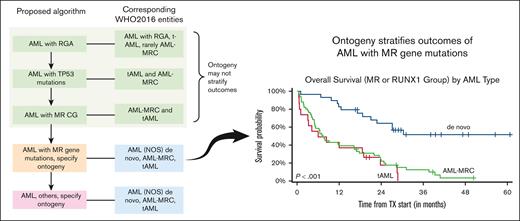
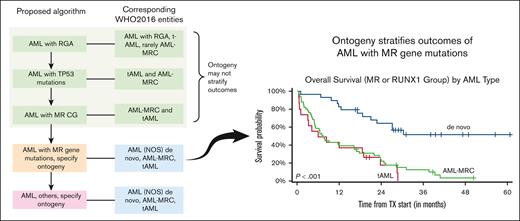
Key Points
Ontogeny assignment from the database registry lacks sensitivity and specificity.
Ontogeny stratifies the outcome of AML with MR gene mutations.
Abstract
Accurate classification and risk stratification are critical for clinical decision making in patients with acute myeloid leukemia (AML). In the newly proposed World Health Organization and International Consensus classifications of hematolymphoid neoplasms, the presence of myelodysplasia-related (MR) gene mutations is included as 1 of the diagnostic criteria for AML, AML-MR, based largely on the assumption that these mutations are specific for AML with an antecedent myelodysplastic syndrome. ICC also prioritizes MR gene mutations over ontogeny (as defined in the clinical history). Furthermore, European LeukemiaNet (ELN) 2022 stratifies these MR gene mutations into the adverse-risk group. By thoroughly annotating a cohort of 344 newly diagnosed patients with AML treated at the Memorial Sloan Kettering Cancer Center, we show that ontogeny assignments based on the database registry lack accuracy. MR gene mutations are frequently observed in de novo AML. Among the MR gene mutations, only EZH2 and SF3B1 were associated with an inferior outcome in the univariate analysis. In a multivariate analysis, AML ontogeny had independent prognostic values even after adjusting for age, treatment, allo-transplant and genomic classes or ELN risks. Ontogeny also helped stratify the outcome of AML with MR gene mutations. Finally, de novo AML with MR gene mutations did not show an adverse outcome. In summary, our study emphasizes the importance of accurate ontogeny designation in clinical studies, demonstrates the independent prognostic value of AML ontogeny, and questions the current classification and risk stratification of AML with MR gene mutations.
Introduction
Acute myeloid leukemia (AML) is a heterogeneous group of clinically aggressive hematologic malignancies characterized by the maturation arrest and accumulation of myeloid blasts.1,2 AML classification is a prerequisite for appropriate disease management and is an evolving process. The French-American-British classification, arguably the first widely adapted classification scheme, was largely based on cytomorphological and cytochemical characteristics, which, despite limitations, informed the formulation of the modern AML classification.3-5 With a deeper understanding of the underlying molecular pathogenesis, it has become clear that an integrated algorithm including clinical history, morphology, immunophenotype, and genetics provides better disease delineation, which can further guide clinical decision making.6 This culminated in the World Health Organization (WHO) 2016 classification of hematolymphoid neoplasms (WHO2016) that classifies AML into 4 major subtypes: AML with recurrent genetic abnormalities, AML with myelodysplasia-related changes (AML-MRCWHO2016), therapy-related AML (t-AML), and AML not otherwise specified.4 Prior cytotoxic therapy for a nonmyeloid disorder is a prerequisite for diagnosing t-AML. The diagnostic criteria for AML-MRCWHO2016 are as follows: (1) history of myelodysplastic syndrome (MDS) or MDS/myeloproliferative neoplasm (MDS/MPN); (2) the presence of MDS-defining cytogenetic abnormalities; or (3) morphologic dysplasia in >50% of cells of at least 2 lineages. The evaluation of dysplasia appears subjective with poor interobserver agreement; therefore, its value as a criterion for AML-MRCWHO2016 has been questioned.7-11 Notably, the ontogeny of AML, as defined in the clinical history, outweighed genomics in the WHO2016 classification.
The genomic landscape of AML has increasingly delineated over the last decade.12-15 Thus, genomic classification of AML has been proposed based on the presence of mutations/fusions, adding prognostic value to risk stratification.15,16 Besides the well-recognized AML with recurrent genetic abnormalities as defined by WHO2016, 2 large genomic clusters of patients with either TP53 or chromatin-spliceosome mutations are also identified,15 presumably corresponding to t-AML or AML-MRCWHO2016, respectively. Furthermore, a study based on a multicenter clinical trial demonstrated that mutations in 8 genes (ie, ASXL1, BCOR, EZH2, STAG2, SF3B1, SRSF2, ZRSR2, and U2AF1) within the chromatin-spliceosome cluster appear to be 95% specific to AML-MRCWHO2016 in comparison to de novo AML.17 Based on these new data, both WHO2022 and ICC2022 include a new AML subtype classifying AMLs with the presence of any of these 8 gene mutations as AML with myelodysplasia-related gene mutations (MR genes) to unify these patients who may share similar biology regardless of antecedent history.18,19 International Consensus classification (ICC) also adds RUNX1 mutations to the list (MR/RUNX1).19 Moreover, although the WHO retains ontogeny in the diagnostic hierarchy,18 genomic features override ontogeny in ICC, which means that ICC removes t-AML and AML-MRCWHO2016 as diagnostic entities.19 Instead, ontogeny becomes a qualifier in the ICC. Concurrently, a few studies have shown inferior outcomes in patients with AML carrying MR gene mutations.16,20-23 Therefore, these mutations have been universally added to the adverse-risk group by the European LeukemiaNet (ELN) 2022.2 Despite these new classifications/risk stratification methods, there is a lack of data demonstrating the relative importance of ontogeny vs genomics in AML prognosis. A thorough and comprehensive review of patient history is essential for an accurate ontogeny assignment, which, unfortunately, has been challenging in many published studies to date. Secondly, whether all the assigned MR gene mutations are specific to AML-MRCWHO2016 and are bona fide adverse-risk factors, as designated by ELN2022, awaits independent validation.
