Key Points
In TRANSFORM, health-related quality of life improved or was maintained with second-line lisocabtagene maraleucel vs standard of care.
HRQOL results coupled with favorable efficacy support liso-cel as a potential second-line option for patients with relapsed/refractory LBCL.
Abstract
Lisocabtagene maraleucel (liso-cel) has shown promising efficacy in clinical trials for patients with relapsed/refractory large B-cell lymphoma (LBCL). We present health-related quality of life (HRQOL) results from the TRANSFORM study, the first comparative analysis of liso-cel vs standard of care (SOC) as second-line therapy in this population. Adults with LBCL refractory or relapsed ≤12 months after first-line therapy and eligible for autologous stem cell transplantation were randomized 1:1 to the liso-cel or SOC arms (3 cycles of immunochemotherapy in which responders proceeded to high-dose chemotherapy and autologous stem cell transplantation). HRQOL was assessed by European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire – 30 items and the Functional Assessment of Cancer Therapy-Lymphoma subscale. Patients with baseline and ≥1 postbaseline assessment were analyzed (liso-cel, n = 47; SOC, n = 43). The proportion of patients with meaningful improvement in global health status/quality of life (QOL) was higher, whereas deterioration was lower in the liso-cel arm vs SOC arm from day 126 to month 6. Mean change scores showed meaningful worsening in global health status/QOL at month 6, fatigue at day 29 and month 6, and pain at month 6 with SOC; mean scores for other domains were maintained or improved in both arms. Time to confirmed deterioration favored the liso-cel arm vs SOC arm in global health status/QOL (median: not reached vs 19.0 weeks, respectively; hazard ratio, 0.47; 95% confidence interval, 0.24-0.94). HRQOL was either improved or maintained from baseline in patients with relapsed/refractory LBCL in the liso-cel arm vs SOC arm as second-line treatment. This study is registered at clinicaltrials.gov as #NCT0357531.
Introduction
Large B-cell lymphomas (LBCL) are potentially curable with first-line combination immunochemotherapy in 50% to 60% of patients.1-3 For patients whose disease is primary refractory to or relapses after first-line therapy and are sufficiently young and fit for high-dose chemotherapy (HDCT), the standard of care (SOC) is a multiagent, platinum-based salvage chemotherapy regimen, including rituximab followed by consolidation with HDCT and autologous stem cell transplantation (ASCT) for chemosensitive disease.4-7 However, because of disease progression or unsatisfactory response to salvage immunochemotherapy, only about 30% of patients eventually receive ASCT.4-7 Among those who complete ASCT, long-term prognosis is also poor, especially for those with high-risk disease (ie, refractory or relapsing within 12 months after first-line therapy),7 indicating a great unmet need in this population.
Chimeric antigen receptor (CAR) T-cell therapy is a new option in the third-line or later setting for patients with relapsed or refractory (R/R) LBCL.8-11 Lisocabtagene maraleucel (liso-cel) is an autologous, CD19-directed, 4-1BB costimulated CAR T-cell product administered at equal target doses of CD8+ and CD4+ CAR+ T cells that has shown promising efficacy results in clinical trials.9 In a prespecified interim analysis of the phase 3 TRANSFORM study, second-line liso-cel showed superiority over SOC (3 cycles of salvage platinum-based immunochemotherapy followed by HDCT and ASCT in responding patients) in the primary end point of event-free survival (EFS); median EFS was 10.1 vs 2.3 months (hazard ratio [HR], 0.35; 95% confidence interval [CI], 0.23-0.53; P < .0001). The liso-cel arm also demonstrated statistically significant improvement in key secondary end points of complete response (CR) rate (66.3% vs 39.1%; P < .0001) and progression-free survival (PFS) (median 14.8 vs 5.7 months; P = .0001); the safety profile was consistent with that observed in third-line or later treatment for LBCL.12
Patients with R/R, aggressive LBCL have reported compromised health-related quality of life (HRQOL) after receiving treatment.13-16 Results of the TRANSCEND NHL 001 study showed short- and long-term improvements in HRQOL after liso-cel infusion in patients with R/R LBCL who had received ≥2 prior lines of treatment.17 Here, we present the first head-to-head comparison of HRQOL between liso-cel and SOC in second-line R/R LBCL from the TRANSFORM study.
