Key Points
D-dimer testing may help to decide on extended treatment after VTE; this was never assessed since DOACs are available.
Exceedingly more outcomes in patients off anticoagulation for negative D-dimer than in those receiving apixaban for positive testing.
Abstract
D-dimer assay is used to stratify patients with unprovoked venous thromboembolism (VTE) for the risk of recurrence. However, this approach was never evaluated since direct oral anticoagulants are available. With this multicenter, prospective cohort study, we aimed to assess the value of an algorithm incorporating serial D-dimer testing and administration of reduced-dose apixaban (2.5 mg twice daily) only to patients with a positive test. A total of 732 outpatients aged 18 to 74 years, anticoagulated for ≥12 months after a first unprovoked VTE, were included. Patients underwent D-dimer testing with commercial assays and preestablished cutoffs. If the baseline D-dimer during anticoagulation was negative, anticoagulation was stopped and testing repeated after 15, 30, and 60 days. Patients with serially negative results (286 [39.1%]) were left without anticoagulation. At the first positive result, the remaining 446 patients (60.9%) were given apixaban for 18 months. All patients underwent follow-up planned for 18 months. The study was interrupted after a planned interim analysis for the high rate of primary outcomes (7.3%; 95% confidence interval [CI], 4.5-11.2), including symptomatic proximal deep vein thrombosis (DVT) or pulmonary embolism (PE) recurrence, death for VTE, and major bleeding occurring in patients off anticoagulation vs that in those receiving apixaban (1.1%; 95% CI, 0.4-2.6; adjusted hazard ratio [HR], 8.2; 95% CI, 3.2-25.3). In conclusion, in patients anticoagulated for ≥1 year after a first unprovoked VTE, the decision to further extend anticoagulation should not be based on D-dimer testing. The results confirmed the high efficacy and safety of reduced-dose apixaban against recurrences. This trial was registered at www.clinicaltrials.gov as #NCT03678506.
Introduction
Venous thromboembolism (VTE), which encompasses deep vein thrombosis (DVT) and pulmonary embolism (PE), tends to recur, especially in patients whose index event was unprovoked or associated with permanent prothrombotic conditions.1 Anticoagulation for 3 to 6 months is recommended in all patients with VTE, whereas indefinite anticoagulation is recommended in patients with unprovoked VTE without a high bleeding risk2 since recurrence after anticoagulation discontinuation does not depend on the duration of initial treatment.3-5 A recent systematic review and meta-analysis of 18 studies involving 7515 patients showed that the cumulative incidence of 10-year recurrence after discontinuation of anticoagulant treatment in patients with a first unprovoked VTE was 36% (with a 95% confidence interval [CI] ranging from 28% to 45%).6 In a previous management study, we tried to stratify patients with unprovoked index events using a serial D-dimer assay. Patients with positive assay were found to be at an increased risk of recurrence and required resumption of anticoagulant treatment, whereas those with persistently negative assay were at low risk and could safely withhold anticoagulation.7 Of note, patients resuming vitamin K antagonists (VKAs), the only oral anticoagulant drugs available at that time, experienced a high rate of bleeding events (2.4% patient-years). In recent years, 4 direct oral anticoagulants (DOACs) were licensed for VTE treatment. Furthermore, specifically designed studies have suggested that 2 of the DOACs (apixaban and rivaroxaban) are as effective and safe when administered at half their usual dose for extended treatment in patients with VTE who had already received a first-line therapy for 6 to 12 months.8,9
The Apidulcis was a multicenter, prospective, management cohort study whose aim was to assess the value of an algorithm incorporating serial D-dimer with low-dose apixaban for the long-term management of patients with a first episode of proximal DVT and/or PE that was either unprovoked or associated with weak risk factors after completing an uneventful 12-month period of conventional anticoagulation.
Methods
Study design and oversight
We conducted a management study to assess whether patients with a first unprovoked VTE event, who had been treated with standard anticoagulation for ≥12 months, could be stratified for the risk of recurrence using a serial D-dimer assay. After this period of anticoagulation (whatever drug was used), patients were tested with a D-dimer assay, first during anticoagulation and then (if the first test turned out negative) 15, 30, and 60 days after anticoagulation was stopped. At the first positive D-dimer, patients were invited to extend treatment by taking apixaban 2.5 mg twice a day for 18 months. Patients with persistently negative D-dimer results were invited to stay off anticoagulation. All patients were planned to be followed up for 18 months.
The study was an independent research initiative promoted by the “Arianna Anticoagulazione” Foundation (Bologna, Italy). The Foundation had responsibility for the study design, its protocol, and oversight, as well as collection, maintenance, verification, and analysis of the data. Public and private institutions, companies, and individuals interested in anticoagulant or antithrombotic treatment (manufacturers of drugs or other goods and services) were asked by the Executive Committee of the Foundation to help fund the studies via unrestricted grants without no right of access to the data.
The study was approved by the institutional review board at each participating center and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients. An independent data monitoring and safety board (DMSB) committee reviewed the outcomes and performed an interim analysis as predefined in the protocol.
Patients
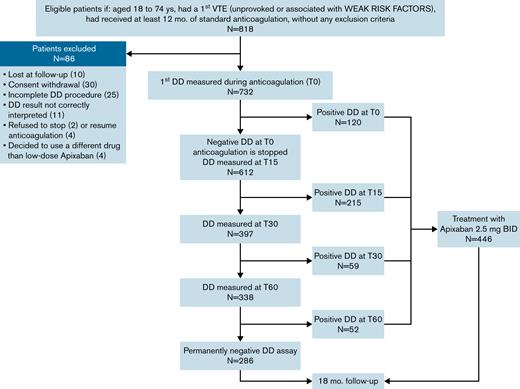
Patients aged 18 to 74 years were eligible for inclusion if they had a first symptomatic proximal DVT and/or PE that was unprovoked or associated with weak and transient risk factors and had undergone standard anticoagulant therapy for ≥12 months without any symptomatic recurrence or bleeding events. Patients were ineligible if they had a contraindication to continued anticoagulant therapy or required anticoagulant treatment for other reasons (for a detailed list of inclusion/exclusion criteria, seeTable 1).