In this study, we manually annotated a cohort of 344 patients with newly diagnosed AML treated at the Memorial Sloan Kettering Cancer Center (MSKCC) and correlated the ontogeny and genomics with the outcomes. We show that (1) ontogeny assignment from the database registry lacks sensitivity and specificity; (2) both ontogeny and genomics provide independent prognostic values in AML; (3) the newly proposed MR gene mutations are enriched in but not specific to AML-MRCWHO2016 and do not necessarily correlate with adverse outcomes; and (4) de novo AML with MR gene mutations shows an outcome that falls between the favorable- and intermediate-risk groups.
Methods
Patient cohort
Patients with newly diagnosed AML from January 2014 to December 2019 at MSKCC were retrospectively enrolled in this study. Patients who lacked genomic data or follow-up data were excluded. The diagnosis of AML was independently confirmed by 2 hematopathologists (M.R. and W.X.). t-AML was defined according to the WHO2016 criteria as a late complication of cytotoxic chemotherapy and/or radiation therapy administered for a prior nonmyeloid neoplastic or non-neoplastic disorder, including alkylating agents, platinum derivatives, topoisomerase II inhibitors, antimetabolites, antitubulin agents, external beam radiotherapy to active marrow sites, and therapeutic systemic radioisotopes.4 AML-MRCWHO2016 was further divided into MR-Hx and MR-CG: AML was classified as MR-Hx when developed at least 3 months after the histologic documentation of antecedent MDS or MDS/MPN and not qualifying as t-AML. In the absence of the aforementioned history, AML with MDS-defining cytogenetic abnormalities was classified as MR-CG. Morphologic dysplasia was not included as 1 of the criteria for AML-MRCWHO2016 in this study. Notably, the criteria for t-AML, MR-Hx, and MR-CG are nearly identical between WHO2008 and WHO2016. The remaining cases were classified as de novo AML. Therefore, AML ontogeny was assigned as t-AML, AML-MRCWHO2016 (specifically MR-Hx vs MR-CG), and de novo AML. This study was approved by the Institutional Review Board at MSKCC.
Chromosome and FISH analysis
Conventional chromosome analysis was performed on fresh bone marrow aspirates and/or peripheral blood specimens following the standard protocol. At least 20 metaphase cells were analyzed, and karyotypes were described per the international system of human chromosome nomenclature, 2016. Fluorescence in situ hybridization (FISH) analysis was performed on bone marrow or peripheral blood pellets following standard protocols. Various commercial FISH probes specific for myeloid neoplasia, such as deletion or loss of chromosomes 5, 7, 17/TP53, gain of chromosome 8, deletion of 20q, MLL/KMT2A (11q23) translocations, EVI1 (3q26.2), and other translocations, were used as appropriate. At least 300 cells were analyzed in each sample, and the results were described per the International System of Human Chromosome Nomenclature, 2016. FISH results were correlated with chromosome analysis findings, when feasible, in metaphase cells for the precise interpretation of chromosome abnormalities. All cytogenetic karyotypes were manually reannotated by a cytogeneticist (Y.Z.) based on numerical and structural chromosomal abnormalities, such as gain, loss, deletion, addition, duplication, balanced or unbalanced translocations, inversion, and derivative chromosomes as well as marker chromosome and ring chromosome to define complex karyotypes (CKs), that is, 3 or more clonal chromosome abnormalities, clonal heterogeneity, and monosomal karyotype (MK).
Mutation profiling
Next-generation sequencing (NGS) studies were performed using the bone marrow or peripheral blood cell that were submitted at diagnosis, using 1 of the 3 clinically validated, hybrid capture-based targeted panels: Raindance Thunderstorm (RDTS, 28-gene panel), Raindance Thunderbolt (RDTB, 49-gene panel) or IMPACT-heme (400-gene panel; supplemental Table 1).24 The FLT3 mutations were detected via polymerase chain reaction tests. Twenty-three patients underwent NGS studies not at diagnosis but only on refractory/persistent disease (>20% blasts) within 4 months of treatment initiation. Patients who had NGS data only at relapse were excluded. Candidate mutations were annotated using the VAGrENT version 3.3.0 (https://github.com/cancerit/VAGrENT) and Ensemble version 91VEP version 92 (https://github.com/Ensembl/ensembl-vep). These annotations were compared with those using COSMIC version 81,25 OncoKB,26 and Genome Aggregation Database27 databases, along with recurrence in a panel of healthy samples, to provide further information about the prevalence of each mutation in patients with cancer and healthy populations. This information was used for the manual curation of each variant to classify it as pathogenic, likely pathogenic, or a variant of uncertain significance. Pathogenic and likely pathogenic mutations were retained for analysis and visualization (supplemental Table 2).
Genomic class
Genomic class assignment was based on the WHO2022 and IC2022 classifications.18,19 The patients were classified in hierarchical order as well-defined AML entities with fusions, including t(15;17), t(8;21), inv(16), t(6;9), MLL rearrangements, and EVI1 rearrangements, followed by NPM1, CEBPA bZIP, TP53, and MR/RUNX1 mutations. Disease-defining fusions override disease-defining mutations when they occur together. None of the patients had NPM1 or TP53 comutations. One patient with a CEBPA bZIP mutation and a subclonal TP53 mutation was classified to have AML with a CEBPA bZIP mutation. AML with MR/RUNX1 mutations was defined as the presence of any MR/RUNX1 mutation but no disease-defining fusions or mutations (ie, NPM1, CEBPA bZIP, or TP53).