Methods
Study design
TRANSFORM (NCT03575351) is a phase 3, randomized, open-label, pivotal study comparing liso-cel treatment with SOC in adult patients with LBCL whose disease was primary refractory to or relapsed within 12 months of first-line immunochemotherapy. The study design has been described previously (Figure 1).18 Briefly, patients were randomized 1:1 to treatment with liso-cel or SOC. Patients in the liso-cel arm underwent lymphodepletion with fludarabine/cyclophosphamide, followed by liso-cel infusion at a target dose of 100 × 106 CAR+ T cells; patients could have also received bridging therapy with 1 cycle of a protocol-defined SOC regimen for disease control during liso-cel manufacturing. Patients in the SOC arm received up to 3 cycles of salvage therapy (rituximab, dexamethasone, cytarabine, and cisplatin; rituximab, ifosfamide, carboplatin, and etoposide; or rituximab, gemcitabine, dexamethasone, and cisplatin) as per physician’s choice. Responding patients (CR or partial response [PR]) were to proceed to HDCT (carmustine, etoposide, cytarabine, and melphalan) and ASCT. Patients on the SOC arm were allowed to cross over and receive liso-cel as third-line treatment if standard second-line treatment failed as assessed by an IRC. Eastern Cooperative Oncology Group performance status (ECOG PS) was documented over time.
Study design andschedule ofassessments. ∗Patients may have received a protocol-defined SOC regimen to stabilize their disease during liso-cel manufacturing. †Only for patients who received bridging therapy. ‡Randomization stratification factors included response to first therapy (stable disease, progressive disease, PR, or CR with relapse before 3 months vs CR with relapse on or after 3 months) and sAAIPI (0/1 vs 2/3). §SOC was defined as physician’s choice of R-DHAP, R-ICE, or R-GDP. IRC, independent review committee; LDC, lymphodepleting chemotherapy; LOT, line of therapy; ORR, objective response rate; OS, overall survival; PET, positron emission tomography; PFS, progression-free survival; PRO, patient-reported outcome; R-DHAP, rituximab, dexamethasone, cytarabine, and cisplatin; R-GDP, rituximab, gemcitabine, dexamethasone, and cisplatin; R-ICE, rituximab, ifosfamide, carboplatin, and etoposide; sAAIPI, secondary age-adjusted International Prognostic Index.
Study design andschedule ofassessments. ∗Patients may have received a protocol-defined SOC regimen to stabilize their disease during liso-cel manufacturing. †Only for patients who received bridging therapy. ‡Randomization stratification factors included response to first therapy (stable disease, progressive disease, PR, or CR with relapse before 3 months vs CR with relapse on or after 3 months) and sAAIPI (0/1 vs 2/3). §SOC was defined as physician’s choice of R-DHAP, R-ICE, or R-GDP. IRC, independent review committee; LDC, lymphodepleting chemotherapy; LOT, line of therapy; ORR, objective response rate; OS, overall survival; PET, positron emission tomography; PFS, progression-free survival; PRO, patient-reported outcome; R-DHAP, rituximab, dexamethasone, cytarabine, and cisplatin; R-GDP, rituximab, gemcitabine, dexamethasone, and cisplatin; R-ICE, rituximab, ifosfamide, carboplatin, and etoposide; sAAIPI, secondary age-adjusted International Prognostic Index.
Patients
Eligible patients were adults ≤75 years of age with an ECOG PS ≤1, histologically confirmed LBCL, defined as diffuse LBCL not otherwise specified or transformed from indolent non-Hodgkin lymphoma, high-grade B-cell lymphoma with rearrangements of MYC and either BCL2, BCL6, or both with diffuse LBCL histology, primary mediastinal LBCL, T-cell/histiocyte-rich LBCL, or follicular lymphoma grade 3B. Patients had either refractory disease (stable disease, progressive disease, PR, or CR with relapse before 3 months) or relapsed disease (defined as CR with relapse on or after lasting ≥3 months but ≤12 months) after first-line treatment containing an anthracycline and a CD20-targeted agent, had adequate organ function, and were eligible for HDCT and ASCT.
