Key Point
In myeloablative allogeneic HCT recipients, pretransplant LRD by any virus was associated with increased mortality.
Abstract
Pretransplant respiratory virus infections (RVIs) have been shown to negatively affect hematopoietic cell transplantation (HCT) outcomes. The impact of and need for delay of HCT for pretransplant infection with human rhinovirus (HRV) or endemic human coronavirus (HCoV; 229E, OC43, NL63, and HKU1) remain controversial. We analyzed the impact of symptomatic RVI within ≤90 days before HCT on overall mortality, posttransplant lower respiratory tract disease (LRD), and days alive and out of hospital (DAOH) by day 100 post-HCT in multivariable models. Among 1,643 adult HCT recipients (58% allogeneic recipients), 704 (43%) were tested for RVI before HCT, and 307 (44%) tested positive. HRV was most commonly detected (56%). Forty-five (15%) of 307 HCT recipients had LRD with the same virus early after HCT. Pretransplant upper respiratory tract infection (URI) with influenza, respiratory syncytial virus, adenovirus, human metapneumovirus, parainfluenza virus, HRV, or endemic HCoV was not associated with increased overall mortality or fewer DAOH. However, in allogeneic recipients who received myeloablative conditioning, LRD due to any respiratory virus, including HRV alone, was associated with increased overall mortality (adjusted hazard ratio, 10.8 [95% confidence interval, 3.29-35.1] for HRV and 3.21 [95% confidence interval, 1.15-9.01] for all other viruses). HRV LRD was also associated with fewer DAOH. Thus, the presence of LRD due to common respiratory viruses, including HRV, before myeloablative allogeneic HCT was associated with increased mortality and hospitalization. Pretransplant URI due to HRV and endemic HCoV was not associated with these outcomes. Improved management strategies for pretransplant LRD are warranted.
Introduction
Respiratory virus infections (RVIs) cause significant morbidity and mortality in hematopoietic cell transplantation (HCT) recipients.1-9 The negative impact of infection before HCT with respiratory syncytial virus (RSV), parainfluenza virus (PIV), adenovirus (ADV), human metapneumovirus (HMPV), and influenza (FLU) viruses is well established, and delay of HCT is recommended when feasible.10-13 One prospective study suggested that pretransplant infection with the 2 most commonly detected respiratory viruses, human rhinovirus (HRV) and endemic human coronavirus (HCoV; 229E, OC43, NL63, and HKU1),14 may also have an impact on transplant outcomes, including overall mortality; they may also be associated with increased health care utilization–measured reduction in days alive out of hospital (DAOH), a commonly used measure accounting for the competing outcome of mortality.15 A recent study found no direct association between pretransplant RVI and mortality in adult HCT patients.16 Using meta-transcriptomic sequencing of pre-HCT bronchoalveolar lavage (BAL) samples, a third study identified distinct signatures of viral infection and commensal bacterial depletion that were associated with significantly increased post-HCT lung injury and fatal lung injury.17 To date, studies have been limited by small size, precluding multivariable statistical analysis of factors associated with outcome such as location of infection or conditioning regimen.
Although delay for RSV, PIV, HMPV, ADV, and FLU RVI is routinely considered,10,11 uncertainty remains on how to manage HRV and HCoV infections. Given the high prevalence of HRV and HCoV in the pretransplant setting, affecting ∼15% of transplant candidates tested for respiratory viruses,14 a recommendation to delay HCT has significant implications with potentially adverse outcomes, including progression of underlying disease, unavailability of the optimal stem cell donor, logistical issues for patients requiring relocation to referral cancer centers, or emergence of other pretransplant complications during this delay. Therefore, more data are needed to define which manifestations of these common infections (ie, upper respiratory tract infection [URI] vs lower respiratory tract disease [LRD]) require a delay of HCT, and whether certain transplant conditions are risk factors for progressive infection early after transplantation.
Our objective was to examine the impact of pretransplant RVI in a large, independent cohort of HCT recipients who were routinely evaluated by using multiplex polymerase chain reaction (PCR) when respiratory symptoms occurred before HCT.
Methods
Patients
Adult autologous and allogeneic HCT recipients undergoing transplant from March 2010 to March 2016 at the Fred Hutchinson Cancer Center were included. Laboratory results and radiology reports were retrieved from the database, and retrospective chart reviews were performed for clinical information. HCT-comorbidity index (HCT-CI) score was calculated in all patients.18 Only patients who received their first HCT during the study period were included.
