Abstract
Bispecific antibodies, a novel immunotherapy with promising efficacy against multiple myeloma, form immune synapses between T-cell surface marker CD3 and malignant cell markers, including B-cell maturation antigen (BCMA), FcRH5, and G protein–coupled receptor GPRC5D. These bispecific antibodies so effectively deplete plasma cells (and to some extent T-cells) that patients are at increased risk of developing infections. A systematic review and meta-analysis of infections in published studies of patients with myeloma treated with bispecific antibodies was conducted to better characterize the infection risks. A literature search used MEDLINE, EMBASE, and Cochrane to identify relevant studies between inception and February 10, 2023, including major conference presentations. Phase 1b-3 clinical trials and observational studies were included. Sixteen clinical trials comprising 1666 patients were included. Median follow-up was 7.6 months and 38% of the cohort had penta-drug refractory disease. Pooled prevalence of all-grade infections was 56%, whereas the prevalence of grade ≥3 infections was 24%. Patients who were treated with BCMA-targeted bispecifics had significantly higher rates of grade ≥3 infections than non-BCMA bispecifics (25% vs 20%). Similarly, patients treated with bispecifics in combination with other agents had significantly higher rate of all-grade infection than those receiving monotherapy (71% vs 52%). In observational studies (n = 293), excluded from the primary analysis to ensure no overlap with patients in clinical trials, several infections classically associated with T-cell depletion were identified. This systematic review identifies BCMA-targeted bispecifics and bispecific combination therapy as having higher infection risk, requiring vigilant infection screening and prophylaxis strategies.
Introduction
Bispecific antibodies, a rapidly advancing treatment for hematologic malignancies, form an immune synapse between T-cells, via surface marker CD3 and surface markers on tumor cells. In multiple myeloma, bispecific antibodies targets include B-cell maturation antigen (BCMA), cell surface marker FcRH5, or G protein–coupled receptor GPRC5D.1 Although they demonstrate promising efficacy, concerns regarding infection-associated morbidity have arisen.2 Mazahreh et al examined infections in bispecific monotherapy trials published from 2019 to 2022, reporting moderate rates of severe infections (24%).3 Longer follow-up from these and other clinical trials, as well as recent publication of observational studies, prompted our systematic review and meta-analysis.
Methods
A systematic review and meta-analysis were conducted according to PRISMA guidelines,4 using the Ovid platform of PUBMED, MEDLINE, EMBASE, and Cochrane to identify relevant publications, between inception and 10 February 2023 using the comprehensive search strategy (supplemental Figure 1). We included clinical trial and observational studies, including patients receiving bispecific antibodies for myeloma as monotherapy or in combination with other agents, using data from the most recent publications and conference proceedings.
Article suitability and data extraction were performed by 2 authors (G.R. and E.R.S.C.), with extracted variables detailed in supplemental Table 1. The primary outcome was the proportion of patients with all-grade infections. Secondary outcomes included grade ≥3 infections, infection-related mortality, and the proportion of bacterial, viral, and fungal infections. Subgroup analyses compared primary and secondary outcomes by therapeutic target (BCMA vs non-BCMA) and monotherapy vs combination therapy. All primary and secondary analyses included only clinical trial data. Patients from observational studies were separately analyzed for infection etiology and timing. Meta-analysis of proportions, using random-effects model (Mantel-Haenszel method) was performed with R, using Cochran Q test to evaluate heterogeneity.5 The Joanna Brigg’s Institute critical appraisal tool assessed study bias.6
Results
After screening 799 studies, 20 studies were identified (supplemental Figure 2). Sixteen clinical trials (1666 patients) were included (Table 1). Twelve trials examined bispecific or trispecific monotherapy (1477 patients), and 4 trials examined combination therapy that included a bispecific antibody (189 patients). Median age was 64.7 years, 55% of patients were male, 78% patients (1218/1559) had triple-class refractory disease and 38% patients (548/1452) had penta drug-refractory disease. Median follow-up was 7.6 months.