HRQOL assessments
HRQOL, one of the secondary study objectives, was assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–30 items (EORTC QLQ-C30) and the Functional Assessment of Cancer Therapy-Lymphoma subscale (FACT-LymS). Questionnaires were completed by patients before any clinical assessments were performed and treatments were administered at any given visit. Assessments were made at baseline (ie, randomization; +3 days), during the treatment period at days 29 (before liso-cel infusion or during SOC cycle 2; ±7 days), 64 (posttreatment for the liso-cel arm/after immunochemotherapy for the SOC arm; ±6 days), and 126 (after stem cell transplantation for the SOC arm; ±7 days), and during the posttreatment period at month 6 (±10 days) and months 9, 12, 18, 24, and 36 (±14 days; Figure 1). No HRQOL data were collected after patients started subsequent antineoplastic treatment.
The EORTC QLQ-C30 comprises 30 items, grouped into the following 15 domains: 5 multi-item functional scales (physical, role, emotional, cognitive, and social), 3 multi-item symptom scales (fatigue, nausea/vomiting, and pain), 6 single-item symptom or financial difficulty scales (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulty), and a global health status/quality of life (QOL) scale.19 The primary domains of interest for this analysis were global health status/QOL, physical functioning, cognitive functioning, fatigue, and pain; all others were considered secondary. Raw scores for all domains are transformed to a 0 to 100 scale; a higher score for a functional domain or global health status/QOL represents a higher or healthier level of functioning or QOL, whereas a higher score for a symptom domain represents a higher level of symptomatology.
Within-group minimally important differences (MID) were used to determine whether a mean change (improvement or deterioration) from baseline in EORTC QLQ-C30 score at a given visit for a treatment group was clinically meaningful.20 The MIDs for between-group differences were based on the lower bound of the MID thresholds reported by Cocks et al,21 as they are considered to be the most conservative set of thresholds among those published when assessing noninferiority. To assess meaningful within-patient change, a responder definition (RD) based on minimal change thresholds (ie, smallest incremental change) as recommended by Cocks and Buchanan was used as follows: 5 points for global health status/QOL, physical, and emotional functioning; 10 points for fatigue; 15 points for role, cognitive, social functioning, nausea/vomiting, and pain; and 30 points for all other domains.22
The FACT-LymS consists of 15 items addressing symptoms and functional limitations that are important to patients with lymphoma.23 Items are scored on a 0 to 4 Likert scale and aggregated to a single score on a 0 to 60 scale, with a higher total score corresponding with lower levels of symptomology. A cutoff value of 3 was defined as the within-group and between-group MID and RD.24
Statistical analyses
Patients in the HRQOL-evaluable population (all randomly assigned patients who completed a baseline and ≥1 postbaseline HRQOL assessment) were included in these analyses. As the power for the study was calculated based on the primary efficacy end point, all HRQOL analyses were descriptive. A linear mixed-effects regression model for repeated measures (MMRM) longitudinal analysis was performed on the HRQOL-evaluable population to estimate treatment effects on change scores for each domain of the EORTC QLQ-C30 and FACT-LymS over time. The difference in change scores between treatment arms was also assessed. Unstructured covariance matrix was used. MMRM results were based on visits up to day 126, where ≥10 patients in each arm completed the questionnaire and it was possible to obtain stable estimates. The dependent variable for the models was the score change from baseline. The models included the intercept and time as random effects and the following covariates as fixed effects: treatment arm, time (as a discrete variable), stratification factors, baseline HRQOL domain score, and treatment arm∗time interaction.
Time to confirmed HRQOL deterioration was calculated using the Kaplan-Meier method, defined as the time from randomization and the date of the first of ≥2 consecutive visits where deterioration greater than the RD threshold was experienced. Patients who did not experience confirmed HRQOL deterioration were censored at the time of the last nonmissing HRQOL assessment; patients who died were censored at the time of the last nonmissing HRQOL assessment. Patients were censored at baseline if they had baseline HRQOL scores low enough as to be impossible to achieve a change score exceeding or equal to the RD threshold for deterioration. HRs (95% CIs) were estimated using a stratified Cox proportional hazards regression analysis by considering treatment group and baseline score as covariates, and the best overall response to first-line therapy (refractory vs relapsed) and sAAIPI (0 or 1 vs 2 or 3) as stratification factors.
