TO THE EDITOR:
Chimeric antigen receptor (CAR)–modified T cells have been reported to successfully treat patients with hematological malignancies.1,2 Several obstacles have limited the availability of autologous CAR T cells; for example, intensively treated patients have an insufficient number of T cells or poor-quality T cells, preventing the manufacture of effective therapeutic products, and eligible patients must wait for the cell products to be generated on schedule.3-5 These challenges may restrict the application of autologous CAR T cells in the clinic.
Recent studies have indicated the feasibility of using allogeneic universal CAR T cells to treat infant, pediatric, and adult patients with leukemia.6,7 However, although allogeneic universal CAR T cells that disrupt the T-cell receptor (TCR) α chain to reduce the expression of TCR could reduce the occurrence of graft-versus-host disease (GVHD), these allogeneic cells can be rapidly rejected by the host’s immune system because of their expression of human leukocyte antigen (HLA). Several studies have indicated that allograft survival can be sustained by disrupting β-2 microglobulin (B2M) to decrease the expression of HLA class I molecules.8-10 Reduced alloreactivity of TCR and B2M double-disrupted CAR T cells in vitro and in animal models has been observed.8,11 Therefore, universal CAR T cells with double disruption of TCR and B2M may be a candidate for the treatment of patients with cancer.
We generated TCR and B2M double-disrupted universal CAR T cells from healthy donor T cells using lentivirus and CRISPR/Cas9 genome-editing technology, and their in vitro characteristics and specific antitumor efficacy were confirmed as shown in supplemental Figure 1. In addition, we report 2 cases of relapsed/refractory diffuse large B-cell lymphoma (DLBCL) in which patients received universal CAR T-cell therapy. Additional details regarding the study procedures are provided in the data supplement.
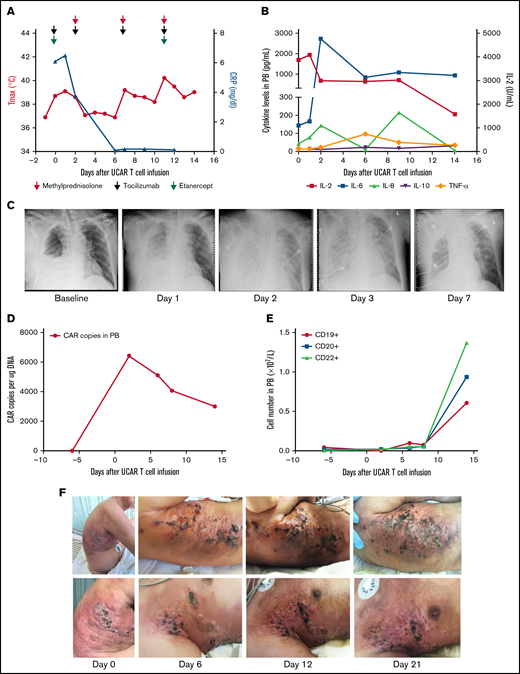
The first patient was a 63-year-old man with rapidly progressive and primary refractory DLBCL after receiving radiation therapy and multiple cycles of chemotherapy within 8.5 months after diagnosis, summarized in supplemental Figure 2. The patient had a bulky tumor burden located on the right trunk skin and in the subcutaneous soft tissue, right pleural space, peritoneum, bilateral cervical lymph nodes, supraclavicular lymph nodes, and bilateral axillary lymph nodes. He received preconditioning treatment with a total dose of 30 mg/m2 of fludarabine and a total dose of 15 mg/kg of cyclophosphamide, followed by a total dose of 1.5 × 106/kg universal CAR T cells in 2 separate doses, without experiencing any immediate infusion-related toxicity (supplemental Figure 2). Within 21 hours after cell infusion, the patient experienced fever, respiratory distress, blood pressure reduction, acute pulmonary edema, and pleural fluid on chest X-ray, accompanied by a significant increase in cytokines, indicating that he had developed grade 4 cytokine release syndrome (CRS; Figure 1A-C). Although multiple cycles of tocilizumab, etanercept, and methylprednisolone were subsequently administered, the patient’s fever and CRS were not effectively ameliorated (Figure 1A-B). Occurrences of thrombocytopenia, anemia, and leukocytopenia were observed after universal CAR T-cell infusion, possibly associated with preconditioning regimens, and these toxicities were reversed after intensive medical intervention (supplemental Figure 3A-D).12 In addition, no GVHD or other toxicities were observed after cell infusion (supplemental Figure 3E-F). CAR gene copy numbers were detectable after universal CAR T-cell infusion and then gradually decreased (Figure 1D). The number of B cells was maintained at a low level within ∼7 days after universal CAR T-cell infusion, and then, the number of B cells increased (Figure 1E). Skin damage was aggravated at the tumor lesion location in the right trunk after cell infusion (Figure 1F; supplemental Figure 3G). In addition, swelling, high temperature, and exudate were observed at the lesion location. The swelling improved after administration of methylprednisolone. Unfortunately, the patient declined all treatment 21 days after cell infusion.