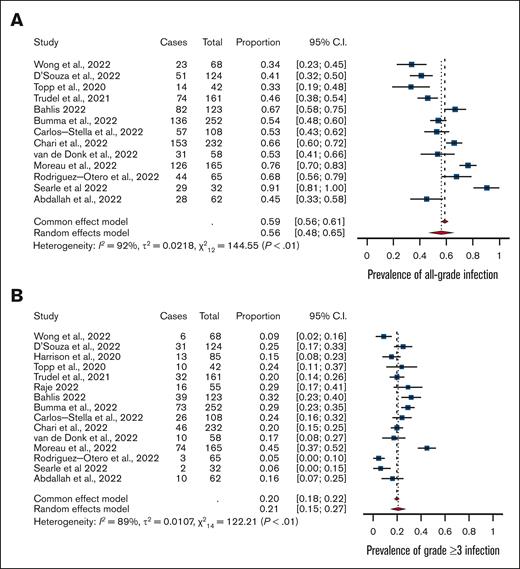
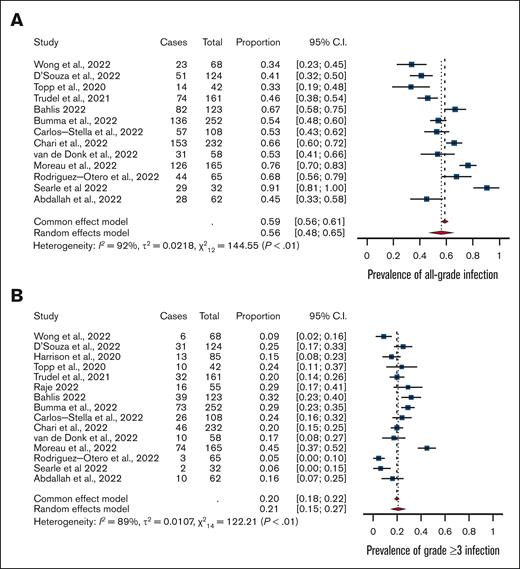
The prevalence of all-grade infections was 56% (95% confidence interval [CI], 0.48-0.65) with high heterogeneity (I2 = 92%) (Figure 1). The prevalence of grade ≥3 infections was 24% (95% CI, 0.19-0.29) with high heterogeneity (I2 = 81).
Meta-analysis. (A) Rates of all-grade infections and (B) grade ≥3 infections among patients with multiple myeloma treated with bispecific antibodies in clinical trials.
Meta-analysis. (A) Rates of all-grade infections and (B) grade ≥3 infections among patients with multiple myeloma treated with bispecific antibodies in clinical trials.
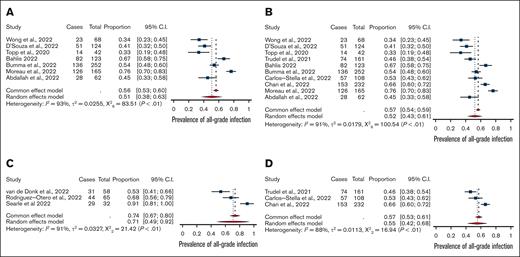
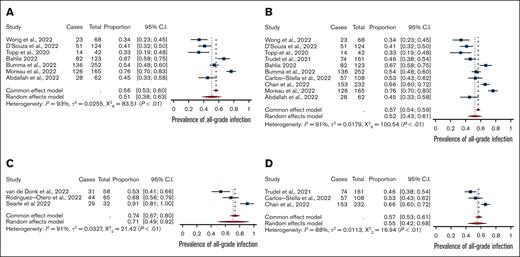
All-grade infections among patients treated on-trial with BCMA-bispecific monotherapy (n = 976; 51%; 95% CI, 0.38-0.63) were comparable with all-grade infections among non-BCMA-bispecific monotherapy (n = 501; 55%; 95% CI, 0.42-0.68). However, grade ≥3 infections were significantly higher among BCMA-targeting bispecifics (25%; 95% CI, 0.17-0.32) than with non-BCMA bispecifics (20%; 95% CI, 0.16-0.23; P < .01; Figure 2). The rates of grade ≥3 neutropenia, all-grade ICANS (immune effector cell–associated neurotoxicity syndrome), and steroid administration were lower in the BCMA-bispecific group (supplemental Table 2). Treatment-emergent hypogammaglobulinemia was infrequently and inconsistently reported, precluding meta-analysis.