An additional MMRM analysis using a “while on treatment” strategy was also used to estimate HRQOL scores for patients experiencing intercurrent events (ie, death or receipt of subsequent antineoplastic treatment). Subsequent scheduled patient-reported outcome assessments after the intercurrent event were imputed with the last nonmissing HRQOL domain scores assessed before the event and analyzed by MMRM.
Trial oversight
All authors had access to the primary clinical trial data. The trial was approved by the institutional review board or independent ethics committee at each study site and conducted in accordance with applicable regulatory requirements, the principles of the Declaration of Helsinki, and Good Clinical Practice guidelines of the International Conference on Harmonization. All patients provided written informed consent.
Results
HRQOL completion rates and patient disposition
At the data cutoff (8 March 2021), 184 patients were included in the intent-to-treat population, with 92 patients in each treatment arm (supplemental Table 1). In the liso-cel arm, 45 patients were not included in the EORTC QLQ-C30 analysis set (28 had no baseline assessment, 1 had no postbaseline assessment, and 16 had no baseline or any postbaseline assessment) and 47 were not included in the FACT-LymS analysis set (30 had no baseline assessment, 1 had no postbaseline assessment, and 16 had no baseline or any postbaseline assessment). In the SOC arm, 49 were not included in the EORTC QLQ-C30 analysis set (20 had no baseline assessment, 6 had no postbaseline assessment, and 23 had no baseline or any postbaseline assessment) and 52 were not included in the FACT-LymS analysis set (21 had no baseline assessment, 6 had no postbaseline assessment, and 25 had no baseline or any postbaseline assessment). A total of 47 (51%) patients in the liso-cel arm and 43 (47%) patients in the SOC arm were eligible for inclusion in the EORTC QLQ-C30 analysis set; 45 (49%) patients in the liso-cel arm and 40 (43%) patients in the SOC arm were included in the FACT-LymS analysis set (supplemental Figure 1). Among patients in the intent-to-treat population who were expected to provide HRQOL assessments at each visit, EORTC QLQ-C30 completion rates were 52% (48/92) at baseline and 39% (22/57) at month 6 among those in the liso-cel arm, and 54% (49/91) at baseline and 43% (10/23) at month 6 among patients in the SOC arm. Missing data at baseline were primarily owing to operational and logistical challenges of implementing an electronic HRQOL data collection method amid the COVID-19 pandemic. It should be noted that 1 patient in the liso-cel arm had an ECOG PS of 1 at screening and was included in this analysis, but their status worsened to an ECOG PS of 2 at the baseline assessment on the day of randomization. After baseline, missing data were due to the start of subsequent antineoplastic treatment (including patients who crossed over from the SOC arm to receive liso-cel), death, limited follow-up at the time of the data cutoff, and other reasons (supplemental Figure 2). Patient deaths over time are reported in supplemental Table 2. Clinical responses over time are shown in supplemental Table 3.
Patient characteristics and HRQOL scores at baseline
The characteristics of patients in the EORTC QLQ-C30 analysis set were generally similar to those of the intent-to-treat population (supplemental Table 1). The median age of patients in the overall EORTC QLQ-C30 analysis set was 58 years (interquartile range, 43-66 years), and 56% of patients were male; baseline characteristics were generally balanced between the 2 treatment arms (Table 1). Most patients had refractory disease to first-line therapy (76%), and over half had a sAAIPI score of 0 or 1 (59%). Overall, most patients (≥60% in each arm) maintained an ECOG PS of 0 or 1 over time. The mean (standard deviation [SD]) EORTC QLQ-C30 score for both arms at baseline was 68.0 (21.7) for global health status/QOL, 85.5 (16.2) for physical functioning, 87.8 (17.3) for cognitive functioning, 30.9 (22.9) for fatigue, and 28.2 (28.6) for pain; the mean (SD) FACT-LymS score was 47.5 (8.1; Table 2). Patients generally had mean baseline scores comparable to a general population with similar age and gender distributions25 across most HRQOL domains, except for role functioning and social functioning of the EORTC QLQ-C30, for which mean scores were much worse than in the general population.