Statistics
Descriptive statistics, including the median and interquartile range (IQR) for continuous variables and percentages for categorical variables, are provided. Fisher exact test or χ2 test was used to evaluate the association between 2 categorical variables. The Wilcoxon rank-sum test or Kruskal-Wallis test was used to assess the difference in a continuous variable between and among patient groups. Univariable logistic regression was performed to assess the association between mutation profiles and AML ontogeny (MR-Hx vs de novo AML, MR-Hx vs t-AML, and t-AML vs de novo AML). Overall survival (OS) was calculated from the start of systematic treatment for AML or the date of diagnosis for patients under supportive care to death or the date of the last follow-up. Event-free survival (EFS) was calculated from the start of systemic treatment for AML to the date of either treatment failure (60 days), hematologic relapse from complete remission with or without hematologic recovery, or death from any cause, whichever occurred first.2 Left truncation was used to account for molecular testing performed after the start of first-line chemotherapy in a small group of patients (n = 23). The Kaplan-Meier method was used to estimate OS and the log-rank test was used to evaluate differences between groups. Univariable Cox proportional hazard regression was used to evaluate OS associated with disease-related determinants, such as AML ontogeny and mutation profiles. We also evaluated AML ontogeny–related risks in multivariable Cox-regression models adjusted for age (modeled by cubic spline), initial treatment at diagnosis, allogenic hematopoietic stem/progenitor cell (allo-HSCT) transplant (modeled as a time-dependent variable), presence of TP53 mutation, ELN2022 risk, cytogenetics, genomics, and sequencing platform. In the sensitivity analysis, we stratified the patients based on ELN2022 risk, cytogenetics, genomics, and sequencing platform while adjusting for the same set of covariates to confirm the association between AML ontology and OS. All analyses and graphics were produced using R version 4.0.3.
Results
Reclassification of AML ontogeny
The entire study cohort comprised 344 patients with newly diagnosed AML (male-to-female ratio, 1.4; median age, 66.7 years; supplemental Table 3). Based on the information extracted from the database registry, ontogeny was assigned as follows: t-AML (n = 48; 14.0%), AML-MRCWHO2016 (n = 139; 40.4%), and de novo AML (n = 157; 45.6%). To accurately classify AML based on ontogeny and WHO2016 criteria, a multidisciplinary team curated each patient’s condition via a thorough chart review, including the history of antecedent hematologic diseases, cancers, chemoradiation therapy, laboratory tests, and cytogenetic reports. The curated data were independently validated by 2 board-certified hematopathologists (M.R. and W.X.). Karyotyping and FISH results were reannotated by a board-certified cytogeneticist (Y.Z.). Any discrepancies were resolved via a group consensus.
The entire cohort was reclassified based on a thorough review as t-AML (92/344 [26.7%]), MR-Hx (107/344 [31.1%]), MR-CG (29/344 [8.4%]), and de novo AML (116/344 [33.7%]) (see “Methods” for definitions; Table 1). Although the initial classification of t-AML was highly specific, the sensitivity was only 52.2%. Half of the t-AMLs were initially misclassified as AML-MRCWHO2016 or de novo AML because of an inadequate history review. The initial AML-MRCWHO2016 classification was moderately specific (79.3%) and sensitive (70.6%). Importantly, the initial designation of de novo AML had a specificity of only 78.1%, with nearly one-third of the cases being t-AML or AML-MRCWHO2016. These findings demonstrate the importance of thorough cohort annotation for accurate assignment of AML ontogeny.
Prediction of AML ontogeny based on the mutations
The clinical characteristics of the AML subtypes are shown in Table 2. Patients with de novo AML were younger than those with t-AML or MR-Hx (median age, 60 vs 70 or 69 years, respectively; P < .001). There were roughly twofold more patients with de novo AML receiving induction chemotherapy and allo-transplant than those with t-AML and MR-Hx (Table 2). The median interval between MDS or MDS/MPN and MR-Hx was 15 months (IQR, 6-26 months). The median latency of t-AML after prior chemoradiation therapy was 71 months (IQR, 29-106 months). A small proportion of patients with de novo AML (8/116 [6.9%]), MR-Hx (13/107 [12%]), and MR-CG (4/29 [14%]) also had a history of solid cancers, mostly at early stages, and were treated solely with surgical resection, for which none of them received chemoradiation therapy.
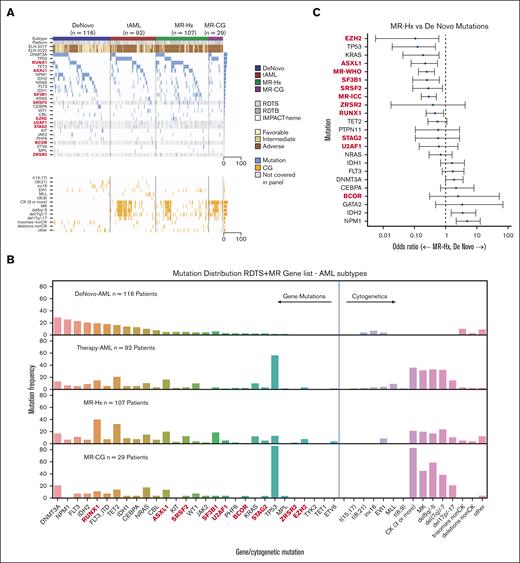
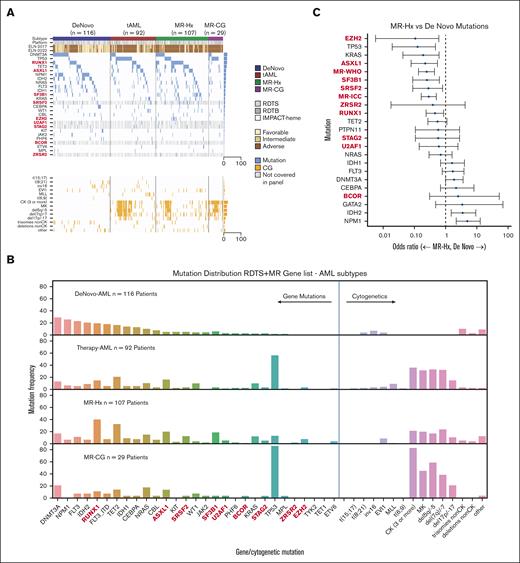
Gene mutations covered by all 3 panels and additional MR genes (BCOR, SRSF2, STAG2, U2AF1, and ZRSR2) only covered by RDTB and IMPACT-heme panels are shown in Figure 1A-B. As expected, mutations in NPM1, DNMT3A, IDH1, IDH2, and FLT3 were predictive of de novo AML, whereas TP53 mutations were highly predictive of t-AML (Figure 1B; supplemental Figure 1A). We also confirmed that mutations in ASXL1, SF3B1, SRSF2, EZH2, and RUNX1 were predictive of MR-Hx over de novo AML17,28 (Figure 1B-C; supplemental Figure 1B). Surprisingly, KRAS mutations were mostly present in MR-Hx. Both the WHO-MR and ICC-MR gene combinations were also predictive of MR-Hx (Figure 1C). A comparison of t-AML and MR-Hx revealed that TP53 mutations were more predictive of t-AML, whereas SF3B1, SRSF2, and RUNX1 mutations were predictive of MR-Hx (supplemental Figure 1C). Notably, the mutational profile of MR-CG resembled that of t-AML with a high prevalence of TP53 mutations (16/29 [55.2%] in MR-CG and 36/92 [39.1%] in t-AML) and a CK, suggesting a shared biology driven by TP53 mutations. TP53 mutations were present in only a small subset of MR-Hx (13/107 [12.1%]) and rarely in de novo AML (2/116[1.7%]).