HCT recipients were grouped as follows: (1) symptomatic patients tested for RVI within ≤90 days to 1 day before transplant; and (2) asymptomatic patients who were not tested. For RSV, PIV, HMPV, ADV, and FLU viruses, HCT was delayed when feasible. For HRV and endemic HCoVs (229E, OC43, NL63, and HKU1), HCT was not routinely delayed (supplemental Figure 1). The lowest lymphocyte count was recorded between day −30 and day −7 before HCT.
The study was approved by the Institutional Review Board at the Fred Hutchinson Cancer Research Center and was conducted according to the Declaration of Helsinki.
Definition of respiratory infections and transplant outcomes
RVI status was classified as either URI or LRD.8,19 URI was defined as respiratory infection confined to the nose, throat, and sinuses as confirmed by respiratory virus detection with URI symptoms but no pulmonary infiltrates. LRD was further classified as (modified from prior definitions): (1) possible infection, respiratory virus detection in upper respiratory tract with new pulmonary infiltrates and with LRD signs and symptoms (eg, cough, wheezing, rales, tachypnea, shortness of breath, dyspnea, or hypoxia); (2) probable infection, respiratory virus detection in the lung with LRD symptoms without new pulmonary infiltrates; and (3) proven infection, respiratory virus detection in the lung with new pulmonary infiltrates with or without LRD symptoms. We also evaluated the possible infection category of LRD as with or without LRD signs and symptoms.
Transplant outcomes included overall mortality by day 100 post-HCT and 3 years’ post-HCT in all HCT recipients, in allogeneic recipients alone, and in allogeneic recipients with myeloablative conditioning separately. DAOH was evaluated by day 100 in allogeneic recipients alone and allogeneic recipients with myeloablative conditioning separately. DAOH was not evaluated in autologous recipients given the shorter period of local follow-up in this population. HCT recipients were also examined for development of posttransplant LRD within 100 days when local follow-up data were available.
Laboratory testing for respiratory viruses
Nasopharyngeal/nasal swab samples were collected when HCT recipients had URI symptoms, and a BAL sample was obtained when patients had LRD symptoms and a radiographic abnormality, at physician discretion. All clinical samples were tested by qualitative laboratory-developed PCR assays for 12 respiratory viruses.14 Samples were considered positive if the PCR amplification plot crossed the threshold at <40 cycles. Qualitative results from clinical specimens were reported to physicians. PCR methods were performed according to the standards of the College of American Pathologists.
Statistical analysis
The Kaplan-Meier estimation method was used to estimate the incidence of overall mortality by day 100 and 3 years’ posttransplant among all subjects, allogeneic recipients, and allogeneic recipients with myeloablative conditioning separately. Cox proportional hazards regression models were used to examine the association between candidate risk factors and overall mortality, and linear regression models to evaluate the association between candidate risk factors and DAOH. Demographic and pretransplant clinical factors evaluated as potential risk factors included: patient age, race, sex, donor relationship, cell source, conditioning regimen, cytomegalovirus serostatus, underlying disease risk,20 HCT-CI score, T-cell depletion (in vivo or ex vivo), and lowest lymphocyte counts 30 to 7 days before HCT. We created a composite variable for the presence of symptoms, virus type, and virus location at the time of HCT to illustrate the varying degrees of association depending on whether each factor was present singly or in combination. Variables with P ≤ .2 in univariable analysis were candidates for inclusion in the multivariable models and were retained in the models if P values were < .1 or modified the effect of another factor. Statistical significance was defined as a two-sided P value <.05. SAS version 9.4 TS1M3 (SAS Institute, Inc.) was used for all statistical analyses.
Results
Pretransplant viral detection
Of 1643 adult HCT recipients, 704 (43%) were tested (supplemental Table 1) and 307 (307 of 704 [44%]; 307 of 1,643 [19%]) had pretransplant RVI documented. HRV was most commonly detected in these patients (173 of 307 [56%]); 250 (81%) of 307 had URI alone and 57 (19%) had LRD. No copathogens were identified among cases of possible LRD. Among 57 patients with LRD, 44 (77%) were possible with symptoms, 2 (4%) probable, and 11 (19%) proven infections. Viruses detected are presented in supplemental Table 2.