Changes from baseline
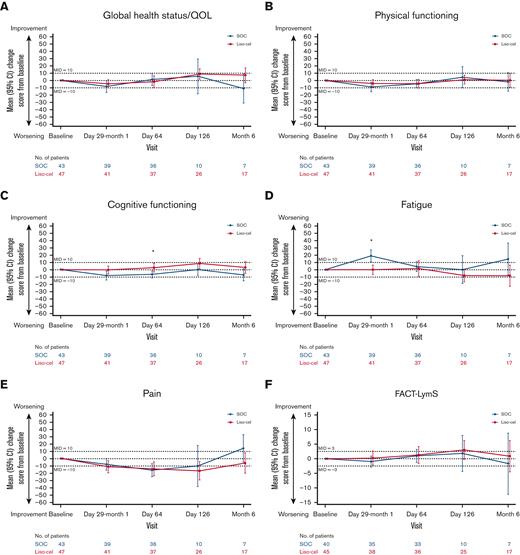
In the SOC arm, observed mean change scores showed clinically meaningful worsening (ie, mean changes exceeded the prespecified within-group MIDs) in global health status/QOL at month 6, fatigue at day 29 and month 6, and pain at month 6; mean scores for the other primary domains of interest were generally maintained or meaningfully improved over time in both treatment arms (Figure 2). Results from the MMRM analyses, which considered all data points through day 126 and controlled for relevant baseline covariates, showed that the overall least squares (LS) mean changes from baseline to day 126 with the liso-cel arm were clinically meaningfully improved (ie, exceeding the prespecified between-group MIDs) compared with those with the SOC arm in cognitive functioning (2.2 vs −2.1) and fatigue (−2.0 vs 3.8) domains (Table 3; supplemental Figure 3). Differences in the remaining primary and secondary domains of interest between arms were small and fell within the between-group MIDs, except for the emotional functioning domain, where it favored the SOC arm.
Observed mean changes from baseline in the primary domains of interest. (A-F) Observed mean changes from baseline in EORTC QLQ-C30 global health status/QOL (A), physical functioning (B), cognitive functioning (C), fatigue (D), pain (E), and FACT-LymS (F). Numbers of evaluable patients at each visit were based on data available for the variable at the given visit. ∗P < .05.
Observed mean changes from baseline in the primary domains of interest. (A-F) Observed mean changes from baseline in EORTC QLQ-C30 global health status/QOL (A), physical functioning (B), cognitive functioning (C), fatigue (D), pain (E), and FACT-LymS (F). Numbers of evaluable patients at each visit were based on data available for the variable at the given visit. ∗P < .05.
Proportion of patients with clinically meaningful changes
The proportion of patients with meaningful improvement in global health status/QOL, cognitive functioning, and fatigue was higher, whereas a lower proportion deteriorated in the liso-cel arm than in the SOC arm from day 126 to month 6 (Figure 3). Results for pain scores trended toward improvement in the liso-cel arm and deterioration in the SOC arm at month 6. For other primary domains of interest (physical functioning and FACT-LymS), the proportions of patients with meaningful improvement or deterioration were generally similar between the liso-cel arm and the SOC arm across visits through month 6.
Proportions of clinically meaningful changes from baseline in the primary domains of interest. (A-F) Proportion of clinically meaningful changes in EORTC QLQ-C30 GH/QOL (A), physical functioning (B), cognitive functioning (C), fatigue (D), pain (E), and FACT-LymS (F). Clinically meaningful change (improvement or deterioration) from baseline in EORTC QLQ-C30 score was determined using a RD based on minimal change thresholds (ie, smallest incremental change) as recommended by Cocks and Buchanan22 as follows: 5 points for GH/QOL, physical, and emotional functioning; 10 points for fatigue; 15 points for role, cognitive, social functioning, nausea/vomiting, and pain; and 30 points for all other domains. For the FACT-LymS, a cutoff value of 3 was defined as the within-group and between-group MID and RD.24 Numbers of evaluable patients at each visit were based on data available for the variable at the given visit. ∗P < .05. Asterisks above bars indicate significant differences in proportions of patients exhibiting clinically meaningful improvement vs no clinically meaningful improvement. Asterisks below bars indicate significant differences in proportions of patients exhibiting clinically meaningful deterioration vs no clinically meaningful deterioration. GH/QOL, global health status/QOL.
