TO THE EDITOR:
The CDKN2A and CDKN2B (CDKN2A/B) locus on 9p21, a tumor suppressor hub, is the second most common genetically inactivated region after TP53 in cancer.1CDKN2A/B deletion has been described in a wide variety of malignancies, including B-cell malignancies; acute lymphocytic leukemia and diffuse large B-cell lymphoma.1-6 In chronic lymphocytic leukemia (CLL), CDKN2A/B loss has been described in a small subset of patients, but its significance is not well understood. It has been reported as the most common acquired abnormality found in ∼19% to 30% of samples by single nucleotide polymorphism microarray or next-generation sequencing (NGS) at the time of transformation of CLL to an aggressive B-cell lymphoma (Richter transformation [RT]).7-12 Previously, loss of CDKN2A/B was thought to only occur at RT but was later reported in 13 patients with CLL with high-risk disease, defined by either TP53 aberration or refractory to purine analogs.7,9,10 Homozygous loss of CDKN2A/B has been described in 3 patients who acquired resistance to venetoclax.13 Most frequently, CDKN2A/B loss co-occurs with TP53 deletion, with the concurrent loss of both tumor suppressors being a potential pathway for RT.10,14 Because of the negative clinical impact of RT and the rarity of this genetic abnormality, we examined a large cohort of patients with CLL using fluorescence in situ hybridization (FISH) for CDKN2A/B deletion to identify the frequency of occurrence, population and genetic characteristics, and outcomes.
After Institutional Review Board approval, a retrospective study with chart review was conducted to identify patients with 1 or more samples submitted for CLL FISH panel analysis over a 3.5-year period. FISH and conventional chromosome analysis were performed on cells stimulated with pokeweed mitogen, phorbol myristate acetate, and CpG oligonucleotides in either peripheral blood or bone marrow aspirate or biopsy. FISH was performed using probes, according to the manufacturer’s recommendations (supplemental Table 1). Patients were screened for abnormal CDKN2A/B results at diagnosis or on subsequent testing at a later stage of the disease. Abnormal CDKN2A/B results included homozygous loss, heterozygous loss, loss of 1 copy with loss of the chromosome 9 centromere, and relative loss of CDKN2A/B in a polyploid background (ie, 2 signals of CDKN2A/B with 4 signals of centromere 9). Karyotype complexity was counted as previously described.15,16 Immunoglobulin heavy-chain variable region (IGHV) mutational status was determined using polymerase chain reaction. For patients who started therapy with a Bruton tyrosine kinase (BTK) inhibitor, mutational testing was performed using NGS or digital droplet polymerase chain reaction. A 50-gene hematologic sequencing panel was performed via ion torrent sequencing and annotated using the GenomOncology platform (supplemental Methods). A Cox regression model was used to evaluate overall survival (OS) after CDKN2A/B deletion. The Fine and Gray model was used to examine the correlation of variables regarding transformation, treating death without transformation as a competing risk.
We identified 636 patients with CLL FISH panel analysis, of whom 43 (6.8%) had CDKN2A/B deletion. The cohort with CDKN2A/B deletion consisted of 28 (65.1%) males and 15 (34.9%) females, with a median age at diagnosis of 54.9 years (range, 41.9-77.7 years). Detection of CDKN2A/B loss occurred at a median of 7.8 years from diagnosis (range, diagnosis to 26.1 years). Five patients (11.6%) had CDKN2A/B loss within 1 year of diagnosis before receiving any therapy. For those who had been previously treated at the time of CDKN2A/B loss detection, the median number of lines of treatment was 5 (range, 1-13). Thirty-two (74.4%) patients had received prior therapy with a BTK inhibitor. BTK C481 mutation testing was performed for 27 (84.4%) patients, with alterations detected in 11 (34.4%). Of the patients with a known IGHV mutational status (n = 33), 30 (90.9%) had unmutated status.