Baseline characteristics of the study patients
| Characteristics, n (%) . | All patients included in the study n = 778∗ . | Patients treated with 2.5 mg BID apixaban (positive D-dimer), n = 446 (60.9) . | Patients who stopped treatment (negative D-dimer), n = 286 (39.1) . | P value <.0001 . |
|---|---|---|---|---|
| Male sex, n (%) | 491 (63.1) | 315 (70.6) | 148 (51.9) | <.0001 |
| Age (y), median (IQR) | 59 (48-67) | 63 (54-69) | 51 (42-61) | <.0001 |
| <51 y, n (%) | 244 (31.4) | 87 (19.5) | 138 (48.2) | <.0001 |
| 51-74 y, n (%) | 534 (68.6) | 359 (80.5) | 148 (51.8) | <.0001 |
| Type of index VTE, n (%) | ||||
| Proximal DVT with/without PE | 597 (76.7) | 355 (79.6) | 206 (72.0) | .0178 |
| Isolated PE | 179 (23.0) | 90 (20.2) | 79 (27.7) | .0190 |
| Missing | 2 (0.3) | 1 (0.2) | 1 (0.3) | — |
| Type of risk factors, n (%) | ||||
| Unprovoked | 588 (75.6) | 370 (83.0) | 185 (64.7) | <.0001 |
| Weak risk factors | 190 (24.4) | 76 (17.0) | 101 (35.3) | <.0001 |
| Minor surgery | 16 | 7 | 9 | — |
| Pregnancy or puerperium | 4 | 0 | 3 | — |
| Hormonal therapy | 79 | 20 | 56 | <.0001 |
| Long travel | 16 | 12 | 2 | — |
| Minor trauma, leg injury | 30 | 15 | 12 | — |
| Reduced mobility | 18 | 7 | 10 | — |
| Hospitalization in a medical ward | 3 | 1 | 2 | — |
| Other diseases | 24 | 14 | 7 | — |
| Anticoagulant drugs used before inclusion | ||||
| VKAs, n (%)† | 68 (8.7) | 42 (9.4) | 23 (8.0) | .5156 |
| DOACs, n normal or low dose (%) | 559 or 151 (91.3) | 305 or 99 (90.6) | 220 or 43 (92.0) | — |
| Apixaban 5 mg or 2.5 mg BID | 149 or 95 (31.4) | 79 or 66 (32.5) | 62 or 27 (31.1) | .6921 |
| Dabigatran 150 mg or 110 mg BID | 80 or 9 (11.4) | 44 or 7 (11.4) | 29 or 1 (10.5) | .7049 |
| Edoxaban 60 mg or 30 mg OID | 112 or 15 (16.4) | 65 or 6 (15.9) | 41 or 7 (16.8) | .7476 |
| Rivaroxaban 20 mg or 10 mg mg OID | 218 or 32 (32.1) | 117 or 20 (30.8) | 88 or 8 (33.6) | .4281 |
| Duration of prior anticoagulant treatment, n (%) | ||||
| 12-18 mo | 583 (74.9) | 319 (71.5) | 230 (80.4) | .0067 |
| >18 mo | 195 (25.1) | 127 (28.5) | 56 (19.6) | .0067 |
| Total duration of follow-up, y | 897 | 533 | 336 | — |
| up to 6 mo, n (%) | 119 (15.3) | 54 (12.1) | 42 (14.7) | .3096 |
| 7-12 mo, n (%) | 161 (20.7) | 95 (21.3) | 56 (19.6) | .5795 |
| 13-18 mo, n (%) | 498 (64.0) | 297 (66.6) | 188 (65.7) | .8018 |
| Median follow-up, median (IQR) | 1.35 (0.73-1.64) | 1.41 (0.77-1.64) | 1.37 (0.77-1.64) | .9621 |
| Associated antiplatelet treatment, n (%) | 26 (3.3) | 19 (4.3) | 5 (1.7) | .0543 |
| Characteristics, n (%) . | All patients included in the study n = 778∗ . | Patients treated with 2.5 mg BID apixaban (positive D-dimer), n = 446 (60.9) . | Patients who stopped treatment (negative D-dimer), n = 286 (39.1) . | P value <.0001 . |
|---|---|---|---|---|
| Male sex, n (%) | 491 (63.1) | 315 (70.6) | 148 (51.9) | <.0001 |
| Age (y), median (IQR) | 59 (48-67) | 63 (54-69) | 51 (42-61) | <.0001 |
| <51 y, n (%) | 244 (31.4) | 87 (19.5) | 138 (48.2) | <.0001 |
| 51-74 y, n (%) | 534 (68.6) | 359 (80.5) | 148 (51.8) | <.0001 |
| Type of index VTE, n (%) | ||||
| Proximal DVT with/without PE | 597 (76.7) | 355 (79.6) | 206 (72.0) | .0178 |
| Isolated PE | 179 (23.0) | 90 (20.2) | 79 (27.7) | .0190 |
| Missing | 2 (0.3) | 1 (0.2) | 1 (0.3) | — |
| Type of risk factors, n (%) | ||||
| Unprovoked | 588 (75.6) | 370 (83.0) | 185 (64.7) | <.0001 |
| Weak risk factors | 190 (24.4) | 76 (17.0) | 101 (35.3) | <.0001 |
| Minor surgery | 16 | 7 | 9 | — |
| Pregnancy or puerperium | 4 | 0 | 3 | — |
| Hormonal therapy | 79 | 20 | 56 | <.0001 |
| Long travel | 16 | 12 | 2 | — |
| Minor trauma, leg injury | 30 | 15 | 12 | — |
| Reduced mobility | 18 | 7 | 10 | — |
| Hospitalization in a medical ward | 3 | 1 | 2 | — |
| Other diseases | 24 | 14 | 7 | — |
| Anticoagulant drugs used before inclusion | ||||
| VKAs, n (%)† | 68 (8.7) | 42 (9.4) | 23 (8.0) | .5156 |
| DOACs, n normal or low dose (%) | 559 or 151 (91.3) | 305 or 99 (90.6) | 220 or 43 (92.0) | — |
| Apixaban 5 mg or 2.5 mg BID | 149 or 95 (31.4) | 79 or 66 (32.5) | 62 or 27 (31.1) | .6921 |
| Dabigatran 150 mg or 110 mg BID | 80 or 9 (11.4) | 44 or 7 (11.4) | 29 or 1 (10.5) | .7049 |
| Edoxaban 60 mg or 30 mg OID | 112 or 15 (16.4) | 65 or 6 (15.9) | 41 or 7 (16.8) | .7476 |
| Rivaroxaban 20 mg or 10 mg mg OID | 218 or 32 (32.1) | 117 or 20 (30.8) | 88 or 8 (33.6) | .4281 |
| Duration of prior anticoagulant treatment, n (%) | ||||
| 12-18 mo | 583 (74.9) | 319 (71.5) | 230 (80.4) | .0067 |
| >18 mo | 195 (25.1) | 127 (28.5) | 56 (19.6) | .0067 |
| Total duration of follow-up, y | 897 | 533 | 336 | — |
| up to 6 mo, n (%) | 119 (15.3) | 54 (12.1) | 42 (14.7) | .3096 |
| 7-12 mo, n (%) | 161 (20.7) | 95 (21.3) | 56 (19.6) | .5795 |
| 13-18 mo, n (%) | 498 (64.0) | 297 (66.6) | 188 (65.7) | .8018 |
| Median follow-up, median (IQR) | 1.35 (0.73-1.64) | 1.41 (0.77-1.64) | 1.37 (0.77-1.64) | .9621 |
| Associated antiplatelet treatment, n (%) | 26 (3.3) | 19 (4.3) | 5 (1.7) | .0543 |
BID, bis intraday; OID, once intraday.