Proportions of clinically meaningful changes from baseline in the primary domains of interest. (A-F) Proportion of clinically meaningful changes in EORTC QLQ-C30 GH/QOL (A), physical functioning (B), cognitive functioning (C), fatigue (D), pain (E), and FACT-LymS (F). Clinically meaningful change (improvement or deterioration) from baseline in EORTC QLQ-C30 score was determined using a RD based on minimal change thresholds (ie, smallest incremental change) as recommended by Cocks and Buchanan22 as follows: 5 points for GH/QOL, physical, and emotional functioning; 10 points for fatigue; 15 points for role, cognitive, social functioning, nausea/vomiting, and pain; and 30 points for all other domains. For the FACT-LymS, a cutoff value of 3 was defined as the within-group and between-group MID and RD.24 Numbers of evaluable patients at each visit were based on data available for the variable at the given visit. ∗P < .05. Asterisks above bars indicate significant differences in proportions of patients exhibiting clinically meaningful improvement vs no clinically meaningful improvement. Asterisks below bars indicate significant differences in proportions of patients exhibiting clinically meaningful deterioration vs no clinically meaningful deterioration. GH/QOL, global health status/QOL.
Time to confirmed deterioration
Time to confirmed deterioration favored the liso-cel arm vs the SOC arm in global health status/QOL (median: not yet reached by the time of data cutoff vs 19.0 weeks; HR, 0.47; 95% CI, 0.24-0.94; supplemental Figure 4). However, there was a trend toward greater risk of deterioration in emotional functioning for liso-cel vs SOC (median in both arms not reached; HR [liso-cel vs SOC] = 1.30; 95% CI, 0.61-2.78). Time to confirmed deterioration in other primary domains of interest was generally comparable between both arms.
Sensitivity analyses
Based on MMRM overall LS mean changes estimated using a “while on treatment” strategy, the liso-cel arm showed clinically meaningful improvement relative to the SOC arm in global health status/QOL (4.88; 95% CI, −1.97-11.73), cognitive functioning (3.73; 95% CI, −1.45-8.91), and fatigue (−5.01; 95% CI, −12.75-2.74), while showing no clinically meaningful worsening relative to the SOC arm in any domain (supplemental Table 4).
Discussion
In the TRANSFORM study, liso-cel demonstrated significantly improved efficacy over SOC in the primary end point of EFS, as well as in key secondary efficacy end points of CR rate and PFS, as second-line therapy in patients with R/R LBCL.12 HRQOL data were collected in the TRANSFORM study to assess the treatment benefits of liso-cel vs SOC from the patient’s perspective through the entire sequence of treatments in each of the study arms (leukapheresis followed by bridging therapy, if needed, lymphodepletion, and liso-cel infusion in the liso-cel arm vs 3 cycles of salvage chemotherapy followed by HDCT and ASCT in responding patients in the SOC arm). These data are the first comparative analysis of HRQOL outcomes in patients receiving liso-cel infusion vs SOC as second-line therapy. If treatment failed in either arm, HRQOL was no longer assessed after patients received subsequent antineoplastic treatment, which reduced the number of patients completing HRQOL assessments over time, especially in the SOC arm, where 50% of patients crossed over to receive liso-cel as third-line therapy.12 This suggests that HRQOL data for the SOC arm were collected mainly from patients who responded to and/or tolerated treatments well; these HRQOL findings should be interpreted within this context.
Patients in this study had baseline HRQOL that was generally comparable to the general population with similar age and gender, except for role functioning and social functioning domains, as expected due to disease burden. Impairment in role functioning and social functioning domains at baseline was consistent with the findings from a qualitative study of HRQOL in patients with diffuse LBCL treated with CAR T-cell therapy.17,18,26
The burden of treatment between the 2 study arms differed in the duration and sequence of treatments unique to each treatment modality. Notably, patients in the SOC arm continued to receive salvage chemotherapy up to around day 64 and were anticipated to be 2 months after completion of HDCT and ASCT by day 126, whereas patients in the liso-cel arm had completed lymphodepletion and liso-cel infusion at around study day 29 (Figure 1). However, the study was designed to compare the entire sequence and burden of treatments in each study arm over time for each of the 2 treatment modalities, and the results demonstrate that the overall burden is lesser with liso-cel than with SOC.