Of 946 (58%) allogeneic recipients, 382 (40%) were tested and 155 (41%) had respiratory virus detection with a median last positive day before HCT of −25.0 (interquartile range, −42.0 to –15.0 days) (Table 1). Of 155 allogeneic recipients with pretransplant RVI, HRV was also most commonly detected (80 of 155 [52%]). Among the 155 patients with pretransplant RVI, 116 (75%) had URI, and 39 (25%) had LRD. Among 39 allogeneic recipients with pretransplant LRD, 30 (77%) had possible, 1 (2%) had probable, and 8 (21%) had proven infection. Viruses detected are presented in supplemental Table 2.
Transplant outcomes
Overall mortality.
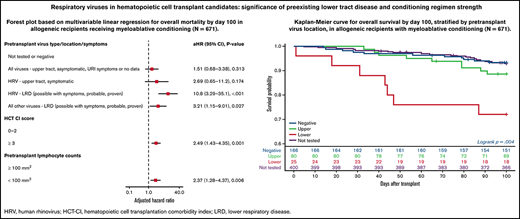
One hundred recipients in the study (of 1643 [6%]) died by day 100. Among the 946 allogeneic recipients, 79 (8%) died by day 100. Overall survival curves by day 100 according to pretransplant virus location are shown in all allogeneic HCT recipients (supplemental Figure 2A), allogeneic recipients with myeloablative conditioning (supplemental Figure 2B), and allogeneic recipients with non-myeloablative conditioning (supplemental Figure 2C). Allogeneic recipients with pretransplant LRD had a significantly lower probability for survival by day 100 (P = .0006); this effect was mainly driven by patients receiving myeloablative conditioning (P = .004). Comparative analyses for autologous recipients were not performed due to an insufficient number of deaths for statistical analysis. Patients with pretransplant LRD also had lower overall survival at 3 years, among both allogeneic HCT recipients and allogeneic HCT recipients receiving myeloablative conditioning (P = .012 and 0.014, respectively) (supplemental Figure 3).
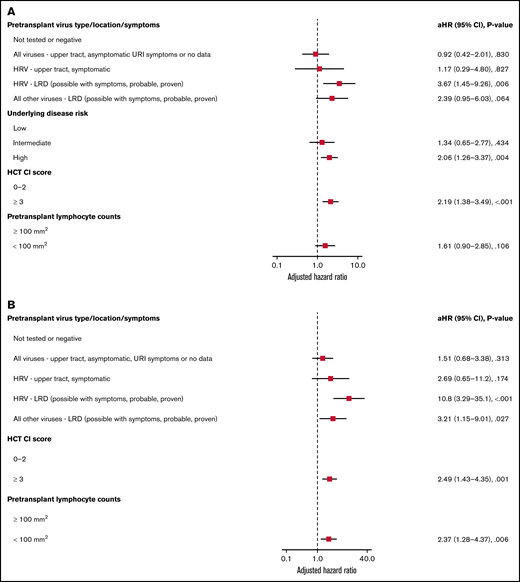
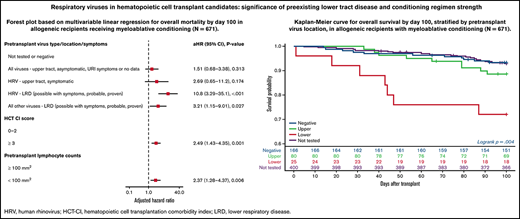
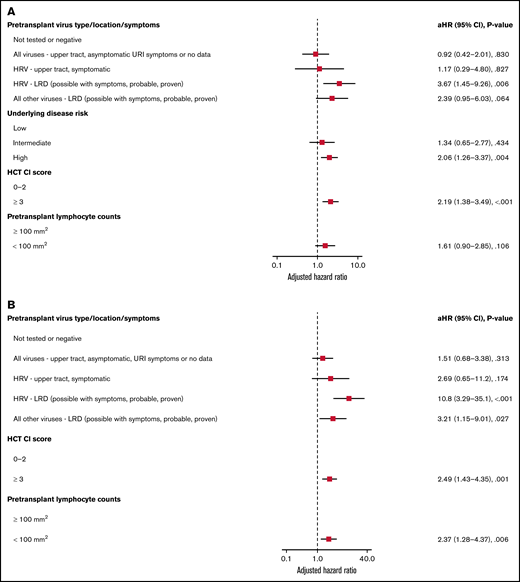
Forest plots based on multivariable Cox regression analyses for overall mortality by day 100 in all allogeneic recipients are shown in Figure 1A; pretransplant LRD by HRV (hazard ratio [HR], 3.67; P = .006), high-risk underlying disease (HR, 2.06; P = .004), and HCT-CI score ≥3 (HR, 2.19; P < .001) were associated with increased mortality. In a separate model with age detached from HCT-CI score, pretransplant HRV LRD (HR, 3.67; P = .006), high-risk underlying disease (HR, 2.06; P = .004), and HCT-CI score ≥3 (HR, 2.15; P = .001) were associated with increased mortality (supplemental Figure 4A).