Meta-analysis of combination versus mono-therapy and of BCMA-targeted versus non-BCMA targeted bispecifics. Comparison of (A) bispecific monotherapy vs (C) combination therapy trials and (B) BCMA-targeting bispecific antibodies vs (D) non-BCMA–targeting bispecific antibodies.
Meta-analysis of combination versus mono-therapy and of BCMA-targeted versus non-BCMA targeted bispecifics. Comparison of (A) bispecific monotherapy vs (C) combination therapy trials and (B) BCMA-targeting bispecific antibodies vs (D) non-BCMA–targeting bispecific antibodies.
The use of a bispecific in combination with another therapy (189 patients) was associated with significantly higher all-grade (71% vs 52%; P < .01) and comparable grade ≥3 infections (26% vs 24%) than monotherapy treatment. This effect was driven by BCMA-targeting agents; only 1 trial involved a non-BCMA agent in combination. Grade ≥3 neutropenia was more prevalent in patients treated with BCMA-combination therapy than those treated with BCMA-bispecific monotherapy (52% vs 44%).
Fourteen trials reported infection-related mortality. Among the 1604 patients enrolled in these trials, there were a total of 65 deaths attributed to infection (random-effects model, 3%; 95% CI, 0.01-0.04). The microbiological cause of infection-related deaths was incompletely reported, as shown in supplemental Table 3. Accepting these limitations, viral infection–associated mortality (including COVID-19 [≥19], disseminated adenovirus [n = 2], and cytomegalovirus [CMV] pneumonia [n = 1]) was as common as bacterial sepsis–associated mortality. Reactivation of pathogens typically associated with T-cell depletion, including Pneumocystis jirovecii pneumonia (PJP), CMV, and invasive aspergillosis, were also reported in low numbers. Other outcomes of infection-related morbidity including intensive care admissions and length-of-stay were not reported in the trials.
The 4 observational studies (141 patients) provided further infection regarding causative pathogen, infection onset, and outcomes (supplemental Table 4).22-25 Of the 293 infection events reported in observational studies, 68% were microbiologically-confirmed, including viral (49%), bacterial (45%), and fungal (6%). When reported, gram-negative pathogens were the predominant cause of bacterial infections (64%). Causative viral and fungal pathogens were inconsistently reported. Studies reported different metrics of infection onset, precluding meta-analysis; however, the median onset of all-grade infection was typically earlier (49-79 days) than that of severe infection (≥3 months). One study reported 2 late (≥12 months) fatal infections and increasing infection incidences despite enduring response.24 Approximately half of infection events required hospitalization.
The bias assessment for included studies found a lower study quality of observational studies (supplemental Table 5).
Discussion
In patients with bispecific antibody–treated myeloma, a high rate of all-grade infection and moderate rate of severe infections warrant caution. The addition of 6 new clinical trials and 481 patients in a short time since a recent analysis resulted in noticeable changes. For example, between the publication of MonumenTAL-1 trial data of talquetamab in the New England Journal of Medicine (data cutoff 17 January 2022) and its presentation at the American Society of Hematology 2022 (data cutoff 12 September 2022), rates of all-grade infections increased from 39.2% to 65.9%. This change may be because of a range of factors, such as time taken for reporting from sites, data cleaning, and/or resolution of queries. Close longer-term follow-up is required to fully appreciate the burden of infections after bispecific therapies.
