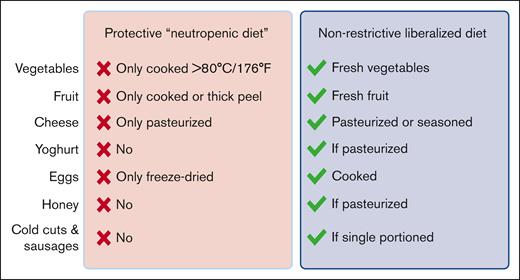
In this issue of Blood Advances, Stella et al report their findings from a multicenter trial that randomly assigned patients undergoing hematopoietic stem cell transplantation (HCT) to a traditional restrictive neutropenic diet or a liberalized diet.1 More than 200 patients who underwent auto- and allo-HCT and underwent treatment at 3 transplant centers in Italy were included in the study, and the authors found that restricting posttransplant dietary options conferred no benefit with regard to the incidence of severe infections or any of the secondary outcomes studied. The key differences between the restrictive and liberalized diet recommendations are summarized in the figure.
The media offers an all-you-can-eat buffet of articles and social-media posts touting the benefits of the superfood du jour, and cancer patients are understandably eager to do anything within their power to optimize their outcomes. The topic of dietary intake looms large for our patients, and discussions regarding food are part of daily clinical practice when caring for patients with cancer. Unfortunately, data to support dietary interventions (or even optimal dietary strategy) during cancer treatment and recovery are sorely lacking. In the earliest decades of transplantation, during the 1960s and 1970s, patients were fed sterile, autoclaved foods, and strict environmental controls were imposed to minimize bacterial exposure.2 Subsequently, some of these restrictions were relaxed and a more liberal neutropenic or low-microbial diet was recommended. This still denied patients fresh fruits and vegetables, eggs, honey, yogurts, and other potentially bacteria-harboring foods but represented a significant improvement in variety and palatability compared with the autoclaved offerings.
In the last decade in the United States, the neutropenic diet recommendations for patients with cancer have fallen from favor, with both the American Society of Clinical Oncology (ASCO) and the Infectious Diseases Society of America (IDSA) citing a lack of evidence that these restrictions reduce risks of infection or all-cause mortality.3,4 A 2015 meta-analysis concluded that neutropenic diet restrictions were unlikely to confer a significant benefit to patients, but the randomized studies included in the meta-analysis did not include patients who underwent transplantation.5 The 1 study in the transplant population that was included in the meta-analysis was a retrospective observational analysis of 726 consecutively treated patients who underwent allo-HCT before and after an institutional policy change from the neutropenic to liberalized diet. These authors reported that the neutropenic diet was actually associated with an increased incidence of microbiologically confirmed infections.6 An additional observational before-and-after analysis of 102 pediatric patients undergoing allo-HCT at a single center did not reveal any association between the liberalized diet and infectious risk after transplantation.7
Despite these studies and expert opinion that following standard food-hygiene practices is probably safe in this patient group, we continue to recommend a variety of restrictions to patients who undergo HCT, with significant variability between institutions. One reason for this is that randomized data have not yet been available in a large cohort of patients who undergo transplantation, a gap that is addressed by Stella et al.1
Two hundred twenty-two patients were randomly assigned to receive advice to follow a strict neutropenic diet or a more liberal dietary strategy (see figure). The majority (79%) received an autologous graft, and 18% received a graft from an allogeneic donor. The primary end point was severe infection (grade ≥2) or death during the posttherapy neutropenic period. Secondary end points included the following: incidence of confirmed infection, nutritional status (eg, weight loss), length of hospital stay, and incidence of acute graft-versus-host disease in the allo-HCT recipients. There was no difference in the primary outcome between the 2 diets, nor was there any difference observed in the secondary outcomes. Further multivariate analysis of the whole cohort revealed that the only factors associated with lower infection risk were the use of a quinolone antibiotic as prophylaxis or, surprisingly, a diagnosis of multiple myeloma. Mucositis was associated with a higher incidence of severe infection. Overall, this prospective randomized study supports recommending a liberalized diet to our patients undergoing both auto and allo-HCT, following general food-safety guidelines but otherwise consuming any foods that they find palatable during the posttransplant period.
Summary of the key differences between the protective diet arm and the liberalized diet arm in the trial of Stella et al (modified from supplemental Table 1). The protective diet in the trial is consistent with traditional neutropenic diet guidelines. The liberalized diet is consistent with current IDSA and ASCO guidelines for patients with cancer. Drawn with BioRender.com.
Summary of the key differences between the protective diet arm and the liberalized diet arm in the trial of Stella et al (modified from supplemental Table 1). The protective diet in the trial is consistent with traditional neutropenic diet guidelines. The liberalized diet is consistent with current IDSA and ASCO guidelines for patients with cancer. Drawn with BioRender.com.
Furthermore, this trial also sets the table for future studies of another potential benefit of a diverse diet: perhaps dietary diversity facilitates a robust and diverse intestinal microbiome? Because microbiome injury is associated with poor outcomes after transplantation,8,9 it may be valuable to consider the role of a diverse diet in promoting optimal microbiome health in the setting of HCT. We view this randomized multicenter trial as practice-changing, although we acknowledge that 1 potential limitation is that all participating centers were within a single European country. As such, there are likely to be some differences between the usual diet consumed by study participants and the usual diet consumed elsewhere in the world. Therefore, we also await the reporting of an ongoing trial that focuses on allograft recipients within the United States (#NCT03016130).
Randomized trials are extremely difficult to perform in this realm, and we, therefore, often resort to conservative recommendations because they seem reasonable and not because they are supported by robust data. Posttransplant anorexia, gastrointestinal symptoms, and taste disruption (dysgeusia) are challenging for many patients, and the additional layer of dietary restriction can not only be a source of frustration but also contribute to malnutrition at a time of increased caloric needs.10 The team performing this study should be commended for addressing this important question in the transplant population, and in clinical practice we should feel comfortable in allowing patients more control over their posttransplant food choices.
Conflict-of-interest disclosure: J.U.P. reports research funding, intellectual property fees, and travel reimbursement from Seres Therapeutics; consulting fees from DaVolterra, CSL Behring, and MaaT Pharma; serves on an advisory board of and holds equity in Postbiotics Plus Research; and has filed intellectual property applications related to the microbiome (reference numbers #62/843 849, #62/977 908, and #15/756 845). Memorial Sloan Kettering Cancer Center has financial interests relative to Seres Therapeutics. K.A.M. reports consulting for Incyte and is a shareholder and board member of Postbiotics Plus.
References
Author notes
The full-text version of this article contains a data supplement.