TO THE EDITOR:
The discovery of somatic alterations activating the MAP kinase signaling pathway has revolutionized the world of histiocytoses.1,2 These hematologic disorders are characterized by the infiltration of various tissues by cells of the macrophage or dendritic cell lineage and include Langerhans cell histiocytosis (LCH), Erdheim-Chester disease (ECD), Rosai-Dorfman-Destombes disease (RDD), juvenile xanthogranuloma, and several other rare entities.3 These entities were once considered inflammatory or reactive conditions to unknown etiology; however, histiocytoses are now widely classified as clonal inflammatory neoplasms harboring genetic alterations leading to aberrant oncological signaling. Although BRAFV600E is the most frequent alteration detected in LCH and ECD,4-6 several other activating genetic alterations have been described in genes encoding proteins of the MAP kinase, PI3K-AKT, or receptor tyrosine kinase signaling pathways.7 Among these genes, MAP2K1 (encoding for MEK1 serine-threonine kinase downstream of BRAF) is the most frequently altered gene after BRAF, affecting 8% to 15% of histiocytoses cases.6,8 However, although large cohorts of patients harboring the BRAFV600E mutation have been reported, only small series have focused on MAP2K1 mutations.9-13 This study includes 284 patients with histiocytosis diagnosis harboring a MAP2K1 alteration. We detected 64 different MEK1 variants and showed a strong correlation between the histiocytosis subtype and the mutational type.
Patients were retrieved from the database and biobanks of the pathology departments of Ambroise Paré hospital (APH) and Memorial Sloan Kettering Cancer Center. Patients signed informed consent, and those from APH were included in Gene Histio or Target Histio studies (NCT04437381). Diagnoses were confirmed by central review of histology, clinical data, and imaging in all cases. Detection of MAP2K1 mutations within biopsies was performed on DNA or RNA extracted from histiocytes-enriched areas using next-generation sequencing (NGS) as previously described.6,14,15 Variants were classified as either small in frame deletions or deletion-insertions (DELINs) or single base substitutions (SBSs). Statistical analyses were performed using R version 3.5.1 software (https://www.R-project.org/).
MAP2K1 alterations were detected in 284 patients with LCH, ECD, RDD, mixed histiocytosis, or other rare histiocytosis subtypes (supplemental Table 1). Median age at diagnosis was 41.1 years (range, 0-91.2 years). Biopsy samples were from bone (n = 77), skin (n = 52), and lymph nodes (n = 49) and of several tissue types.
Samples contained 140 DELINs and 158 SBSs involving MAP2K1, corresponding to 72 complementary DNA variants coding for 64 protein variants. One patient had 2 simultaneous DELINs, and another had 2 SBS. For 14 patients, 2 different samples were analyzed in which the same mutations were detected. MAP2K1 DELINs and SBSs corresponded to 35 and 37 different mutations, respectively. The MEK1-activating alterations were localized to the negative regulatory domain (encoded by exon 2) or the serine-threonine kinase catalytic domain (encoded by exon 3; Figure 1). Despite selection of histiocyte-enriched regions, the variant allele frequency (VAF) was too low to validate the mutation by NGS on genomic DNA in a subset of samples with poor quantity/quality of DNA. In such cases, we observed that the VAF detected on transcripts using RNA sequencing was higher than that observed within DNA of the same FFPE biopsy specimen (median VAF, 4.9 [range, 2.6-28.4] vs 1 [range, 0.5-2.4], respectively; P = .002, Wilcoxon paired test). Altogether, 22 samples underwent both DNA and RNA NGS, with 16 validated only by RNA analysis. Thus, the frequency of MAP2K1 alterations may be underestimated at centers using DNA analysis alone.