Including patients who did not complete the D-dimer procedure (25), those with D-dimer results that were not correctly evaluated by the investigators (11), who refused to stop anticoagulation (2), to resume anticoagulation (4), or decided to use a different drug than low-dose apixaban (4).
One subject received fondaparinux.
Management procedures
All included patients underwent serial D-dimer assessment starting at baseline during anticoagulation (T0). Patients with positive baseline D-dimer were instructed to take apixaban 2.5 mg twice a day for 18 months, while those with negative D-dimer at T0 were asked to stop anticoagulant therapy and repeat D-dimer testing after 15 ± 3 (T1), 30 ± 4 (T2), and 60 ± 5 (T3) days from T0. At the first positive D-dimer result, apixaban 2.5 mg twice a day was given by the local investigator for a treatment duration of 18 months. Three boxes containing apixaban 2.5 mg tablets (each box sufficient for 1 month of treatment) were distributed to patients directly by the participant investigators and refilled every 3 months. Patients with persistently negative D-dimer results were invited to stay off anticoagulant treatment.
Surveillance and follow-up
Patients underwent assessment, either at the clinic or by phone, every 3 to 6 months during the study period. Patients were invited to report to the study center if they had symptoms suggestive of recurrent VTE or bleeding. An independent adjudication committee that was unaware of patient name, D-dimer testing result at inclusion, management, and enrolling center assessed all outcomes. Recurrent DVT was diagnosed if a previously fully compressible segment at compression ultrasonography (CUS; contralateral or ipsilateral) could no longer be compressed or if an increase of ≥4 mm in the diameter of the residual thrombus during compression was detected.10 In patients with suspected PE, recurrence was diagnosed following objective algorithms incorporating clinical probability, ventilation-perfusion lung scanning, or computerized tomography pulmonary angiogram, CUS, and/or D-dimer testing as appropriate.11
D-dimer assays and prespecified cutoff values
D-dimer concentrations were assessed with the quantitative assay routinely used in each participating center, provided it was one of the following: Cobas h232 (Roche Diagnostics; Switzerland), HemosIL D-dimer HS 500 (Instrumentation Laboratory; Milan, Italy), HemosIL D-dimer HS (Instrumentation Laboratory), HemosIL D-dimer (Instrumentation Laboratory), Innovance D-DIMER (Siemens; Deerfield, IL), Sclavo Auto D-dimer (Dasit; Milan, Italy), STA Liatest D-dimer (Diagnostica Stago; Asnieres-sur-Seine, France), and VIDAS D-dimer Exclusion (bioMerieux; Lyon, France). For the assays expressing results as fibrinogen equivalent units, the cutoffs were 350 ng/mL and 500 ng/mL for males and females, respectively; the cutoffs for assays expressing the results as D-dimer units were 175 ng/mL and 250 ng/mL for males and females, respectively. These cutoffs were selected by the study team after a critical evaluation of the Dulcis study results,7 taking into account that (1) patients aged ≥75 years were excluded from the present study (and therefore, there was no need for different cutoff levels according to the age) and (2) male patients are unanimously recognized at higher risk of recurrence than females (for this reason the decided cutoff level was lower for males than females).
Outcome measures
The primary outcome for efficacy was the incidence of recurrent symptomatic proximal DVT and/or PE, or death from VTE. The secondary efficacy outcomes included arterial cardiovascular events, isolated distal DVT, superficial vein thrombosis, death from any cause, and venous thrombosis in other sites. The primary safety outcome was major bleeding (MB), classified as such according to International Society of Thrombosis and Haemostasis (ISTH).12 The secondary safety outcome was clinically relevant nonmajor bleeding.13 Minor bleeds were also recorded.
Statistical analysis, sample size, and interim analysis
Based on the Dulcis study results,7 about 52% of eligible patients were expected to discontinue anticoagulation for persistently negative D-dimer tests with an annual rate of VTE recurrence around 3% (95% CI, 2.0-4.4). The rate of recurrent VTE in the remaining 48% of patients with a positive D-dimer assay to be treated with low-dose apixaban was, based on the results of the Amplify Extension study,8 expected to be around 1.7% after the first year of treatment. Based on these assumptions, we expected a recurrence rate at 12 months of 2.2% (0.45 × 0.03 + 0.55 × 0.017 = 2.2%). With a projected recurrence rate of 2.2% per year, we estimated that a sample size of 1150 patients, followed for 18 months, was needed to demonstrate that the upper 95% CI of the recurrence rate in the whole management cohort was <3.5%, with a study power of 80%.14 We expected around 38 recurrences and 2 major bleeding events in our study.
However, an interim analysis was planned when 20 primary outcomes (combination of the primary efficacy and safety outcomes) were registered. The interim analysis preestablished that the study would have to be interrupted prematurely if the relative risk of primary endpoints (hazard ratio [HR]) in the observational nontreatment arm was >3 HR, with a P value < .0001, compared with that recorded in the arm of patients receiving low-dose apixaban.
Continuous variables are expressed as the median and interquartile range (IQR), and categorical variables are presented as frequencies and percentages. Differences between groups were assessed using the χ-square test with Yates’ correction for categorical variables and the Mann-Whitney U test for continuous variables. Kaplan-Meier survival curves were plotted to estimate the cumulative incidence of primary outcomes (symptomatic recurrent VTE and major bleeding) in the 2 groups. HR and their 95% CI were calculated with the use of the Cox proportional-hazard model (after checking the proportional hazards assumption). Initially, an unadjusted HR was calculated. Then a multivariable model was constructed, including sex, patient age (≤50 vs >50 y), duration of anticoagulation before enrollment (12 to 18 vs >18 mo), the type of index VTE event (DVT with or without PE vs isolated PE), the nature of the index event (unprovoked vs secondary to weak risk factors), and body mass index (BMI) values. The data were analyzed with the use of Prism software (Version 9.3.1, GraphPad Software Incorporated; San Diego, CA) and SPSS software (version 11.0 SPSS Inc., IBM; Armonk, NY).
Role of funding source
The Bristol-Myers Squibb and Pfizer Alliance contributed to the present study by providing all apixaban 2.5 mg tablets and an unrestricted research grant with no right of access to data collected in the database.