A higher rate of infections was observed in patients treated with BCMA-bispecifics than in patients treated with non-BCMA targeting bispecifics, despite a lower prevalence of both penta drug-refractory disease and treatment complications in the BCMA cohort, including neutropenia, CRS, ICANS, and steroid administration. Comparatively, BCMA CAR-T studies have reported similar all-grade and grade ≥3 infection rates to patients treated with BCMA-bispecific antibodies, both in clinical trial settings and observational studies.23,26 Translational research into the on-target, off-tumor effects of BCMA blockade on cellular immunity may help mechanistically explain this effect, appreciating the fact that BCMA therapies are currently delivered to patients with refractory disease, who have been heavily pretreated.27
This analysis highlights the potential relationship between combination therapy and higher incidence of infection, with all-grade infection rates 19% higher in patients on combination trials than those receiving bispecific antibody monotherapy, perhaps, because of more comprehensive plasma cell aplasia. Daratumumab can be associated with neutropenia, and higher rates of grade ≥3 neutropenia were observed in the combination group.28 Consequently, patients receiving combination therapy including bispecific antibodies should be considered as a higher-risk group requiring further evaluation and targeted prophylaxis.
Although data are evolving, the high rates of viral infection and the occurrence of infections classically associated with T-cell depletion highlight the importance of antimicrobial prophylaxis in patients treated with bispecific antibodies. In anticipation, pretreatment screening for hepatitis B and seropositivity for CMV, varicella zoster, and herpes simplex viruses should be performed to guide subsequent management.29 Strong consideration should be given to routine herpes simplex and varicella zoster and PJP prophylaxis. A low threshold for investigating viral reactivation and dissemination, including adenovirus, CMV, and varicella should be considered. The role of antifungal and routine antibacterial prophylaxis in this population is not yet known. Intravenous immunoglobulin is recommended with hypogammaglobulinemia and was associated with reduced rates of severe bacterial infections in a retrospective analysis.24,29,30 Fixed-duration treatment with bispecific antibodies and/or response-adapted treatment should also be evaluated in clinical trials.
This systematic review suggests patients treated with bispecific antibodies in combination with other therapies may represent a cohort with higher infection risk, requiring more vigilant screening and considered approaches to prophylaxis. Frequent analysis of longer-term infection outcomes is particularly important for this population to define duration of infection risk and assess the functional effect of long-term T-cell redirection despite enduring disease control.
Authorship
Contribution: GR and E.R.S.C. contributed equally to the manuscript. G.R., E.R.S.C., and B.W.T. designed the research; G.R., E.R.S.C., M.N.L.H. performed the research; G.R. analyzed the data; and G.R., E.R.S.C., G.R.M., R.P., S.M., M.N.L.H., S.J.H., A.S.K., B.W.T. prepared the manuscript.
Conflict-of-interest disclosure: E.R.S.C. receives research funding from Arnold Ventures (institutional). G.R. receives research funding from NHMRC. R.P. receives honoraria from Janssen, Celgene, GlaxoSmithKline, AbbVie, and Sanofi; holds a consulting or advisory role in GlaxoSmithKline, Celgene, Roche, BeiGene, and Janssen; and receives research funding from GlaxoSmithKline (institutional) and travel and accommodation expenses from Janssen and GlaxoSmithKline. B.W.T. discloses stock and other ownership interests in CSL Behring; research funding from MSD and Seqirus; and uncompensated relationships with CSL Behring (institutional), Takeda (institutional), and Moderna Therapeutics (institutional). A.S.K. receives research funding from Arnold Ventures (institutional). S.J.H. discloses having a leadership role at Haemalogix; receives honoraria from AbbVie, Amgen, Celgene/BMS, GKS, Janssen Cilag, Novartis, Roche, Genentech, Haemalogix, Eusa, and Terumo BCT; has a speaker’s bureau membership at AbbVie, Amgen, Celgene/BMS, GSK, Janssen Cilag, Novartis, Roche Genetech, and Eusa; and research funding from Celgene/BMS, GSK, Janssen Cilag, and Haemalogix. The remaining authors declare no competing financial interests.
Correspondence: Gemma Reynolds, National Centre for Infections in Cancer, Peter MacCallum Cancer Centre and Austin Health, 145 Studley St, Melbourne 3084, VIC, Australia; e-mail: gemma.k.reynolds@gmail.com.
References
Author notes
∗G.R. and E.R.S.C. contributed equally as first authors.
Data are available upon reasonable request from the corresponding author, Gemma Reynolds (gemma.k.reynolds@gmail.com).
The full-text version of this article contains a data supplement.