, designed and wrote the TMI radiation portion of the study and supervised the TMI treatment; Y.H., K.Y, J.P., and A.S.A. contributed to the design and writing of the protocol; M.C.T., and A.W. collected and analyzed the data; and W.S., R.A.L., S.K., J.L.L., J.K., P.A.R., R.W., and M.R.B. conducted the research; and all authors contributed to the writing of the manuscript.
Conflict-of-interest disclosure: H.L. has research support from Karyopharm and Bristol Myers Squibb; served at the advisory board meeting for Agios, Pfizer, Nkarta, and CTI Biopharm; consultant for NGM Biopharma; and received consulting fees from BeiGene. Other co-authors reported research funding and membership on advisory boards from different companies but reported no conflicts of interest with this study. The remaining authors declare no competing financial interests.
Correspondence: Hongtao Liu, The University of Chicago, 5841 South Maryland Ave, MC 2115, Chicago, IL 60637; e-mail: hliu2@medicine.bsd.uchicago.edu.
References
Author notes
The full-text version of this article contains a data supplement.
B.A. and H.L. are joint senior authors.