Genomic profiling predicts the AML ontogeny. (A) Oncoplot of AML subtypes (de novo AML, t-AML, MR-Hx, and MR-CG). NGS panels are indicated as platforms (RDTS, RDTB, and IMPACT-heme). Genes not covered by RDTS are indicated in gray. The ELN2017 and ELN2022 risk groups are listed. EVI1 indicates EVI1 rearrangements. MLL indicates MLL rearrangements. (B) Bar plots of genomic aberrations for each AML subtype. Proportions are shown. The MR/RUNX1 genes are bolded. (C) Association between individual gene mutations and AML ontogeny. Odds ratio was depicted on a log10 scale. The comparison is between MR-Hx and de novo AML.
Genomic profiling predicts the AML ontogeny. (A) Oncoplot of AML subtypes (de novo AML, t-AML, MR-Hx, and MR-CG). NGS panels are indicated as platforms (RDTS, RDTB, and IMPACT-heme). Genes not covered by RDTS are indicated in gray. The ELN2017 and ELN2022 risk groups are listed. EVI1 indicates EVI1 rearrangements. MLL indicates MLL rearrangements. (B) Bar plots of genomic aberrations for each AML subtype. Proportions are shown. The MR/RUNX1 genes are bolded. (C) Association between individual gene mutations and AML ontogeny. Odds ratio was depicted on a log10 scale. The comparison is between MR-Hx and de novo AML.
Correlation between AML ontogeny and genomic classification
Based on previous studies and the new WHO and IC classifications,15,18,19 patients with AML were divided into 8 genomic classes: favorable fusions (t[15;17], t[8;21] or inv[16], n = 19; 5.5%), MLL rearranged (n = 10; 2.9%), t(6;9) (n = 2; 0.6%), EVI1 rearranged (n = 18; 5.2%), NPM1 mutations (n = 43; 12.5%), CEBPA bZIP mutations (n = 11; 3.2%), TP53 mutations (n = 64; 18.6%), and MR/RUNX1 mutations (n = 102; 29.7%) (Table 2; supplemental Table 4). Sixty-two (18%) patients had mutations that did not belong to any of these subgroups (not otherwise specified). Thirteen patients (3.8%) had no mutations detected (negative).
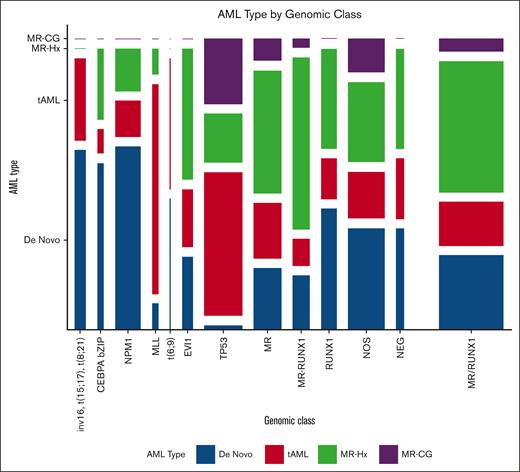
Patients with favorable fusions and CEBPA bZIP mutations were younger than those in other subgroups (supplemental Table 4). NPM1 mutated and MLL rearranged AML showed female preponderance. Most of the AML with favorable fusions, CEBPA bZIP or NPM1 mutations and a small proportion of t-AMLs were de novo (Figure 2). Interestingly, 3 of 11 (27.3%) patients with CEBPA bZIP and 7 of 43 (16.3%) patients with NPM1 mutated AML had a history of MDS (1 had MDS/MPN) with a median interval of 6 months (IQR, 3.8-10.5 months) before progressing to AML (supplemental Table 5). Among them, 2 CEBPA bZIP and 2 NPM1 mutated patients had MDS-EB2 (10%-15% blasts). In addition, 1 of 10 (10%) patients with MLL and 9 of 18 (50%) patients with EVI1 rearranged AML had antecedent MDS or MDS/MPN, with a median interval of 12 months (IQR, 4-24 months) before AML (supplemental Table 5). Six patients were evaluated for EVI1 rearrangements at the stage of MDS, and 5 were positive: 4 had ∼10% to 15% blasts, and 1 had 5% blasts. Notably, nearly all these patients would be classified as having de novo AML based on the WHO2022, and ∼50% would, if ICC2022 was applied.
Distribution of AML ontogeny subtypes in each genomic class. The width of each bar indicates the number of patients. MR indicates patients with AML with MR gene mutations (including ASXL1, BCOR, EZH2, STAG2, SF3B1, SRSF2, ZRSR2, and U2AF1, but no RUNX1 mutations). MR-RUNX1 indicates patients with AML with both MR gene and RUNX1 gene mutations. RUNX1 indicates patients with AML with RUNX1 but no MR gene mutations. MR/RUNX1 indicates patients with AML with MR and/or RUNX1 gene mutations. NOS indicates patients with AML with mutations detected but cannot be assigned to a well-defined entity. NEG indicates patients with AML with no mutations or rearrangements detected.
Distribution of AML ontogeny subtypes in each genomic class. The width of each bar indicates the number of patients. MR indicates patients with AML with MR gene mutations (including ASXL1, BCOR, EZH2, STAG2, SF3B1, SRSF2, ZRSR2, and U2AF1, but no RUNX1 mutations). MR-RUNX1 indicates patients with AML with both MR gene and RUNX1 gene mutations. RUNX1 indicates patients with AML with RUNX1 but no MR gene mutations. MR/RUNX1 indicates patients with AML with MR and/or RUNX1 gene mutations. NOS indicates patients with AML with mutations detected but cannot be assigned to a well-defined entity. NEG indicates patients with AML with no mutations or rearrangements detected.