Results
Patients and management
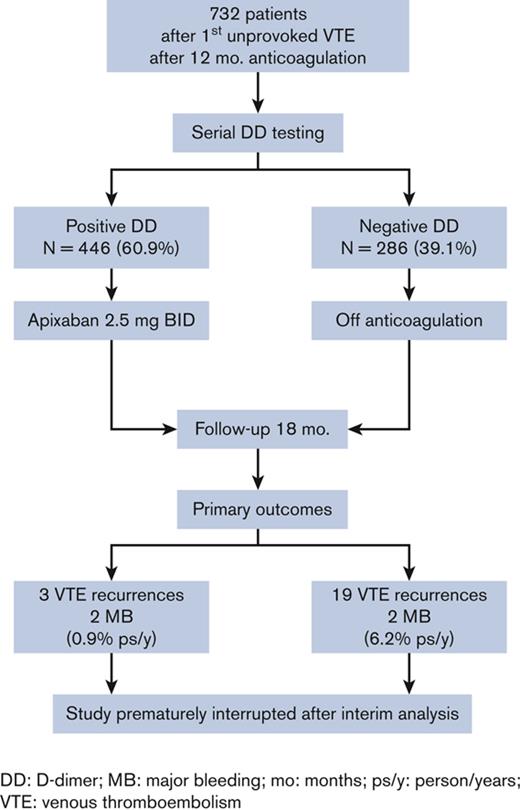
Figure 1 shows the study flowchart. In the 49 participating centers, 818 patients were enrolled in the study between August 2018 and November 2021. Of these patients, 40 were excluded because of consent withdrawal (n = 30), or they were lost to follow-up after the first D-dimer test (n = 10). In accordance with the study protocol, a formal interim analysis was performed in November 2021 when the first 20 primary events had occurred (encompassing recurrent VTE events or death because of VTE and major bleeding events). Based on the interim analysis, the DMSB recommended early termination of inclusion of new patients, having observed an unacceptable higher rate of primary outcomes in patients who had remained without treatment for negative D-dimer results compared with those who had received the extended treatment with apixaban. Indeed, the estimated HR of event incidence in the former vs the latter group was 7.8 (CI, 3.1-19.4), a value above the stopping-rule preestablished in the protocol (HR cutoff value >3.0). The inclusion of new patients was stopped at the end of November, and in line with the DMSB recommendations, the patients received a final follow-up visit by the end of December 2021.
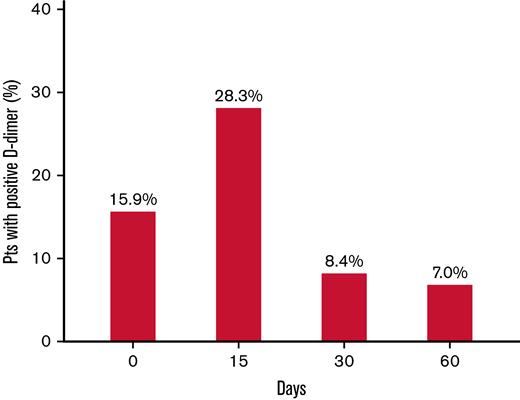
A total of 778 patients (491 males) were included in the study (Table 1). The median age of participants was 59 years (IQR, 48-67). The index event was unprovoked in 588 (75.6%) patients and associated with weak risk factors (listed in Table 1) in the remaining patients. The anticoagulant treatment before inclusion was performed with a DOAC in 559 patients (91.3%), which was a low-dose drug in 27.1% of them. As shown in Figure 2, the prevalence of positive D-dimer results was high (15.9%) during anticoagulation and highest (28.3%) 15 days after its interruption. Some patients were excluded from further evaluation because they did not complete D-dimer testing (n = 25), their D-dimer results were not correctly interpreted as negative by the investigators (n = 11), refused to stop anticoagulation (n = 2), refused to resume anticoagulation (n = 4), or decided to use a different anticoagulant drug than 2.5 mg apixaban (n = 4). Of these patients, 40 remained without any anticoagulant treatment; they were followed up but not included in the final analysis. D-dimer testing was completed in 732 subjects: 446 (60.9%) had positive D-dimer results and received apixaban 2.5 mg twice daily, whereas 286 (39.1%) had persistently negative results and remained without anticoagulation. The prevalence of positive D-dimer at first measurement (during anticoagulation) was significantly higher in patients treated with a reduced-dose DOAC than in those receiving standard dose (25.3% vs 14.1%, respectively; P = .0014). The patients with positive or negative D-dimer had significantly different baseline characteristics (Table 1). Patients with negative D-dimer were younger (more subjects aged <51 y), more frequently women, and presented more frequently with isolated PE as an index event that was more often associated with weak risk factors, especially hormonal therapy. The median follow-up time was 1.41 and 1.37 years in patients receiving apixaban or going without treatment, respectively (Table 2).
Prevalence of first-time-ever positive D-dimer result (above the predefined cutoff levels) in the investigated study population at the serial measurement during (0) and days after anticoagulation withdrawal. The percentages are calculated vs the total number of patients tested.
Prevalence of first-time-ever positive D-dimer result (above the predefined cutoff levels) in the investigated study population at the serial measurement during (0) and days after anticoagulation withdrawal. The percentages are calculated vs the total number of patients tested.