Treatment with liso-cel (ie, lymphodepleting chemotherapy and liso-cel infusion with or without bridging chemotherapy) did not have a detrimental effect on most HRQOL measures. In many domains, patients in the liso-cel arm reported more favorable HRQOL results compared with those in the SOC arm. In the liso-cel arm, low rates of severe cytokine release syndrome (1%) or neurological events (4%) did not appear to affect mean HRQOL outcomes. At the group level (based on observed mean changes from baseline), patients in the SOC arm showed clinically meaningful worsening in global health status/QOL, fatigue, and pain at ≥1 assessment visits; these were not observed among patients in the liso-cel arm. The MMRM results suggest that patients in the liso-cel arm had meaningfully better overall LS mean change from baseline through day 126 than those in the SOC arm (after HDCT and ASCT) in cognitive functioning and fatigue domains. At the individual level, the proportion of patients with clinically meaningful improvement or no change by month 6 was higher in patients treated in the liso-cel arm compared with those in the SOC arm across most of the primary HRQOL domains, particularly in global health status/QOL, cognitive functioning, and fatigue.
In this longitudinal analysis of HRQOL, time to confirmed deterioration in global health status/QOL was not reached in the liso-cel arm compared with 19 weeks in the SOC arm; however, there seemed to be a greater risk of deterioration of emotional functioning in the liso-cel arm than in the SOC arm. Worsening in emotional functioning during or immediately after CAR T-cell therapy was also reported in a qualitative study, which was mainly ascribed to concerns about the efficacy of treatment and side effects such as cytokine release syndrome and neurological events.26 However, by 6 months after treatment, the proportion of patients reporting a concern with emotional impact was reduced by half.26 A short- and long-term study of patients with lymphomas and other malignancies assessed EORTC QLQ-C30 scores at baseline before transplant and once a week through week 4 after ASCT, once a month from month 6, and once every 6 months for up to 3 years.27 The patients with lymphoma experienced the greatest deterioration 2 weeks after beginning treatment, but scores gradually improved to baseline by 3 years. The most pronounced decline in functional domain items was the role of function (ie, ability to participate in work, daily activities, hobbies, and leisure time).
This study was limited by lower than anticipated questionnaire completion rates, with only 51% of patients in the liso-cel arm and 47% of patients in the SOC arm included in the HRQOL analyses, primarily owing to issues caused by unfamiliarity with electronic clinical outcome assessment implementation for this study, as well as operational and logistical challenges due to the COVID-19 pandemic. However, questionnaire completion rates were similar between both arms across most visits. The causes for missing data affected both arms in a similar way, suggesting that results are unlikely to be biased against either treatment arm, and patients’ baseline characteristics were similar to those of the overall intent-to-treat population (supplemental Table 1). In addition, the follow-up period was relatively short to see the long-term effect on HRQOL, as assessments were only done for study visits with ≥10 patients in each arm. Many patients in the study had not yet reached the 6-month time point or required additional antineoplastic treatment and were no longer eligible for HRQOL evaluation. Although the follow-up of this study allowed assessment of the effects on HRQOL of some CAR T-cell therapy–associated adverse events with shorter onset and duration times, such as cytokine release syndrome and neurological events, it may not be sufficient for assessment of other adverse events, such as prolonged cytopenia and infections, which may have long-term effects on HRQOL. Additional longer-term analyses based on further follow-up are needed to inform the impact of longer-term adverse events on HRQOL beyond this study and time period.
The effect of liso-cel compared with SOC on HRQOL may have been underestimated, as patients in the SOC arm who did not respond to treatment could start the next line of therapy, after which HRQOL data were not collected. Therefore, our results from the SOC arm represent HRQOL findings for patients who were responding to and/or tolerating treatments well. In addition, the timing of the HRQOL assessment at day 126 (ie, on average 55 days after the date of ASCT) may be too late to capture the negative short-term effect on HRQOL caused by HDCT and ASCT among patients in the SOC arm.