TP53 mutated AML comprised predominantly t-AML (35/64 [54.7%]), MR-CG (16/64 [25%]), and MR-Hx (12/64 [18.8%]) groups. Only 1 of 64 (1.6%) patients with TP53 mutated AML was de novo (supplemental Table 4). This patient had de novo monocytic AML with TET2 and TP53 mutations and extensive necrosis in the marrow. FISH analysis of the peripheral blood samples showed negative results. The patient had refractory disease and died within 6 months. Thirty-one of 64 (48.4%) patients with TP53 mutated AML had prior MDS (19 t-MDS) or MDS/MPN stage, with a median interval of 11 months (IQR, 4-15 months) before the development of AML. Strikingly, 61 of 64 (95.3%) patients with TP53 mutated AML had CG abnormalities involving −5/del5q, −7/del7q, −17/del17p, and/or CK. Conversely, 61 of 119 (51.3%) patients with such CG abnormalities harbored TP53 mutations. Specifically, 46 of 55 (83.6%) patients with concurrent CK and MKs harbored TP53 mutations.
MR/RUNX1 mutated AML mostly comprised MR-Hx (51/102 [50%]) and t-AML (18/102 [17%]) cases (Figure 2; supplemental Table 4). Only 5 patients (4.9%) had MR-CG. Importantly, 29 of 102 (28%) patients had de novo AML. Of note, 10 (9.6%) patients had isolated trisomy 8 and/or del20q only, which was newly added by ICC but not WHO as MDS-defining CG abnormalities, and 5 of them had de novo AML (supplemental Table 6).
AML ontogeny predicts the outcome independent of ELN2022 risk stratification
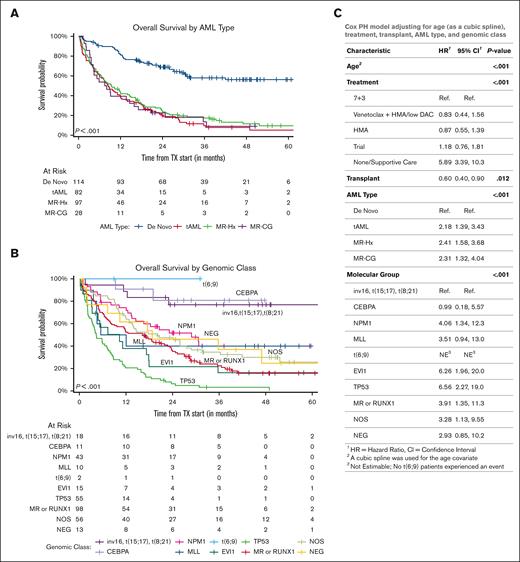
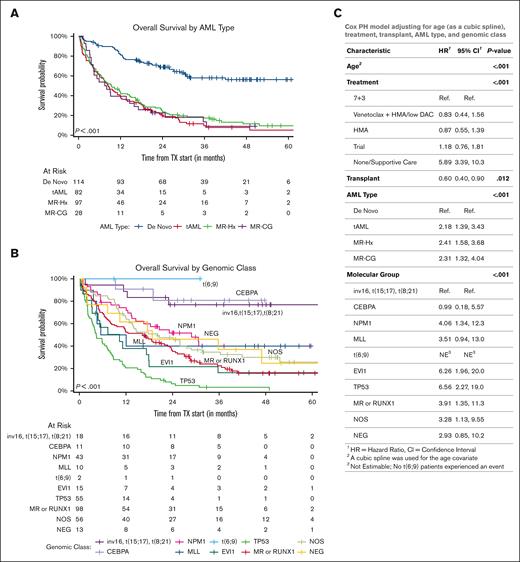
De novo AML had a significantly better EFS and OS than all 3 other subtypes (median, not reached vs 8.4 months [95% confidence interval (CI), 6-12.1 months] in t-AML, 9.4 months [95% CI, 6.5-14.7 months] in MR-Hx, and 7.7 months [95% CI, 4.3-17.1 months] in MR-CG; P < .0001; Figure 3A; supplemental Figure 2A). This difference remained statistically significant in patients treated with daunorubicin + cytarabine (7 + 3) induction therapy, if TP53 mutated patients were excluded or after stratification based on age, ELN2022 risk groups, genomic classes, CG risks, and NGS platforms (supplemental Figures 3-5). AML ontogeny further stratified the outcomes of all 3 ELN2022 risk groups but only of the intermediate (neither favorable nor adverse) CG risk group (supplemental Figure 6).
Both AML ontogeny and ELN risk have independent prognostic values. (A) Kaplan-Meier curves of OS divided by AML ontogeny subtypes. (B) Kaplan-Meier curves of OS divided by genomic classes. Both patients with t(6;9) underwent allo-HSCT. (C) AML ontogeny–related risk was evaluated using multivariable Cox-regression models adjusted for age (modeled by cubic spline), initial treatment at diagnosis, allogenic transplant (modeled as a time-dependent variable), and genomic classes. 7 + 3, daunorubicin + cytarabine, including CPX-351. HMA, hypomethylating agents; low DAC, low-dose cytarabine.
Both AML ontogeny and ELN risk have independent prognostic values. (A) Kaplan-Meier curves of OS divided by AML ontogeny subtypes. (B) Kaplan-Meier curves of OS divided by genomic classes. Both patients with t(6;9) underwent allo-HSCT. (C) AML ontogeny–related risk was evaluated using multivariable Cox-regression models adjusted for age (modeled by cubic spline), initial treatment at diagnosis, allogenic transplant (modeled as a time-dependent variable), and genomic classes. 7 + 3, daunorubicin + cytarabine, including CPX-351. HMA, hypomethylating agents; low DAC, low-dose cytarabine.