Clinical events occurring in the investigated patients
| Events . | Patients treated with 2.5 mg BID apixaban (positive D-dimer), n = 446, FU 533 y . | Patients who stopped treatment (negative D-dimer), n = 286, FU 336 y . | P value . |
|---|---|---|---|
| Primary outcomes, n % (95% CI) (Including VTE recurrences and MB; no death could be attributed to VTE) | 5 (4 males) | 21 (14 males) | — |
| Incidence per 100 person-years (95% CI) | |||
| Primary outcomes | 0.9 (0.3-2.2) | 6.2 (3.9-9.5) | <.0001 |
| MB | 0.3 (0-1.3) | 0.6 (0-2.1) | — |
| CRNMB | 1.1 (0.4-2.4) | 0 | — |
| Primary outcomes and arterial events | 1.7 (0.8-3.2) | 6.5 (4.1-9.9) | .0002 |
| Recurrent VTE, n | |||
| DVT | 2 | 10 | — |
| Isolated PE | 1 | 9 | — |
| DVT + PE | 0 | 0 | — |
| Deaths for VTE∗ | 0 | 0 | — |
| MB, n† | 2 | 2 | — |
| Other outcomes, n | |||
| Arterial vascular event, n‡ | 4 (1 death) | 1 | — |
| Isolated distal DVT, n | 2 | 5 | — |
| SVT, n | 1 | 13 | — |
| CRNMB, n | 6 | 0 | — |
| Minor bleeds, n | 21§ | 0 | — |
| Retinal vein occlusion | 1 | 0 | — |
| Patients with duration of follow-up <18 moǁ | 184 | 127 | — |
| Events . | Patients treated with 2.5 mg BID apixaban (positive D-dimer), n = 446, FU 533 y . | Patients who stopped treatment (negative D-dimer), n = 286, FU 336 y . | P value . |
|---|---|---|---|
| Primary outcomes, n % (95% CI) (Including VTE recurrences and MB; no death could be attributed to VTE) | 5 (4 males) | 21 (14 males) | — |
| Incidence per 100 person-years (95% CI) | |||
| Primary outcomes | 0.9 (0.3-2.2) | 6.2 (3.9-9.5) | <.0001 |
| MB | 0.3 (0-1.3) | 0.6 (0-2.1) | — |
| CRNMB | 1.1 (0.4-2.4) | 0 | — |
| Primary outcomes and arterial events | 1.7 (0.8-3.2) | 6.5 (4.1-9.9) | .0002 |
| Recurrent VTE, n | |||
| DVT | 2 | 10 | — |
| Isolated PE | 1 | 9 | — |
| DVT + PE | 0 | 0 | — |
| Deaths for VTE∗ | 0 | 0 | — |
| MB, n† | 2 | 2 | — |
| Other outcomes, n | |||
| Arterial vascular event, n‡ | 4 (1 death) | 1 | — |
| Isolated distal DVT, n | 2 | 5 | — |
| SVT, n | 1 | 13 | — |
| CRNMB, n | 6 | 0 | — |
| Minor bleeds, n | 21§ | 0 | — |
| Retinal vein occlusion | 1 | 0 | — |
| Patients with duration of follow-up <18 moǁ | 184 | 127 | — |
BID, bis intraday; CRNMB, clinically relevant nonmajor bleeding; FU, follow-up; MB, major bleeding; SVT, superficial vein thrombosis.
No death could be attributed to VTE.
Two MB events in the patients receiving apixaban and 1 in patients off anticoagulant treatment were posttraumatic.
The 4 arterial events occurring in patients receiving apixaban were 2 acute myocardial infarctions, 1 stroke, and 1 sudden death attributed to a cardiovascular cause; the arterial event occurring in patients with negative D-dimer was a peripheral arterial thrombosis.
The minor bleeds occurred in 14 patients.
Patients with lower than established follow-up because of the premature interruption of the study.
Primary study outcomes and distribution in the 2 study groups
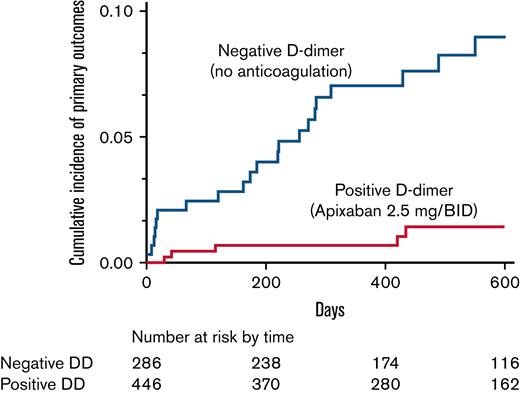
During the entire follow-up of 869 person-years, 26 primary outcomes occurred in the 732 subjects who correctly followed the study protocol (12 proximal DVTs with or without PE, 10 isolated PEs, and 4 MB events), for an incidence rate of 3.0 per 100 person-years (95% CI, 1.9-4.4). The study was prematurely stopped when a planned interim analysis found an unacceptable (according to a preestablished stopping rule) higher prevalence of recurrences in patients who remained off anticoagulation compared with those receiving reduced-dose apixaban. Twenty-one primary events (19 thrombotic recurrences) occurred in patients (14 males) with negative D-dimer (6.2 per 100 person-years; 95% CI, 3.9-9.5), and 5 (3 recurrences; 4 males) in those receiving apixaban after positive D-dimer results (0.9 per 100 person-years; 95% CI, 0.3-2.2). Details of primary and secondary outcomes are reported in Table 2. Figure 3 shows the Kaplan-Meier curves of cumulative event rates for the primary outcomes. The unadjusted HR for primary outcomes between the 2 groups of patients was 6.6 (95% CI, 2.7-19.8), and the adjusted HR was 8.2 (95% CI, 3.2-25.3). Nine events (incidence rate of 33.3 per 100 person-years) occurred in the 40 subjects who did not correctly follow the algorithm and remained without anticoagulant treatment.
The Kaplan-Meier cumulative event rates for the primary outcomes in patients receiving low-dose apixaban (dotted line) for positive D-dimer and in patients with persistently negative D-dimer in whom anticoagulation was definitively stopped (continuous line).
The Kaplan-Meier cumulative event rates for the primary outcomes in patients receiving low-dose apixaban (dotted line) for positive D-dimer and in patients with persistently negative D-dimer in whom anticoagulation was definitively stopped (continuous line).
Subgroup analysis in patients with negative D-dimer testing
As shown in Table 3, among patients with negative D-dimer, the incidence rate of primary outcomes was significantly lower in those whose index event was associated with weak risk factors (P = .0362) and in those aged <51 years. Though no other difference reached statistical significance, it can be noted that recurrences occurred less frequently in women whose index event was associated with hormonal therapy.