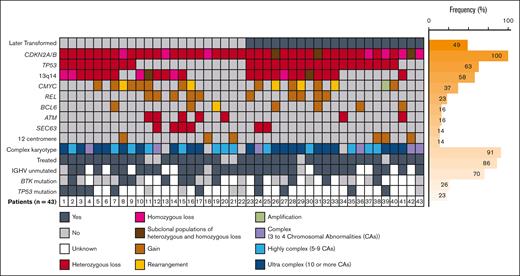
Regarding CDKN2A/B deletion, 33 (76.7%), 7 (16.3%), and 3 (7.0%) patients had heterozygous, homozygous, or subclonal populations of heterozygous as well as homozygous loss, respectively. Complex (3-4 abnormalities), highly complex (5-9 abnormalities), and ultra complex (>10 abnormalities)15 karyotypes were found in 6 (14.0%), 13 (30.2%), and 20 (46.5%) patients, respectively (Figure 1). A chromosomal abnormality involving the 9p21 locus was visible by conventional karyotyping in 72.1% of patients (supplemental Table 2). Additional FISH testing showed that 27 patients (62.8%) had TP53 deletion; 25 (58.1%) had deletion of 13q14; 16 (37.2%) had gain, rearrangement, or amplification of MYC; 10 (23.3%) had gain of REL; 7 (16.3%) had BCL6 gain or rearrangement; 7 (16.3%) had ATM deletion; 6 (14.0%) had SEC63 deletion; and 6 (14.0%) had trisomy 12. Sequencing data were available for 15 (34.9%) patients; of note, 10 (66.7%) had mutations in TP53, with 4 (26.7%) also having mutations in SF3B1. BRAF mutations occurred in 2 patients, and 1 patient had mutations in NOTCH1 and XPO1.
Genetic profiles of patients with CLL with CDKN2A/B deletion. Bone marrow or peripheral blood analysis at the time of the first CDKN2A/B deletion detection using FISH. Fine and Gray model for RT (n = 20) or prolymphocytic leukemia (n = 1). IGHV, immunoglobulin heavy-chain variable region.
Genetic profiles of patients with CLL with CDKN2A/B deletion. Bone marrow or peripheral blood analysis at the time of the first CDKN2A/B deletion detection using FISH. Fine and Gray model for RT (n = 20) or prolymphocytic leukemia (n = 1). IGHV, immunoglobulin heavy-chain variable region.
In terms of outcomes, there were 30 deaths (69.8%) in the cohort. With a median follow-up period of 10.6 years among survivors, there was a median OS of 10.8 years from diagnosis (95% confidence interval [CI], 8.0-14.4). The median OS from the time of detection of CDKN2A/B deletion was 1.7 years (95% CI, 0.3-4.7), with a median follow-up period of 4.9 years. The treatments after CDKN2A/B deletion are provided in supplemental Table 3. Of the 43 patients in the cohort, 21 (49%) progressed to RT (n = 20) or prolymphocytic leukemia (n = 1). Two additional patients each underwent a bone marrow biopsy which noted concern for prolymphocytic leukemia transformation at the time of CDKN2A/B deletion; however, no follow-up data were available for confirmation. The median age at RT was 65.8 years (range, 56.0-83.9 years), and 1 patient was treatment naïve at the time of RT. The characteristics at the time of RT are shown in supplemental Figure 1. Of the 30 deaths, 17 occurred among those with RT and 13 among those without RT due to progressive disease (n = 8), infection (n = 2), complications of CLL therapy (n = 1), secondary cancer (n = 1), and respiratory failure (n = 1).