The genomic classes significantly correlated with EFS and OS (Figure 3B; supplemental Figure 2B). The impact of the genomic class was more significant in de novo AML and only borderline significant in t-AML (supplemental Figure 7). Patients with favorable fusions and CEBPA mutations had the most favorable OS (median, not reached; Figure 3B). NPM1-mutated and MR/RUNX1-mutated AMLs had median OSs of 28.9 months (95% CI, 14.6 months to not reached) and 16.3 months (95% CI, 9.1-23.4 months), respectively. Interestingly, the outcomes of both CEBPA- and NPM1-mutated AML (but not AML with favorable fusions) were further stratified based on ontogeny (supplemental Figure 8A-C), somewhat in contrast to the outcomes of a recent large cohort showing NPM1 mutated t-AML has a similar OS to its de novo counterpart after excluding early death.29 As expected, either TP53 mutated or EVI1 rearranged AML had dismal outcomes (median OS, 4.4 months [95% CI, 3.4-7.5 months] and 10.3 months [95% CI, 4.9-35.7 months], respectively) regardless of ontogeny (supplemental Figure 8D-E). Further analysis of TP53 mutated AML showed that neither mutation burden, biallelic status, CK, nor comutations in signaling molecules affected the outcomes (supplemental Figure 9).
In the univariate analysis, CEBPA, DNMT3A, NPM1, and IDH2 mutations were associated with a superior OS (supplemental Figure 10A). In contrast, TP53, KRAS, NOTCH2, EZH2, and SF3B1 mutations were associated with an inferior OS. Of note, mutations in most MR genes and RUNX1 were not significantly associated with outcome. To study the interaction between mutations and ontogeny, the impact of mutations on OS was adjusted for the AML subtype. To this end, the prognostic values of many gene mutations (DNMT3A, NPM1, IDH2, KRAS, NOTCH2, EZH2, and SF3B1) were no longer significant, whereas TP53 and CEBPA mutations remained prognostic (supplemental Figure 10B). In a multivariate analysis adjusted for age, initial treatment for induction, and allo-HSCT, both AML subtypes and genomic classes or ELN2022 risks were significantly associated with outcomes (Figure 3C; supplemental Figure 10C), demonstrating that ontogeny and genomics provide independent prognostic values.
Specificity of MR gene mutations in MR-Hx AML
Next, we focused on MR/RUNX1-mutated AML group. Although a previous study had suggested that MR gene mutations are highly specific to MR-Hx (with 95% specificity),17 the specificity of these gene mutations to MR-Hx remains controversial.28,30 We decided to address these issues using our well-annotated cohort. We first examined the MR/RUNX1 group, which was sequenced using targeted NGS panels covering all 8 MR and RUNX1 genes. Among 42 patients, mutations in MR/RUNX1 genes were present in 14 de novo AML and 28 MR-Hx AML, resulting in a positive predictive value of only 66% for MR-Hx. This is similar to that of our entire cohort (positive predictive value 51/80 (64%); specificity, 74%) regardless of the NGS panels used (Figure 2), as well as to those cohorts of other studies.28,30 If RUNX1 mutations are excluded, the positive predictive value and specificity of MR gene mutations for MR-Hx are 70% and 75%, respectively.
De novo AML with MR gene mutations does not have an adverse outcome
Nearly one-third of patients with AML with MR/RUNX1 mutations had de novo conditions. The ELN2022 guideline risk stratifies these patients into an adverse-risk group, regardless of ontogeny. We, thus, investigated the impact of ontogeny on EFS and OS in patients carrying these mutations. In this group, there were very few patients with MR-CG, which was insufficient for the statistical analysis. However, patients with de novo AML had remarkably better EFS and OS than those with MR-Hx or t-AML (median OS, not reached vs 7.3 [95% CI, 5.1-17 months] or 6 months [95% CI, 3 months to not reached]; P < .001; Figure 4A; supplemental Figure 11A; supplemental Table 7). This was also true in patients with MR only or RUNX1 only mutations (supplemental Figure 12A-B). Comparable OS was observed between the ELN2017 intermediate- and adverse-risk groups, suggesting that even ASXL1 and RUNX1 mutations may not be able to risk stratify this patient population (supplemental Figure 12C). The proportion of adverse CG abnormalities was significantly lower in the de novo AML subgroups (supplemental Table 7); however, the difference in OS was small between the intermediate and adverse CG risk groups (supplemental Figure 12D). Univariate analysis after stratifying the cases based on AML subtypes showed that mutations in NRAS and KRAS, but not in any of the individual MR/RUNX1 genes, were associated with an inferior OS (supplemental Figure 13A). Multivariate analysis showed that age, initial treatment for induction, allo-HSCT, and AML ontogeny, but not NRAS or KRAS mutations or CG risks, remained statistically significant (supplemental Figure 13B). The prognostic values of AML ontogeny were even more significant if the analysis was restricted to patients uniformly treated with induction chemotherapy with a daunorubicin+cytarabine regimen (Figure 4B). De novo AML with MR/RUNX1 mutations had an outcome that fell between the favorable- and intermediate-risk groups when compared with the remainder of the patients stratified based on either ELN 2017 or ELN2022 risks (Figure 4C; supplemental Figures 11B and 14). Interestingly, de novo AML with WHO-MR mutations had an outcome overlapping with that of the ELN2022 intermediate-risk group; however, de novo AML with only RUNX1 mutations had a rather favorable outcome (supplemental Figure 14D).
AML ontogeny determines the outcome of AML with MR/RUNX1 gene mutations. (A) Kaplan-Meier curves of OS of AML with MR/RUNX1 gene mutations divided by ontogeny. (B) AML ontogeny–related risk was evaluated in multivariable Cox-regression models adjusting for age (modeled by cubic spline), allogenic transplant (modeled as time-dependent variable), NRAS/KRAS mutations, and CG risks in patients with AML with MR/RUNX1 mutations uniformly treated with 7+3 induction therapy. (C) Kaplan-Meier curves of patients with AML were divided into ELN2022 risk groups. De novo AML with MR/RUNX1 gene mutations separated from the ELN2022 adverse group show an outcome falling in between the favorable and intermediate-risk groups.