Overall mortality by day 100 in allogeneic recipients. Forest plots based on multivariable Cox regression for overall mortality by day 100 in all allogeneic recipients (A, n = 946) and in allogeneic recipients receiving myeloablative conditioning (B, n = 671). HRV, human rhinovirus; HCT-CI, hematopoietic cell transplantation comorbidity index; LRD, lower respiratory tract disease.
Overall mortality by day 100 in allogeneic recipients. Forest plots based on multivariable Cox regression for overall mortality by day 100 in all allogeneic recipients (A, n = 946) and in allogeneic recipients receiving myeloablative conditioning (B, n = 671). HRV, human rhinovirus; HCT-CI, hematopoietic cell transplantation comorbidity index; LRD, lower respiratory tract disease.
Forest plots based on multivariable Cox regression analyses for overall mortality in allogeneic recipients with myeloablative conditioning only are shown in Figure 1B; pretransplant LRD by HRV (HR, 10.8; P < .001), LRD due to all other viruses (HR, 3.21; P = .027), HCT-CI score ≥3 (HR, 2.49; P = .001), and pretransplant lymphocyte count <100/mm3 (HR, 2.37; P = .006) were associated with increased mortality. In a separate model with age detached from HCT-CI score, pretransplant LRD by HRV (HR, 8.94; P < .001), LRD due to all other viruses LRD (HR, 3.56; P = .018), age > 60 years (HR, 2.35; P = .01), and high-risk underlying disease (HR, 3.79; P < .001) were associated with increased mortality (supplemental Figure 4B).
When we evaluated the subjects with possible LRD with or without LRD signs and symptoms, the results were qualitatively similar, but the effect was less pronounced (supplemental Figures 5 and 6). All patients with possible LRD who did not have LRD symptoms survived.
In univariable analyses, age at transplant, high-risk underlying disease, high HCT-CI score, and lower lymphocyte counts were associated with 3-year mortality (supplemental Table 3). LRD due to any virus continued to show a trend toward association with mortality compared with negative or not tested individuals. However, only LRD due to HRV was significant among all allogeneic HCT recipients, whereas LRD due to any other virus was significant in the myeloablative group.
Days alive and out of hospital.
In allogeneic recipients, median DAOH was 80 days (interquartile range, 69-88 days; data not shown). There was no difference for DAOH between patients not tested for respiratory viruses pretransplant and those tested (P = .09; data not shown). DAOH for autologous recipients could not be accurately calculated because many of these patients were treated at outpatient clinics and/or did not have prolonged follow-up within our center.
In allogeneic recipients, there was no difference in DAOH for not tested or negative, presence of symptoms, virus type, or virus location (URI vs LRD; data not shown).
Allogeneic recipients with myeloablative conditioning generally had shorter DAOH compared with allogeneic recipients with non-myeloablative conditioning, although this finding was not significant (data not shown). In addition, among all allogeneic recipients, there was a trend for shorter DAOH among recipients with myeloablative conditioning who had LRD due to HRV, but there was no significant difference between myeloablative and non-myeloablative recipients with all variables.