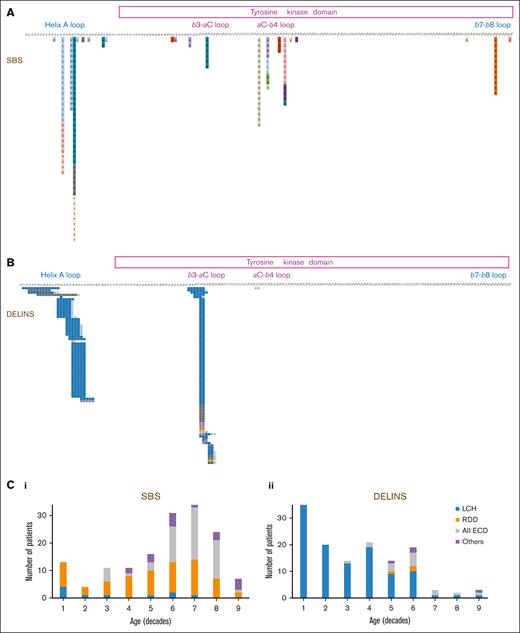
Mutations of MAP2K1 in histiocytoses. Description of all mutation detected, corresponding to either (A) SBSs or (B) DELINs. Correlation of the type of genetic alteration with age and subtype of histiocytosis. C1: SBSs and C2: DELINs
Mutations of MAP2K1 in histiocytoses. Description of all mutation detected, corresponding to either (A) SBSs or (B) DELINs. Correlation of the type of genetic alteration with age and subtype of histiocytosis. C1: SBSs and C2: DELINs
The main histiocytosis subtypes drastically differed in their MAP2K1 mutational type: DELINs arose in 109 of 119 (91.6%) LCHs but in only 3 of 70 RDDs (4.3%; Fisher exact test, P < 2.2 × 10–16). DELINs were detected in 15 of 71 ECDs (21.1%), including mixed forms (Figure 1B). Age also correlated with MAP2K1 mutational type, with DELINs being more frequent than SBSs in younger patients: 57 of 74 (77.0%; <18 years), 68 of 138 (49.3%; 18-60 years), and 8 of 72 (11.1%; >60 years; χ2 testChi2, P < 10-14). However, the median ages at the time of biopsy were 17.9, 50, and 62.4 years for LCH, RDD, and ECD, respectively. When restricting the analysis to the LCH, RDD, or ECD subgroups, the age was no longer associated MAP2K1 mutational type.
In the APH database of patients with LCH (712 samples), the mean age at the date of biopsy was 14.1 years, whereas it was 22.9 years in this series of LCH with MAP2K1 mutations (P < 1.5 × 10-6, t test). The most frequent alteration in LCH is BRAFV600E, which is detected in 54.6% of children.5 However, several cases of LCH have been shown to have DELINs activating BRAF.16,17 These DELINs are extremely rare in other types of histiocytosis. Indeed, in our database of histiocytoses among 57 samples with DELINs of BRAF, 54 (95%) had a LCH diagnosis, although LCH accounted for only 40.4% of the samples of histiocytosis of the APH series. Thus, DELIN DNA alterations of both MAP2K1 and BRAF were specifically enriched in LCH compared with other histiocytoses. These DELIN variants might be coding for specific functional alterations of MEK1 and BRAF driving the differentiation of precursor cells to LCH rather than to other histiocytoses. However, this hypothesis is unlikely, because DELIN alterations involve distinct domains of both proteins. Alternatively, DELINs may occur preferentially in precursors of LCH cells, because of genetic or epigenetic specificity.
Patients with ECD can have mixed histiocytosis, defined by the presence of histology of either LCH or RDD in 1 of the biopsy samples. ECD is associated with LCH in ∼10% of patients and frequently has BRAFV600E.18 Only rare cases of ECD have histologically been shown to have RDD despite typical clinical and radiological features of ECD.19 These latter cases were reported mainly in males and showed MAP2K1 mutation in most cases. In this series, 15 of 72 patients with ECD (20.8%) had a mixed histiocytosis with RDD (Table 1), which is far more frequent than expected. Interestingly, males were more frequent in the ECD and LCH groups of this series than in those published in large unselected series (83.6% vs 72% for ECD and LCH, respectively).20,21
Characteristics of the main histiocytoses subtypes
| . | LCH . | ECD . | RDD . | Other histiocytoses . | Total . | Significance∗ . | ||
|---|---|---|---|---|---|---|---|---|
| Pure . | Mixed RDD . | All . | ||||||
| Number of patients | 119† | 53 | 15 | 72‡ | 70† | 24§ | 284 | |
| Median age (y) | 17.9 | 64 | 56.3 | 62.4 | 50.0 | 57.3 | 41.05 | P < 10-16 (Kruskall-Wallis rank sum test) |
| Sex (M/F; % of males) | 77/42,;64.7% | 46/7; 86.7% | 11/4;73.3% | 60/11; 84.5% | 34/36; 48.6% | 11/13; 45.8% | 182/102; 64.1% | P < 10-4 (χ2 test) |