Toxicities, persistence, and response in patient 1 after administration of universal CAR T cells. (A) The change in maximum temperature (Tmax) and the serum level of C-reactive protein (CRP) were tested in patient 1 before and after universal CAR (UCAR) T-cell infusion and CRP recovery after multicycle of methylprednisolone (red arrow), tocilizumab (light green arrow), and etanercept (purple arrow) administration. (B) The serum levels of cytokines, including interleukin-2 (IL-2), IL-6, IL-8, IL-10, and tumor necrosis factor-α (TNF-α), were tested after UCAR T-cell infusion. (C) Chest X-ray showed the appearance of acute pulmonary edema after UCAR T-cell infusion. (D) Persistence of infused UCAR T cells in patient peripheral blood (PB) before and after cell infusion. The level of UCAR T cells was analyzed using quantitative polymerase chain reaction to detect the CAR gene copy number in genomic DNA obtained from PB of patient 1. (E) The change in B-cell number before and after UCAR T-cell infusion. (F) Skin damage in the right trunk developed after UCAR T-cell infusion. The swelling improved after methylprednisolone treatment.
Toxicities, persistence, and response in patient 1 after administration of universal CAR T cells. (A) The change in maximum temperature (Tmax) and the serum level of C-reactive protein (CRP) were tested in patient 1 before and after universal CAR (UCAR) T-cell infusion and CRP recovery after multicycle of methylprednisolone (red arrow), tocilizumab (light green arrow), and etanercept (purple arrow) administration. (B) The serum levels of cytokines, including interleukin-2 (IL-2), IL-6, IL-8, IL-10, and tumor necrosis factor-α (TNF-α), were tested after UCAR T-cell infusion. (C) Chest X-ray showed the appearance of acute pulmonary edema after UCAR T-cell infusion. (D) Persistence of infused UCAR T cells in patient peripheral blood (PB) before and after cell infusion. The level of UCAR T cells was analyzed using quantitative polymerase chain reaction to detect the CAR gene copy number in genomic DNA obtained from PB of patient 1. (E) The change in B-cell number before and after UCAR T-cell infusion. (F) Skin damage in the right trunk developed after UCAR T-cell infusion. The swelling improved after methylprednisolone treatment.
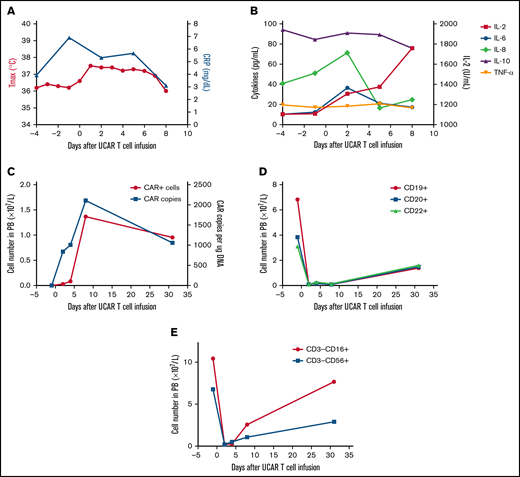
The second patient was a 64-year-old man treated using a similar protocol in January 2018 (supplemental Figure 4). He was diagnosed with relapsed/refractory DLBCL after administration of multiple types and cycles of cancer treatment, summarized in supplemental Figure 4. The patient had a tumor burden located in the bilaterally cervical, right submandibular, right clavicular, mediastinal, and bilateral hilar lymph node regions. Lymphodepletion was performed with a total dose of 20 mg/kg of cyclophosphamide and a total dose of 52.6 mg/m2 of fludarabine, followed by a total dose of 1.21 × 106/kg universal CAR T cells (supplemental Figure 4). No immediate infusion-related toxicity was observed. After universal CAR T-cell infusion, the patient experienced fever accompanied by a significant increase in C-reactive protein and cytokines (Figure 2A-B). Hyperpyrexia and the levels of serum C-reactive protein and several cytokines, except interleukin-2, recovered without any treatment >1 week after cell infusion. In addition, no GVHD or other toxicities developed after cell infusion (supplemental Figure 5). Laboratory investigations confirmed the presence of universal CAR T cells by quantitative polymerase chain reaction and flow cytometry in peripheral blood (Figure 2C). Rapid increases in universal CAR T cells were observed after cell infusion, and universal CAR T cells then decreased after 1 month. In addition, the number of B cells in peripheral blood decreased after cell infusion and recovered after 1 month (Figure 2D). By 1 month after cell infusion, he had disease progression.
Toxicities, persistence, and response in patient 2 after administration of universal CAR T cells. (A) The change in maximum temperature (Tmax) and the serum level of C-reactive protein (CRP) were monitored in patient 2 before and after universal CAR (UCAR) T-cell infusion, and Tmax and CRP recovered without any treatment. (B) The serum levels of cytokines, including interleukin-2 (IL-2), IL-6, IL-8, IL-10, and tumor necrosis factor-α (TNF-α), were tested before and after UCAR T-cell infusion. (C) Persistence of the infused UCAR T cells in peripheral blood (PB) of patient 2 before and after cell infusion. Flow cytometry and quantitative polymerase chain reaction were used to detect the level of UCAR T cells in PB. (D) The change in B-cell number before and after UCAR T-cell infusion. (E) The change in NK cell number before and after UCAR T-cell infusion.