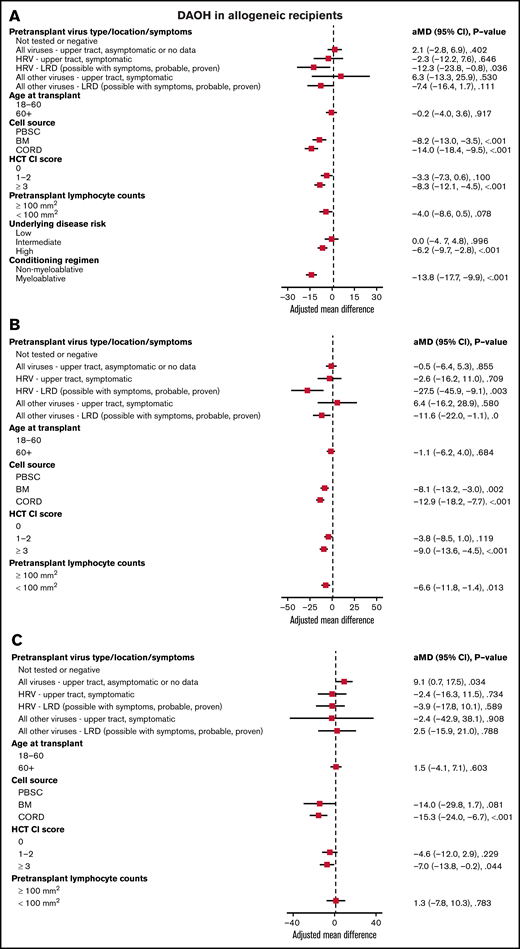
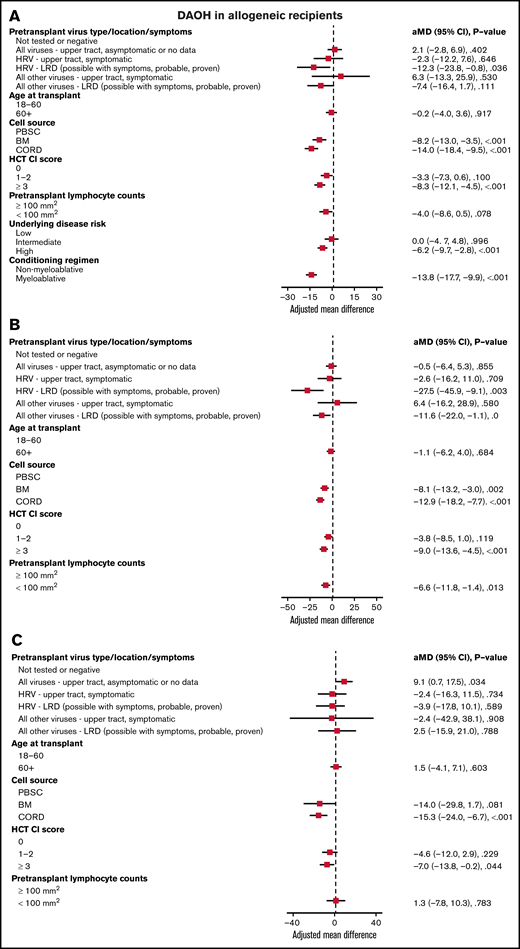
In all allogeneic recipients, LRD by HRV (mean difference [MD], −12.3; P = .036), bone marrow (MD, −8.2; P < .001), or cord blood (MD, −14.0; P < .001) as a cell source, HCT-CI score ≥3 (MD, −8.3; P < .001), high-risk underlying disease (MD, −6.2; P < .001), and myeloablative conditioning regimen (MD, −13.8; P < .001) were associated with shorter DAOH (Figure 2A). In allogeneic recipients with myeloablative conditioning, LRD by HRV (MD, −27.5; P = .003), bone marrow (MD, −8.1; P = .002), or cord blood (MD, −12.9; P < .001) as a cell source, HCT-CI score ≥3 (MD, −9.0; P < .001) and pretransplant lymphocyte count <100/mm3 (MD, −6.6; P = .013) were associated with shorter DAOH (Figure 2B). For LRD due to viruses other than HRV, there was a trend toward fewer DAOH. In allogeneic recipients with non-myeloablative conditioning, cord blood as a cell source (MD, −15.3; P < .001) and HCT-CI score ≥3 (MD, −7.0; P = .044) were associated with shorter DAOH, whereas pretransplant RVI was not (Figure 2C).
Days alive and out of hospital (DAOH) in allogeneic recipients. Forest plot based on multivariable linear regression for DAOH in all allogeneic recipients (A), in myeloablative allogeneic recipients (B), and in allogeneic recipients receiving non-myeloablative conditioning (C, n = 275). HRV, human rhinovirus; HCT-CI, hematopoietic cell transplantation comorbidity index; LRD, lower respiratory tract disease.
Days alive and out of hospital (DAOH) in allogeneic recipients. Forest plot based on multivariable linear regression for DAOH in all allogeneic recipients (A), in myeloablative allogeneic recipients (B), and in allogeneic recipients receiving non-myeloablative conditioning (C, n = 275). HRV, human rhinovirus; HCT-CI, hematopoietic cell transplantation comorbidity index; LRD, lower respiratory tract disease.