AML ontogeny determines the outcome of AML with MR/RUNX1 gene mutations. (A) Kaplan-Meier curves of OS of AML with MR/RUNX1 gene mutations divided by ontogeny. (B) AML ontogeny–related risk was evaluated in multivariable Cox-regression models adjusting for age (modeled by cubic spline), allogenic transplant (modeled as time-dependent variable), NRAS/KRAS mutations, and CG risks in patients with AML with MR/RUNX1 mutations uniformly treated with 7+3 induction therapy. (C) Kaplan-Meier curves of patients with AML were divided into ELN2022 risk groups. De novo AML with MR/RUNX1 gene mutations separated from the ELN2022 adverse group show an outcome falling in between the favorable and intermediate-risk groups.
Discussion
The most striking finding of our study was the inaccuracy of ontogeny assignment based on the initial database registry, which is likely not unique to this study and/or our cancer center. It may also have been encountered in many large cohorts of AML studies, as several landmark genomic studies on de novo AML included at least 15% or 20% cases harboring apparent MDS-defining cytogenetic abnormalities with or without TP53 mutations,13,17,31-35 consistent with either t-AML or AML-MRCWHO2016. This number is certainly an underestimate because not all t-AML or AML-MRCWHO2016 cases had MDS-defining cytogenetic abnormalities. The inaccurate assignment of ontogeny is due to (1) inadequate history documentation of antecedent MDS or MDS/MPN, or cytotoxic treatment for other types of malignancies/disorders; (2) delayed and/or overlooked cytogenetic information showing MDS-defining abnormalities; and (3) overcalling AML-MRCWHO2016 solely based on morphologic dysplasia. Our study emphasizes the importance of cohort annotation in rendering an accurate assignment of AML ontogeny.
Our study demonstrated the prognostic value of AML ontogeny, independent of genomics and/or ELN risk stratification. The designation of ontogeny as either MR-Hx/MR-CG or t-AML has clinical implications. Many studies have shown that secondary AML, mostly MR-Hx/MR-CG and t-AML, is associated with an inferior outcome with or without allo-HSCT.16,33,34,36-40 Our study confirmed these findings by showing that MR-Hx, MR-CG, and t-AML all confer worse outcomes than de novo AML, even after adjusting for age, treatment, transplant, and ELN risk. Importantly, several clinical trials have shown that CPX-351, a liposomal form of daunorubicin+cytarabine, improves the outcomes of AML-MRCWHO2016 and t-AML compared with conventional daunorubicin+cytarabine.41-43 Therefore, an accurate designation of AML ontogeny remains important for clinical decisions.
The best way to incorporate ontogeny and genomics into AML classification and risk stratification to guide clinical management remains to be determined, particularly for AML with MR gene mutations. Although many MR genes are moderately predictive of MR-Hx, our study found that they are also frequent in de novo AML, resulting in only ∼70% specificity to MR-Hx, significantly lower than the 95% previously reported.17 The discrepancy may be due to different cohorts but is more likely related to misclassification. The suboptimal specificity of MR gene mutations has also been observed in a few recent studies.28,30 This questions the approach of classifying these MR genes into 1 entity, largely aiming to replace MR-Hx or AML-MRCWHO2016.
Several studies have demonstrated inferior outcomes in AML with MR gene mutations, but its independent prognostic value in de novo AML has been controversial.21-23,44 Recent studies have shown that MR gene mutations in a favorable-risk group (mostly de novo) do not affect outcome.30,45 Our findings indicate that the outcome of AML with MR gene mutations in the ELN2017 intermediate/adverse-risk group is stratified by ontogeny and de novo patients had an OS not inferior to the intermediate-risk group. Therefore, it would be reasonable to restrict the diagnostic criteria of AML-MR to: (1) history of MDS or MDS/MPN (ie, MR-Hx) and/or (2) MDS-defining CGA (ie, MR-CG). Most of the patients with AML with MR gene mutations will meet 1 or both of these 2 criteria and therefore be classified as AML-MR. In order to better study the biology and management of de novo AML with MR gene mutations that do not meet either of these 2 criteria, a provisional entity could be established: (de novo) AML with MR gene mutations. Our data demonstrate that the assignment of mutations in all of these MR genes into adverse-risk groups is likely to be oversimplified and inaccurate and may lead to mismanagement in a subset of these patients. Our data also suggest that the ontogeny of MR-Hx and t-AML bears more prognostic weight than MR gene mutations and should be included in AML risk stratification for patients lacking favorable-risk factors. The findings that ontogeny carries prognostic value independent of genomics are intriguing and may be attributed to clonal architectural complexity, immune dysregulations, and the microenvironment, among other factors.
Our findings support the classification of AML with TP53 mutations as a distinct entity because of its dismal prognosis, regardless of ontogeny, which has recently been demonstrated in several studies.35,37,46,47 We and others have shown that nearly >90% of AML with TP53 mutations harbor CK or a MK involving chromosomes 5, 7, and/or 17.46 Notably, the similar mutational profile between MR-CG and t-AML was largely driven by TP53 mutations,47-49 further supporting the separation of AML with TP53 mutations from others. Unifying the classification of AML with TP53 mutations will facilitate the development of novel therapies (ie, hypomethylating agents, anti-CD47, and APR-246) for this adverse group of patients with urgent unmet needs.50-52
Because this was a retrospective, single-center study at a tertiary cancer center, there is a potential inherent bias toward more adverse-risk patients. However, we were able to review the detailed clinical history and pathologic findings of every patient in this cohort. To our knowledge, this is, to date, the most well-annotated cohort with a large number of patients with therapy-related or antecedent MDS history, which is essential for addressing the clinical impact of ontogeny. Although not all patients were uniformly treated, most patients received standard intensive induction chemotherapy with the daunorubicin + cytarabine regimen, and the conclusion remained valid when the analysis was restricted to this subset of patients. Moreover, preliminary data from several recent studies performed in different centers support our findings regarding AML with MR gene mutations,53-56 arguing against the inclusion of all MR gene mutations in the adverse-risk group. A recent study suggested that the number of MR mutations predicts the outcome.16 Unfortunately, our study was not powered to validate the results because of the incomplete coverage of all MR genes in a subset of patients. More studies are urgently needed to delineate the role of individual MR genes, their combinations, and allelic burden in AML classification and risk stratification.57,58
Acknowledgments
The authors thank Michael R. Waarts for his critical reading.