Incidence of primary outcome events in patients with negative D-dimer (who stopped anticoagulation)
| Characteristic . | Patients with event vs all patients with the characteristic . | Incidence per 100 person-years, % (95% CI) . | P value . |
|---|---|---|---|
| Overall | 21/286 | 6.2 (3.9-9.5) | — |
| Nature of index event | |||
| Unprovoked | 18/185 | 8.4 (5.0-13.3) | .0321 |
| Weak risk factors | 3/101 | 2.5 (0.5-7.2) | — |
| Age, y | |||
| <51 | 6/138 | 3.6 (1.3-7.9) | .0460 |
| 51-74 | 15/148 | 8.9 (5.0-14.7) | — |
| Type of index event | |||
| DVT with/without PE | 14/206 | 5.8 (3.2-9.7) | .5661 |
| Isolated PE | 7/79 | 7.5 (3.0-15.5) | — |
| 1 missing | |||
| Sex | |||
| Male | 14/148 | 8.1 (4.4-13.6) | .1520 |
| Female | 7/138 | 4.3 (1.7-8.9) | — |
| Women | |||
| With unprovoked events | 5/62 | 6.7 (2.2-15.8) | .3131 |
| With events associated with HT | 2/56 | 3.0 (0.4-10.8) | — |
| Duration of anticoagulation before inclusion, mo | |||
| 12-18 | 16/230 | 5.9 (3.4-9.6) | .5732 |
| >18 | 5/56 | 7.8 (2.5-18.2) | — |
| BMI | |||
| ≤24.9 | 8/118 | 5.9 (2.6-11.7) | .9155 |
| 25-30 | 9/105 | 7.0 (3.2-13.3) | — |
| >30 | 4/69 | 5.8 (1.6-14.8) | — |
| 4 missing | |||
| Characteristic . | Patients with event vs all patients with the characteristic . | Incidence per 100 person-years, % (95% CI) . | P value . |
|---|---|---|---|
| Overall | 21/286 | 6.2 (3.9-9.5) | — |
| Nature of index event | |||
| Unprovoked | 18/185 | 8.4 (5.0-13.3) | .0321 |
| Weak risk factors | 3/101 | 2.5 (0.5-7.2) | — |
| Age, y | |||
| <51 | 6/138 | 3.6 (1.3-7.9) | .0460 |
| 51-74 | 15/148 | 8.9 (5.0-14.7) | — |
| Type of index event | |||
| DVT with/without PE | 14/206 | 5.8 (3.2-9.7) | .5661 |
| Isolated PE | 7/79 | 7.5 (3.0-15.5) | — |
| 1 missing | |||
| Sex | |||
| Male | 14/148 | 8.1 (4.4-13.6) | .1520 |
| Female | 7/138 | 4.3 (1.7-8.9) | — |
| Women | |||
| With unprovoked events | 5/62 | 6.7 (2.2-15.8) | .3131 |
| With events associated with HT | 2/56 | 3.0 (0.4-10.8) | — |
| Duration of anticoagulation before inclusion, mo | |||
| 12-18 | 16/230 | 5.9 (3.4-9.6) | .5732 |
| >18 | 5/56 | 7.8 (2.5-18.2) | — |
| BMI | |||
| ≤24.9 | 8/118 | 5.9 (2.6-11.7) | .9155 |
| 25-30 | 9/105 | 7.0 (3.2-13.3) | — |
| >30 | 4/69 | 5.8 (1.6-14.8) | — |
| 4 missing | |||
BMI, body mass index; HT, hormonal therapy.
Discussion
In the Apidulcis study, patients who had completed ≥12 months of anticoagulant treatment after a first VTE episode that was unprovoked or associated with weak risk factors underwent serial D-dimer testing. Those with positive D-dimer assay received treatment with apixaban 2.5 mg twice daily for secondary prophylaxis, while patients who had persistently negative D-dimer results discontinued anticoagulation; the planned follow-up was 18 months for all the patients. Based on the results of previous studies, we expected a similarly low recurrence rate in both patient groups.7,8 However, after the preestablished interim analysis, planned when the first 20 outcomes had occurred in patients who had complied with the algorithm, the DMSB decided to stop the patient recruitment because of an alarming distribution of outcomes among the study groups. At the time of the study interruption, 26 primary events had occurred, 21 among patients who remained off anticoagulation and 5 among those given low-dose apixaban, accounting for an incidence of 6.2 and 0.9 × 100 patient-years, respectively. While the study confirmed the optimal efficacy and safety of the reduced apixaban dose for extended treatment after unprovoked VTE, it clearly showed the inability of the serial D-dimer testing procedure to identify patients at such a low risk of recurrent VTE to justify the exclusion from extended treatment.
The reasons why the recurrence rate in patients stopping anticoagulation for negative D-dimer testing was higher than that recorded in our study,7 as well as other management studies,15 that adopted a similar approach are unclear. First, it is likely that the patients included in the Apidulcis study were at a higher risk of recurrences than those in other studies, mainly because they were included after having completed ≥12 months of anticoagulation before enrolment, as compared with that of “≥3 months” adopted in other studies. As in clinical practice, most clinicians still favor shorter durations of anticoagulation because of the fear of bleeding complications16; the long anticoagulation before inclusion in the study is likely to have impacted the selection of patients perceived as being at a higher risk of recurrent events.17 This is also confirmed by the very high rate of recurrence (incidence rate of 33.3 per 100 person-years) in the few patients who remained without anticoagulation because they did not comply with the D-dimer testing procedure. Another factor that may have contributed to the unsatisfactory results of D-dimer predictivity is that, unfortunately, the study was initiated and performed in part before and after the spread of the COVID-19 pandemic in our country (starting in February 2020). In addition, to have affected the patient enrollment in the study for the difficult working conditions of clinical centers, the pandemic may also have affected the results through direct or indirect effects.18
Other differences were present between the designs of the present and other studies.7,15 In the present study, patients >74 years were excluded because of their high risk of bleeding when assuming anticoagulant drugs2 and the nonpredictive value of D-dimer in elderly subjects.19 A D-dimer cutoff level lower than that used for VTE diagnosis has been adopted in the present study for males to compensate for their higher risk of recurrence.20
An important and unexpected result of the present study was the very high prevalence of patients with positive D-dimer (60.9%), which was higher not only vs Dulcis (47.7%) but also vs the results (45.5% to 48%) calculated in 2 meta-analyses of the studies focused on the same issue,15,21 and especially compared with that (22%) found in a study in which D-dimer was assessed by a qualitative assay.22 Interestingly, the prevalence of positive testing was already higher at first measurement during anticoagulation (15.8% in Apidulcis vs 4.7% in Dulcis). This result was not attributable to the slight differences between the cutoffs used in the 2 studies; in fact, the difference remained almost unchanged if results were recalculated by using the cutoffs for VTE diagnosis in both studies (Apidulcis: 12.6%; Dulcis:4.8%; P < .0001). Other factors should therefore be considered. While all patients in Dulcis were receiving VKAs at enrollment, >90% of patients in Apidulcis received a DOAC (at a low dose in more than one-fourth of patients) as anticoagulant when the first D-dimer was measured. The anticoagulant action of DOACs and VKAs is based on completely different mechanisms; possible different effects on the formation and degradation of fibrin cannot be excluded, and though data on possible differences are to date missing,23 this issue deserves further analysis. Other factors may contribute to a less constant anticoagulant effect, such as the high inter- and intraindividual variability in the drug blood concentrations,24,25 and problems in adherence to treatment.26 Finally, lower than recommended doses and regimens of DOACs are noninfrequently used in patients with VTE.27 In this study, we found that the prevalence of positive D-dimer at first measurement was significantly higher in patients treated with a low dose than in those receiving a standard dose of DOAC (P = .0014), thus suggesting a lower degree of anticoagulant effect, though not leading to clinical events.
Despite the administration in patients at increased risk of recurrent VTE (because of a baseline positive D-dimer), in our study, the extended treatment with low-dose apixaban proved very effective against VTE recurrence, with a good safety profile. Indeed, of the 446 patients allocated to the extended treatment with reduced-dose apixaban, only 3 (0.56 per 100 person-years) experienced recurrent VTE, and only 2 (0.37 per 100 person-years) experienced major bleedings, which were both posttraumatic in nature. Our results confirm and extend those recorded in the Amplify Extension study.8
The strength of our investigation lies in its study design. Selection bias was prevented by excluding patients with previous VTE. Observation bias was prevented by including among the study outcomes only clinically symptomatic relevant events, which were all objectively confirmed. The compliance with the study was high, as was the consistency of the study results among the participating centers. The adjudication of both recurrent and bleeding events was done by an independent committee whose members were unaware of the patient’s clinical details as well as of the modality of their management. Predefined stopping rules were adopted to prevent undesirable harm to attending patients.