The time from CDKN2A/B deletion detection to RT ranged from 0 to 5.5 years, with all but 2 patients progressing to RT within 2 years. The cumulative incidence rates of RT from the initial CLL diagnosis were 4.7% (95% CI, 0.8-14.0) at 12 months and 9.3% (95% CI, 2.9-20.3) at 24 months. The cumulative incidence rates of RT after CDKN2A/B deletion were 23.5% (95% CI, 10.9-38.9) at 12 months and 29.4% (95% CI, 15.1-45.3) at 24 months. Although there were limited patients progressing to RT with the detection of CDKN2A/B deletion, the multivariable model found that loss of TP53 (P = .02; hazard ratio, 4.53 [95% CI, 1.33-15.43]) and homozygous and heterozygous loss of CDKN2A/B (P = .0008; hazard ratio, 6.08 [95% CI, 2.13-17.39]) were independent significant variables associated with RT (Table 1). The frequencies of MYC, REL, and BCL6 abnormalities and karyotype complexity were similar between patients with and without RT.
To our knowledge, this study examined the largest cohort of patients with CLL and CDKN2A/B deletion and demonstrated poor outcomes after detection. This retrospective study has various limitations. Serial samples were unavailable for many patients (supplemental Table 4); thus, the timing of acquisition of deletion remains unclear. We were unable to assess whether CLL and subsequent RT were clonally related, and NGS data were available for a limited subset of patients with CDKN2A/B not part of the panel. Loss of CDKN2A/B was a rare event in patients with CLL. Among those with the abnormality, 18.6% died due to progressive CLL and 48.8% progressed with RT. Here, we show that FISH analysis is a clinically practical option that can be incorporated into routine FISH testing for CLL, particularly for high-risk patients with TP53 abnormalities. Our findings indicate that future prospective analysis of CDKN2A/B deletion as a prognostic variable is warranted.
Contribution: S.M.T. compiled data from chart review; S.M.T. and C.R.M. wrote the manuscript; Y.H. conducted the statistical analysis; S.M.T., Y.H., A.S.K., S.A.B., M.G., K.A.R., W.Z., D.J., J.C.B., M.R.A., N.A.H., J.A.W., and C.R.M. provided clinical data, reviewed the manuscript, and approved the final version; and J.A.W. and C.R.M. supervised the study.
Conflict-of-interest disclosure: Y.H. provides statistical support for AstraZeneca. A.S.K. consults for AstraZeneca, AbbVie, BeiGene, Loxo@Lilly, Janssen, and Kite; receives research funding from AstraZeneca; and is on a speakers bureau for BeiGene. S.A.B. consulted for Pharmacyclics, Janssen, BeiGene, and AstraZeneca; received an honorarium from OncLive; and received a travel grant from ArQule. M.G. consults for Pharmacyclics, Acerta, Serono, AstraZeneca, and Axio Inc; receives research funding from Innate Pharma; and has membership in the board of directors advisory committees for the Hairy Cell Leukemia Foundation. K.A.R. consulted for Pharmacyclics, BeiGene, Genentech, AstraZeneca, AbbVie, Janssen, and Loxo@Lilly; received travel funding from AstraZeneca; and receives research funding from Genentech, AbbVie, Janssen, and Novartis. J.C.B. receives honoraria from Pharmacyclics LLC, TG Therapeutics, and Novartis; is a current equity holder in the publicly traded company Vincerx Pharma; consults for Kura Oncology, Syndax, Novartis, AstraZeneca, and Janssen Pharmaceuticals; and receives research funding from Xencor Inc and Vincerx Pharma. M.R.A. receives funding from Phase Scientific. J.A.W. receives research funding from Karyopharm Therapeutics, Loxo@Lilly, Schrodinger, AbbVie, and MorphoSys, and consults for ArQule, AstraZeneca, BeiGene, Janssen, Pharmacyclics, Newave, AbbVie, MorphoSys, and Genentech. C.R.M. receives funding from AbbVie. The remaining authors declare no competing financial interests.
Correspondence: Cecelia R. Miller, Pathology, Clinical Cytogenetics Laboratory, The Ohio State University, 680 Ackerman Rd, Room D426, Columbus, OH 43202; e-mail: cecelia.miller@osumc.edu.
References
Author notes
∗J.A.W. and C.R.M. contributed equally to this study.
Data are available on request from the corresponding author, Cecelia Miller (cecelia.miller@osumc.edu).
The full-text version of this article contains a data supplement.