Toxicities, persistence, and response in patient 2 after administration of universal CAR T cells. (A) The change in maximum temperature (Tmax) and the serum level of C-reactive protein (CRP) were monitored in patient 2 before and after universal CAR (UCAR) T-cell infusion, and Tmax and CRP recovered without any treatment. (B) The serum levels of cytokines, including interleukin-2 (IL-2), IL-6, IL-8, IL-10, and tumor necrosis factor-α (TNF-α), were tested before and after UCAR T-cell infusion. (C) Persistence of the infused UCAR T cells in peripheral blood (PB) of patient 2 before and after cell infusion. Flow cytometry and quantitative polymerase chain reaction were used to detect the level of UCAR T cells in PB. (D) The change in B-cell number before and after UCAR T-cell infusion. (E) The change in NK cell number before and after UCAR T-cell infusion.
More than 5 × 104 mismatched T cells per kilogram is often considered a threshold effect for GVHD in HLA-haploidentical stem cell transplantation after αβ T and B cells are removed,13 and in our practice, 2.28 × 104/kg and 5.46 × 104/kg residual TCR-αβ+ cells were infused in patients 1 and 2, respectively. Despite the number of infused TCR-αβ+ cells in patient 2 being >5 × 104/kg, GVHD was not observed in either of the 2 patients after universal CAR T-cell infusion.
Several trials have indicated that CRS directly correlates with tumor burden at the time of CAR T-cell infusion.14,15 In this study, both patients developed CRS after universal CAR T-cell infusion. Patient 1, who had a bulky tumor burden, developed grade 4 CRS that did not improve despite intensive medical intervention. Patient 2, who had a lower tumor burden than patient 1, developed grade 1 CRS that recovered without any treatment >1 week after cell infusion. Therefore, administering salvage chemotherapy in the context of a bulky tumor burden could reduce the risk of CRS.
Recent studies have indicated that clinical outcome is closely associated with persistence of CAR T cells in peripheral blood circulation.16,17 Our current data showed a rapid increase in universal CAR T cells after cell infusion and then a decrease within 1 month after universal CAR T-cell infusion. With the universal CAR T cells in our study, HLA class I− cells may be lysed by host natural killer (NK) cells.8,18,19 In patient 2, the number of NK cells was increased after universal CAR T-cell infusion (Figure 2E); in contrast, the universal CAR T cells were decreased. Lymphodepletion via chemotherapy or NK cell–specific antibodies was shown to effectively deplete most host NK cells to reduce the risk of NK cell–mediated lysis in HLA class I− cells.20,21 However, lymphodepletion regimens may be toxic and may affect the antitumor activity of universal CAR T cells. Constitutive expression of nonclassic HLA class I molecules, such as HLA-E and HLA-G, on HLA class I− cells could protect against allogeneic NK cell–mediated lysis.22-24 In addition, in our preclinical study, we constitutively expressed the mutant B2M-HLA-E and B2M-HLA-G fusion proteins in anti-CD19 universal CAR T cells to reduce allogeneic NK cell–mediated lysis, indicating that this approach could improve the persistence of universal CAR T cells in patients.25
In conclusion, our study shows that immunotherapy with universal CAR T cells negative for TCR and HLA class I molecules is a potential treatment for patients with DLBCL. This study had a number of limitations, such as safety and clinical response; however, our pooled analysis provides a substantial step forward in the development of universal CAR T cells to improve safety, efficacy, and feasibility for patients with hematological malignancies.
Acknowledgments: The authors thank the patients and families for allowing us to share their stories.
This work was supported by National Natural Science Foundation of China grants 81830002 and 31991171 (W.H.) and 81903151 (Y.G.), and Translational Research Grant of NCRCH (National Clinical Research Center for Hematologic Diseases) grant 2021WWC04 (W.H.).
Authorship: Contribution: W.H. conceived and designed the experiments; Y.G., C.T., Z.W., and Y.W. performed the experiments; W.Z., H.J., Y.L., and Q.Y. provided patient management (patient enrollment, treatment, and data collection); Y.G. and L.S. analyzed the data; and Y.G. wrote the manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Weidong Han, No. 28 Fuxing Rd, Beijing, China; e-mail: hanwdrsw69@yahoo.com; Yao Wang, No. 28 Fuxing Rd, Beijing, China; e-mail: wangyao_301@hotmail.com; and Zhiqiang Wu, No. 28 Fuxing Rd, Beijing, China; e-mail: wuzhiqiang1006@163.com.
References
Author notes
Y.G., C.T., and L.S. contributed equally to this study.
Requests for data sharing may be submitted to Weidong Han hanwdrsw69@yahoo.com.
The full-text version of this article contains a data supplement.