Symptomatic patients at the time of HCT and posttransplant outcomes.
In all 1643 HCT recipients, and of 307 patients with RVI, only 41 (41 of 1,643 [2.5%]; 41 of 307 [13%]) patients were still symptomatic at time of HCT. In 946 allogeneic HCT recipients, and of 155 patients with RVI, 22 (22 of 946 [2%]; 22 of 155 [14%]) patients were still symptomatic at the time of HCT (supplemental Table 4).
Among 41 symptomatic patients at the time of HCT, 37 (90%) had pretransplant URI only and 4 (10%) had pretransplant LRD. Among the 37 patients with pretransplant URI, 2 (5%) continued to have URI posttransplant, 11 (30%) developed LRD posttransplant, and 24 (65%) did not have any viral detection during posttransplant period. Among the 4 patients with pretransplant LRD, 2 (50%) had LRD posttransplant and 2 did not have any viral detection during the posttransplant period.
Among 22 symptomatic allogeneic recipients at the time of HCT, 19 (86%) had pretransplant URI only and 3 (14%) had pretransplant LRD. Among the 19 patients with pretransplant URI, 2 (10%) had URI posttransplant, 6 (32%) developed LRD posttransplant, and 11 (58%) did not have any viral detection in the 100 days posttransplant. Among the 3 patients with pretransplant LRD, 2 (67%) had LRD posttransplant, and 1 did not have any viral detection during the posttransplant period.
LRD early after transplantation among all HCT recipients.
Among 307 patients with pretransplant RVI, 45 patients (14.7%, both allogeneic and autologous HCT recipients) had posttransplant LRD caused by the same virus by day 100 (33 possible, 2 probable, and 10 proven). HRV was responsible for possible posttransplant LRD in 26 patients, probable LRD in 1 patient, and proven LRD in 8 patients (Table 2). Detailed information on 12 patients who developed probable or proven LRD posttransplant within 100 days with the same pretransplant virus is shown in supplemental Table 5.
We evaluated possible risk factors for posttransplant progression to LRD among patients with pretransplant RVI. Univariable Cox regression analysis of 155 allogeneic recipients showed that high underlying disease risk was associated with LRD by posttransplant day 100 (data not shown). Detectable lymphopenia (<100/mm3) at posttransplant day 30 was also associated with higher risk of LRD between day 30 and day 100 (P < .05; data not shown).
In addition, we assessed the frequency with which transplantation was delayed for reasons related to respiratory infection. We observed that delay was less frequent in patients with HRV infections (18% vs 53% of patients positive for infection with HRV vs other viruses, respectively) (supplemental Table 6). The potential effects of transplantation delay were also evaluated. Among those who received myeloablative conditioning, there was no apparent difference in overall mortality and nonrelapse mortality between those who had their transplant delayed compared with those who did not (supplemental Figure 7).
Discussion
In this study, the impact of pretransplant symptomatic RVI on posttransplant LRD, overall mortality, and DAOH was investigated in a large cohort of 1,643 adult HCT recipients. We found that URI due to viruses such as RSV, PIV, FLU viruses, ADV, and HMPV, for which delay is typically recommended, were not associated with poor posttransplant outcomes. Likewise, pretransplant HRV or HCoV URI was not associated with poor outcomes. LRD due to any virus was associated with increased mortality in allogeneic recipients, with the effect primarily seen in recipients of myeloablative conditioning. In addition, pretransplant LRD due to HRV alone was associated with increased overall mortality in all allogeneic HCT recipients in multivariable models. Pretransplant LRD due to HRV was also associated with shorter DAOH in all allogeneic recipients, again with the effect primarily seen in recipients of myeloablative conditioning.
The morbidity and mortality of RVIs in HCT recipients have been frequently reported with respiratory viruses known to cause severe disease such as RSV, PIV, FLU viruses, and ADV.21 Numerous efforts have been made to identify strategies to decrease the negative impact of RVIs on transplant outcomes. With advances in diagnostics, including the widespread use of multiplex respiratory virus PCR,22,23 screening for respiratory viruses before HCT is performed in several centers but not routinely recommended in clinical guidelines due to the balance of cost and clinical benefit.10,24 However, pretransplant RVI has been shown to have a negative impact on HCT outcomes, and delay of HCT is often recommended for RSV, PIV, influenza viruses, ADV, and HMPV.10,11 Whether delay is needed for HRV and HCoV, the 2 most commonly detected respiratory viruses, remains controversial. Our group previously reported in a prospective study that allogeneic recipients with pretransplant RVI had fewer DAOH and increased overall mortality when symptomatic.14 Of note, this negative impact was also observed in patients with HRV alone.