The study has limitations. Patients with <12 months duration of anticoagulation were excluded from the study, and therefore the study conclusions are not applicable to these patients. The lower-than-expected proportion of patients with negative D-dimer, as well as the premature interruption of the study, meant fewer patients were enrolled, especially those with negative D-dimer results, thus limiting the chances of achieving statistical significance in some results regarding the prevalence of VTE recurrence in subjects remained without anticoagulation. Finally, the investigators who performed follow-up evaluations were not blind to the treatment of the patient.
Conclusions
In a setting of patients with VTE characterized by a high risk of recurrences, the results of this study show that a serial D-dimer procedure, in isolation, fails to identify those in whom anticoagulation can be safely discontinued, whereas reduced-dose apixaban confers protection that is effective and reassuringly safe.
Acknowledgments
Serena Zorzi obtained authorization for the study from the National Health Authority and local ethical committees. Stephen Jewkes corrected the English.
The Bristol-Myers Squibb and Pfizer Alliance provided the 2.5 mg tablets of apixaban used in the study and also provided financial support.
Authorship
Contribution: Conception and design: G.P., P.P., C.L., D.P., S.T., A.T., V.P., and W.A. Analysis and interpretation of the data: G.P., C.L., D.P., P.P., S.T., A.T., L.B., V.P., and W.A. Drafting of the article: G.P. Critical revision of the article for important intellectual content: G.P., D.P., P.P., C.L., W.A., S.T., A.T., L.B., and V.P. Final approval of the article: all the authors. Provision of study materials or patients: D.P., E.B., S.T., O.P., A.C., A.S., I.M., P.B., A.F., A.T., L.S., D.M., B.C., A.V., R.C.S., N.Z., E.G., L.C., and W.A. Administrative, technical, or logistic support: C.L. and E.A. Collection and assembly of data: C.L.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of participating clinical centers appears in “Appendix.”
Correspondence: Gualtiero Palareti, Fondazione Arianna Anticoagulazione, Via Paolo Fabbri 1/3, 40138 Bologna, Italy; e-mail: gualtiero.palareti@unibo.it.
Appendix: participating clinical centers
Daniela Poli, Elena Lotti, Felice Crudele - Malattie Aterotrombotiche, AOU Careggi - Florence (134 recruited cases)
Walter Ageno, Alessia Abenante, Lucia Caiano, Giovanna Colombo, Matteo Guarascio – UOC Pronto Soccorso, Medicina d’Urgenza e Centro Trombosi ed Emostasi, ASST dei Sette Laghi - Varese (70)
Sophie Testa, Emilia Cancellieri, Rossella Morandini, Oriana Paoletti, Silvia Zambelli - Centro Emostasi e Trombosi, UUOO Laboratorio Analisi chimico-cliniche e microbiologiche, ASST Cremona - Cremona (48)
Eugenio Bucherini, Sauro Martini, Monica Vastola - SS Medicina Vascolare e Angiologia, Medicina 2, AUSL Romagna - Ravenna (45)
Antonio Chistolini, Alessandra Serrao - Dipartimento di Medicina Traslazionale e di Precisione Sapienza Università di Roma - Rome (43)
Ida Martinelli, Paolo Bucciarelli, Maria Abbattista, Andrea Artoni, Marco Capecchi, Francesca Gianniello, Barbara Scimeca - Centro Emofilia e Trombosi A. Bianchi Bonomi, Fondazione IRCCS Ca' Granda, Ospedale Maggiore Policlinico - Milan (42)
Anna Falanga, Luca Barcella, Sara Gamba, Teresa Lerede, Anna Maggioni, Francesca Schieppati, Laura Russo, Federica Zunino - Divisione di Immunoematologia e Medicina Trasfusionale & Centro Emostasi e Trombosi, ASST Papa Giovanni XXIII - Bergamo (38)
Alberto Tosetto, Anna Artuso, Stefania Bellesso, Jessica Cadau, Giuseppe Carli, Ilaria Nichele, Omar Perbellini - UOC Ematologia, Centro Malattie Emorragiche e Trombotiche (CMET), AULSS 8 Berica Ospedale S. Bortolo - Vicenza (35)
Luca Sarti, Antonella Caronna, Filippo Gabrielli, Francesca Lami, Alberto Nicolini, Federica Scaglioni - Centro per la diagnosi e la sorveglianza della malattia tromboembolica, UO Medicina interna d'urgenza, Azienda Ospedaliero Universitaria Policlinico di Modena, Ospedale Civile Baggiovara - Modena (32)
Daniela Mastroiacovo, Mauro Pinelli, Giovambattista Desideri - UOSD Angiologia e Diagnostica Vascolare, Dipartimento Medico, Ospedale Civile SS Filippo e Nicola - Avezzano (AQ) (27)
Benilde Cosmi, Laura Borgese, Elisabetta Favaretto, Alessia Libra, Ludovica Migliaccio, Michelangelo Sartori - UO di Angiologia e Malattie della Coagulazione, Azienda Ospedaliero Universitaria di Bologna, Policlinico S. Orsola – Malpighi – Bologna (25)
Adriana Visonà, Chiara Panzavolta, Tatiana Scandiuzzi, Beniamino Zalunardo - UOC Angiologia, Dipatimento di Medicina Clinica, Azienda ULSS 2 Marca Trevigiana, Ospedale San Giacomo Apostolo - Castelfranco Veneto (TV) (20)
Rita Carlotta Santoro, Antonella Ierardi, Marzia Leotta, Alessandra Strangio - Centro Emostasi e Trombosi, Dipartimento Emato- Oncologico, Presidio Ospedaliero deLellis, Az. Osp. “Pugliese-Ciaccio” – Catanzaro (18)
Nello Zanatta, Samuele Guzzon - UOSD di Angiologia, Ospedale di Conegliano - Conegliano (TV) (18)
Elvira Grandone, Donatella Colaizzo, Giovanni Favuzzi - UO Emostasi e Trombosi, Centro Ricerca Poliambulatorio Giovanni Paolo II, IRCCS Ospedale Casa Sollievo della Sofferenza - San Giovanni Rotondo (FG) (17)