Conclusion
Most HRQOL domains at baseline among the study population were generally comparable to the general population. The results of this analysis demonstrate that treatment with liso-cel improved some HRQOL domains, including global health status/QOL, cognitive functioning, and fatigue, and maintained HRQOL in most of the remaining domains, compared with SOC. Coupled with the superior efficacy results, these data further support the use of liso-cel as a potential new SOC for second-line treatment in patients with early R/R LBCL.
Acknowledgments
The authors would like to thank the patients and families who made this trial possible and the investigators and clinical study teams who participated in the trial.
This study was funded by Celgene, a Bristol-Myers Squibb Company. Writing and editorial assistance were provided by Brooke Middlebrook, CMPP, of Evidera Inc. and Jeremy Henriques, PhD, CMPP, of the Lockwood Group (Stamford, CT), funded by Bristol Myers Squibb.
Authorship
Contribution: J.S.A., P.B.J., and S.R.S. contributed to data acquisition and interpretation; M.K., S.I., K.I., J.A., B.G., P.M., and M.L. contributed to data acquisition; J.B., F.F.L., A.C., S.M., and A.P. contributed to conception or design, data analysis, and data interpretation; S.G. and L.S. contributed to data analysis and data interpretation; and all authors contributed to and approved the final version of the manuscript for submission and agree to be accountable for all aspects of the work.
Conflict-of-interest disclosure: J.S.A. reports consultancy from AbbVie, Allogene Therapeutics, AstraZeneca, BeiGene, Bluebird Bio, Bristol Myers Squibb, C4 Therapeutics, Celgene, a Bristol-Myers Squibb Company, Eli Lilly, EMD Serono Inc., Genentech, a member of the Roche Group, Genmab, Incyte Corporation, Karyopharm Therapeutics, Kite Pharma, Kymera Therapeutics, MorphoSys, Novartis, and Regeneron; and research grants from Bristol-Myers Squibb and Seagen Inc. M.K. reports consultancy from AbbVie, Adaptive Biotechnologies, ADC Therapeutics, AstraZeneca, BeiGene, and Bristol Myers Squibb; honoraria from Seagen; and grants for research from Genentech and TG Therapeutics. S.I. reports divested equity in a private or publicly-traded company in the past 24 months from Karyopharm Therapeutics. K.I. reports honoraria from Daiichi Sankyo, Eisai, Fuji Film Toyama Chemical, Janssen, Kyowa Kirin, Novartis, Ono Pharmaceutical, Symbio, Takeda, Chugai, Celgene, AstraZeneca, Allergan Japan, and AbbVie and research funding from Daiichi Sankyo, Eisai, Genmab, Incyte, Huya Biosciences, Janssen, Kyowa Kirin, MSD, Novartis, Ono Pharmaceutical, Pfizer, Solasia, Takeda, Yakult, Chugai, Celgene, BeiGene, Bayer, and AstraZeneca. J.A. reports honoraria from Juno Therapeutics, a Bristol-Myers Squibb Company. B.G. reports consultancy from Bristol-Myers Squibb and Roche; research funding from Riemser Pharma GmbH. M.L. reports consultancy from Karyopharm, AstraZeneca, Legend, Verastem, Janssen, Myeloid Therapeutics, Daiichi Sankyo, Novartis, Spectrum, Celgene, a Bristol-Myers Squibb Company AbbVie, Acrotech, BeiGene, ADC Therapeutics, TG Therapeutics, MorphoSys, Kite Pharma, a Gilead Company, and Kyowa Kirin. S.G. is a current employee at Evidera, which received research funding from Bristol Myers Squibb; and reports consultancy from Bristol Myers Squibb, Gilead, and Janssen. L.S. is a current employee at Evidera, which received research funding from Bristol Myers Squibb. J.B., F.F.L., A.C., S.M., and A.P. are current employees and equity holders at Bristol Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Jeremy S. Abramson, Massachusetts General Hospital Cancer Center, 0 Emerson Place, Boston, MA 02114; e-mail: jabramson@mgh.harvard.edu.
References
Author notes
Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
The full-text version of this article contains a data supplement.