| Type of mutation (DELINs/SBSs, %DELINs) | 109/10, 91.6% | 10/43, 18.9% | 3/12, 20% | 15/56, 21.1% | 3/67, 4.3% | 6/18, 25.0% | 133/151, 46.8% | P < 10-16 (Fisher exact test) |
| . | LCH . | ECD . | RDD . | Other histiocytoses . | Total . | Significance∗ . | ||
|---|---|---|---|---|---|---|---|---|
| Pure . | Mixed RDD . | All . | ||||||
| Number of patients | 119† | 53 | 15 | 72‡ | 70† | 24§ | 284 | |
| Median age (y) | 17.9 | 64 | 56.3 | 62.4 | 50.0 | 57.3 | 41.05 | P < 10-16 (Kruskall-Wallis rank sum test) |
| Sex (M/F; % of males) | 77/42,;64.7% | 46/7; 86.7% | 11/4;73.3% | 60/11; 84.5% | 34/36; 48.6% | 11/13; 45.8% | 182/102; 64.1% | P < 10-4 (χ2 test) |
| Type of mutation (DELINs/SBSs, %DELINs) | 109/10, 91.6% | 10/43, 18.9% | 3/12, 20% | 15/56, 21.1% | 3/67, 4.3% | 6/18, 25.0% | 133/151, 46.8% | P < 10-16 (Fisher exact test) |
Comparing the 3 main groups LCH, all ECD, and RDD.
The 2 cases of mixed LCH-RDD are excluded from LCH and RDD and counted within “other histiocytoses.”
3 cases of ECD are mixed ECD-LCH.
12 malignant histiocytoses.
MAP2K1 mutations are frequent in histiocytoses6,8 and accounted for 23.7% of the kinase alterations detected in the APH histiocytosis samples during the past 4 years (not shown). Forty-eight of the 64 MEK1 protein variants that we detected have already been demonstrated as gain-of-function in vitro and/or are associated with inherited disease such as RASopathies.13,22,23 Altogether, 275 of 284 (96.8%) were reported to activate MEK1. Histiocytoses carrying 12 of the 16 unpublished MEK1 variants were positive for pERK, confirming activation of the MAP kinase pathway.
The largest published series of histiocytoses with MAP2K1 mutations included <40 cases each.8,9 Meanwhile, analysis of this series of 284 cases demonstrates previously unknown correlative features of MAP2K1-mutant histiocytic neoplasms, with a major difference noted between the LCH and RDD subtypes, in which DELINs account for 91.6% (LCH) vs 4.3% (RDD) of MAP2K1 mutations. To the best of our knowledge, this is the first example of a type of mutation within a given gene correlating with the subtype of a neoplasm.
Acknowledgments: The authors thank all pathologists who referred biopsy samples and physicians who referred data and imaging. The authors also acknowledge M. Bakari, R. Ben Jannet, C. Chauvin, G. Kotokpo-Youkou, T. Satour, and N. Teronnes for excellent technical assistance.
This work was supported by grants from PRTK 19-143 (J.-F.E.) and by the National Institutes of Health/National Cancer Institute (P30 CA008748) as well as the National Cancer Institute (R37CA259260) (E.L.D.). This was supported by the Frame Family Fund (E.L.D.), the Joy Family West Foundation (E.L.D.), and the Applebaum Foundation (E.L.D.).
Contribution: J.-F.E. wrote the draft of the manuscript; J.-F.E., E.L.D., and J.D. obtained fundings J.-F.E., E.L.D., J.D., and J.H. designed the study; and all the authors contributed to data collection and data analysis.
Conflict-of-interest disclosure: E.L.D discloses unpaid editorial support from Pfizer Inc and serves on an advisory board for Day One Biotherapeutics, Springworks Therapeutics, and Opna Bio, all outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Jean-François Emile, Pathology Department, Ambroise Paré Hospital, 9 Ave Charles de Gaulle, 92104 Boulogne, France; e-mail: jean-francois.emile@uvsq.fr.
References
Author notes
∗E.L.D., J.D., and J.H. contributed equally to this work.
Data are available on request from the corresponding author, Jean-François Emile (jean-francois.emile@uvsq.fr).
The full-text version of this article contains a data supplement.