Maria Rosa Lombardi, Piera Maria Ferrini, Maria Ilaria Tassoni - Medicina interna a indirizzo angiologico e coagulativo Azienda Ospedaliero-Universitaria di Parma - Parma (12)
Sara Corradini, Matteo Iotti, Isabella Lambertini, Maria Rosaria Veropalumbo - S.C. Medicina Cardiovascolare, Dip. Internistico, AUSL di Reggio Emilia, Arcispedale S. Maria Nuova - Reggio Emilia (11)
Gianfranco Lessiani - Ambulatorio Medicina Vascolare, UOC Medicina e Geriatria, CDC Villa Serena, Città Sant’Angelo (PE) (11)
Roberto Parisi, Cristiano Bortoluzzi, Hong Ngoc Vo - UOSD Ipertensione e Patologie Endocrine Metaboliche Angiologiche, Ospedale SS. Giovanni e Paolo, AULSS3 Serenissima - Venice (11)
Paolo Chiarugi, Monica Casini - Ambulatorio Antitrombosi, U.O Laboratorio Analisi Chimico Cliniche Edificio 2W, Azienda Ospedaliero-Universitaria Pisana, Nuovo S. Chiara - Pisa (10)
Caterina Violo, Marco Nuti - Sezione Dipartimentale Cardio-Angiologia, Dipartimento Cardio-Toraco-Vascolare, Azienda Ospedaliera Universitaria - Pisa (10)
Lucia Angeloni - Ospedale “G. Dossetti” - Dipartimento Cure Primarie Appennino, Reno, Lavino e Samoggia - AUSL Bologna - V.le dei Martiri 10/b, 40053 Valsamoggia (BO) (9)
Laura Carrozzi, Roberta Pancani, Davide Chimera, Valentina Conti, Claudia Meschi - U.O. Pneumologia, Dipartimento Cardiotoraco Vascolare, Azienda Ospedaliero-Universitaria Pisana, Ospedale di Cisanello - Pisa (9)
Marco Cattaneo, Gianmarco Podda, Simone Birocchi - Medicina 3, ASST Santi Paolo e Carlo, San Paolo - Milan (9)
Stefano Cuppini, Marco Marzolo, Marta Milan - UOS Angiologia Medica, AULSS 5 Polesana - Rovigo (9)
Giuliana Martini, Sara Merelli, Sara Pontoglio, Nicola Portesi - Laboratorio Analisi Chimico Cliniche, ASST Spedali Civili di Brescia - Brescia (9)
Sabina Villalta, Lara De Lucchi, Alessandra Sponghiado - Medicina Interna 1, Ospedale Cà Foncello, AULSS2 Marca Trevigiana - Treviso (9)
Cecilia Becattini, Michela Giustozzi, Alessandra Vinci - Medicina Vascolare e D'urgenza, Stroke Unit, Ospedale S. Maria della Misericordia - Perugia (8)
Pasquale Pignatelli, Tommaso Bucci, Danilo Menichelli, Daniele Pastori - Sapienza Università di Roma - Rome (8)
Fulvio Pomero, Sara Casalis, Eleonora Galli - Medicina Interna, Ospedale San Lazzaro - Alba (CN) (8)
Maurizio Ciammaichella, Rosa Maida - UOC Medicina Interna ad Alta Intensità di Cura, Azienda Ospedaliera S. Giovanni Addolorata – Rome (7)
Raimondo De Cristofaro, Maria Adele Alberelli, Maria Rosaria Basso, Erica De Candia, Leonardo Di Gennaro - Dipartimento di Oncologia ed Ematologia, Istituto di Medicina Interna e Geriatria, Centro per emorragie e malattie trombotiche ed emofilia, Ospedale “A. Gemelli”, Università Cattolica - Rome (7)
Nicola Mumoli, Riccardo Capra, Mariantonia Orlando, Cesare Porta, Giuseppe Rotiroti - UOC Medicina Interna, Ospedale Fornaroli, - Magenta (MI) (6)
Monica Demarco, Paola Petrillo - UO Medicina Interna, Humanitas Mater Domini - Castellanza (VA) (5)
Elena Rossi, Francesca Bartolomei, Denise Soldati - Sezione di Ematologia, Dipartimento di Scienze Radiologiche ed Ematologiche, Università Cattolica, Fondazione Policlinico Gemelli, IRCCS - Rome (5)
Umberto Russo, Ilaria Burgo - UOC Ematologia e Medicina Trasfusionale, ASST Fatebenefratelli Sacco - Milan (5)
Maurizio Ziliotti, Corrado Pataccini, Lorenza Terroni, Maria Chiara Ugolotti - Divisione di Medicina, Ospedale di Vaio - Fidenza (PR) (5)
Angela Di Giorgio - Dipartimento di Scienze Cardiovascolari, Fondazione Policlinico Universitario A. Gemelli IRCCS - Rome (4)
Laura Cavagna, Francesca Mete, Miriam Gino - Laboratorio Analisi Unificato Rivoli-Pinerolo/Medicina Interna, Ospedale degli Infermi di Rivoli - Rivoli (TO) (3)
Angelo Santoro, Armando De Carlo - UOC Patologia Clinica e Centro Trombosi, Ospedale Antonio Perrino - Brindisi (3)
Roberto Cappelli, Maurizio Bicchi, Lediona Dyrmo - UOS Angiologia Centro Trombosi, Dipartimento di Scienze Mediche, Chirurgiche e Neuroscienze, Università di Siena - Siena (2)
Elisa Grifoni, Luca Masotti - SOC Medicina Interna II, Ospedale S. Giuseppe, Azienda USL Toscana Centro - Empoli (FI) (2)
Luigi Ria, Marina Spagnolo - UOC Medicina Interna e Centro Trombosi ed Emostasi, P.O. “S. Cuore di Gesù”, ASL LECCE - Gallipoli (LE) (2)
Serena Rupoli, Irene Federici, Erika Morsia, Anna Rita Scortechini, Elena Torre - Clinica di Ematologia, AOU Ospedali Riuniti Umberto I, GM Lancisi, G Salesi - Ancona (2)
Massimo Franchini, Paolo Montorsi - SIMT - Ospedale “Carlo Poma” - Mantua (1)
Giuseppe Galgano, Anna De Luca - UOC Cardiologia e UTIC, Ospedale Generale Regionale “F. Miulli” - Acquaviva delle Fonti (BA) (1)
Maria Lorenza Muiesan, Anna Paini, Deborah Stassaldi - Dipartimento Scienze Cliniche e Sperimentali Università di Brescia/UOC Medicina Generale 2, ASST Spedali Civili di Brescia - Brescia (1)
Vittorio Pengo, Gentian Denas - Clinica Cardiologica, Azienda Ospedaliera di Padova - Padua (1)
Raffaele Pesavento, Davide Ceccato - U.O.C. Clinica Medica 3, Azienda Ospedale Università di Padova – Padua (1)
References
Author notes
For original data, please contact gualtiero.palareti@unibo.it.