This study was funded by the Center for Hematologic Malignancies at Memorial Sloan Kettering Cancer Center and in part through National Institutes of Health/National Cancer Institute Cancer Center support grant P30 CA008748. A.J.S. is supported by the Young Investigator Award from the Edward P. Evans Foundation. A. Dunbar is a William Raveis Charitable Fund Physician-Scientist of the Damon Runyon Cancer Research Foundation (PST- 24-19). S.F.C. is supported by National Cancer Institute grant K08 CA241371-01A1. J.L.G. is supported by National Cancer Institute grant NCI K08CA230172 and Equinox Cycle for Survival. O.I.A.-W. is supported in part by the Edward P. Evans Foundation, National Institutes of Health/National Cancer Institute grants R01 CA251138 and R01 CA242020, National Institutes of Health/National Heart, Lung, and Blood Institute grant R01 HL128239, National Institutes of Health/National Cancer Institute grant P50 CA254838-01, and the Leukemia & Lymphoma Society. R.L.L. is supported by a Cycle For Survival Innovation grant and National Cancer Institute grant R35 CA197594. W.X. is supported by Alex's Lemonade Stand Foundation, the Runx1 Research Program, Cycle for Survival’s Equinox Innovation Award in Rare Cancers, Memorial Sloan Kettering Leukemia SPORE Career Enhancement Program (P50 CA254838-01), and National Cancer Institute grant K08CA267058-01.
Authorship
Contribution: J.G.W.M. performed computational analysis and curated the mutations; D.N. and A. Derkach performed statistical analysis; C.A.F., Z.S.S.-M., J.C., B.J.B., Z.D.E.-P., A.D.G., M.R., and W.X. performed the chart review; N.F., A.S.M., and M.E.A. assisted in mutation analysis; A.J.S., A. Dunbar, S.F.C., J.L.G., M.B.G., R.K.R., E.B., O.I.A.-W., E.M.S., A.D.G., M.S.T., and R.L.L. provided patient care; E.P. supervised the computational analysis; Y.Z. reviewed the cytogenetic data; M.R. and W.X. reviewed the pathology; W.X. initiated, designed, and supervised the study, and drafted the manuscript; and all authors finalized and approved the manuscript.
Conflict-of-interest disclosure: M.E.A. served as a consultant for Janssen Global Services, Bristol Myers Squibb (BMS), AstraZeneca, and Roche, and has received honoraria from Biocartis, Invivoscribe, physician educational resources, PeerView Institute for Medical Education, clinical care options, and RMEI Medical Education. A.J.S. reports his spouse is an employee of BMS. B.J.B. served on the advisory board for Oncovalent and BMS. S.F.C. is a consultant for and holds an equity interest in Imago BioSciences, none of which is directly related to the content of this paper. J.L.G. received consulting fees from GLG. M.B.G. receives research support from Actinium, Amgen, and Sanofi, and has served in an advisory role for Sanofi, Novartis, and Allogene. R.K.R. has received consulting fees from Incyte Corporation, Celgene/BMS, Blueprint, AbbVie, CTI, Stemline, Galecto, Pharmaessentia, Constellation/MorphoSys, Sierra Oncology/GlaxoSmithKline, Sumitomo Dainippon, Kartos, Servier, Zentalis, Karyopharm, and research funding from Constellation Pharmaceuticals, Ryvu, Zentalis, and Stemline Therapeutics. O.I.A.-W. served as a consultant for H3 Biomedicine, Foundation Medicine Inc, Merck, Prelude Therapeutics, and Janssen; is on the scientific advisory board of Envisagenics Inc, AIChemy, Harmonic Discovery Inc, and Pfizer Boulder; and has received prior research funding from H3 Biomedicine, Nurix Therapeutics, Minovia Therapeutics, and Loxo Oncology, unrelated to the current manuscript. A.D.G. received research funding from Celularity, ADC Therapeutics, Aprea, AROG, Pfizer, Prelude, and Trillium; received research funding from and served as a consultant for Aptose and Daiichi Sankyo; served as a consultant and member of the advisory committees for Astellas, Celgene, and Genentech; received research funding from, served as a consultant for, and was a member of the advisory committees for AbbVie; and received honoraria from Dava Oncology. M.S.T. received research funding from AbbVie, Orsenix, BioSight, Glycomimetics, Rafael Pharmaceuticals, and Amgen; is on the advisory boards of AbbVie, Daiichi Sankyo, Orsenix, KAHR, Jazz Pharmaceuticals, Roche, BioSight, Novartis, Innate Pharmaceuticals, Kura, Syros Pharmaceuticals, and Ipsen Biopharmaceuticals; received royalties from UpToDate; and is on the DSMB of HOVON protocol Ho156 and adjudication committee of Foghorn protocol FHD-286. R.L.L. is on the supervisory board of Qiagen and is a scientific adviser to Imago, Mission Bio, Syndax, Zentalis, Ajax, Bakx, Auron, Prelude, C4 Therapeutics, and Isoplexis for which he receives equity support; receives research support from Ajax and AbbVie; has consulted for Incyte, Janssen, MorphoSys, and Novartis; and received honoraria from AstraZeneca and Kura for invited lectures and from Gilead for grant reviews. E.P. is a founder and equity holder, and holds a fiduciary role in Isabl Inc. M.R. is on the scientific advisory board in Auron Pharmaceutical for which he received equity support; receives research funding from Celularity, Roche-Genentech, Beat AML, and NGM; and receives travel funds from BD Biosciences. W.X. received research support from Stemline Therapeutics. The remaining authors declare no competing financial interests.
The current affiliation for Z.S.S.-M. is Jacobs School of Medicine and Biomedical Sciences at the University at Buffalo, Buffalo, NY.
The current affiliation for J.C. is Weill Cornell Medicine, New York City, NY.
The current affiliation for B.J.B. is City of Hope National Medical Center, Duarte, CA.
The current affiliation for M.S.T. is Northwestern University, Chicago, IL.
Correspondence: Wenbin Xiao, Department of Pathology and Laboratory Medicine, Memorial Sloan Kettering Cancer Center, Pathology, 1275 York Ave, Pathology-Bobst, New York, NY 10065; e-mail: xiaow@mskcc.org.
References
Author notes
Data are available on request from the corresponding author, Wenbin Xiao (xiaow@mskcc.org).
The full-text version of this article contains a data supplement.