Because HRV is the most commonly detected virus in HCT recipients and was previously considered as a cause of only mild respiratory infection, clarification of the significance of pretransplant HRV infection has become more crucial. One key finding of the present study, that HRV LRD pretransplant but not URI adversely affects posttransplant outcomes, is therefore a significant advance in our understanding of pretransplant RVIs. These results potentially could limit patients from having unnecessary delays while identifying a high-risk group that should be considered for delay of transplantation. In the context of the continuing COVID-19 pandemic, our findings also underscore the importance of continuing to screen for other RVIs (in addition to SARS-CoV-2) in HCT candidates presenting with respiratory symptoms to correctly identify the infectious etiology and assess potential negative impacts. In addition, our results suggest that HCT candidates with pretransplant HRV should undergo careful clinical assessment for LRD, which may include thorough physical examination, imaging, and/or BAL. Importantly, the present study found that possible LRD with LRD signs and symptoms had a greater effect on outcomes, suggesting that the presence of LRD symptoms is an important risk factor that should be considered when evaluating patients for HCT.
Our data are consistent with a study by Versluys et al, which suggested that pretransplant respiratory virus positivity in BAL (mainly HRV) was a predictor posttransplant of alloimmune-mediated lung syndrome, or idiopathic lung syndrome, in pediatric HCT recipients.13,25 No difference in predictive value was found between various viral species in the lower respiratory tract, consistent with our finding that the impact of HRV lower respiratory tract infection on outcomes is comparable to that of other respiratory viruses. That study, in contrast with ours, did not identify an association between cord blood as a cell source and improved outcomes. However, Versluys et al observed a trend toward a protective effect of grade II to IV acute graft-versus-host disease related to steroid treatment; for our data set, steroid data were not available, precluding analysis of its effect.
The present study adds to the evidence that HRV is a significant pathogen in the HCT setting, especially when there is clinical LRD or virus is detected in the lower respiratory tract. Seo et al26 observed that among 569 HCT recipients with HRV URI and 128 HCT recipients with HRV LRD, the probabilities of overall mortality at 90 days were 6% and 41%, respectively. Mortality after HRV LRD was similar to that after LRD by RSV, PIV, or influenza viruses in an adjusted model, meaning that transplant recipients with HRV detection in the lower respiratory tract had high mortality rates comparable to viral pneumonia associated with other well-established respiratory viruses. In addition, other prospective studies showed that HCT recipients with HRV infection shed virus for a median duration of 3 weeks,27 and an initially high viral load was associated with prolonged shedding.28 Finally, even posttransplant HRV URI can be clinically significant as it can progress to LRD.29,30
URI due to any of the 4 types of endemic HCoVs, which occurred in 38 patients pretransplant (5% among HCT recipients tested for respiratory viruses and 2% among all HCT recipients), did not appear to be associated with the most severe outcomes (mortality) or DAOH, although progression to LRD early after HCT was occasionally observed (Table 2). Only limited literature regarding HCoV infection in HCT recipients is available.31,32 Our study was conducted before SARS-CoV-2 emerged33 but can serve as a benchmark once data on pretransplant SARS-CoV-2 become available. Interestingly, the current epidemiology of RVIs during the COVID-19 pandemic has revealed ongoing detection of HRV, whereas detection of all the other respiratory viruses has been reduced significantly.34-36 HRV transmission seems to be highly difficult to prevent in the community, even with ongoing social distancing at the community level and increased health care–associated infection prevention practices. Therefore, the impact of pretransplant HRV detection on posttransplant outcomes remains highly relevant.
Our study has strengths and limitations. The large sample size and the systematic virologic evaluation allowed us to separate URI from LRD, a key strength that adds to our understanding of specific high-risk scenarios. However, the retrospective nature of the study and our inability to evaluate possible differences between possible, probable, and proven LRD are limitations. Moreover, due to the limited number of cases for some viruses, our study was not powered to evaluate the negative effect of viruses other than HRV (eg, HMPV, PIV), which may nonetheless herald poor outcomes. We were unable to assess the impact of viral shedding duration or cycle threshold values; however, cycle threshold values have not been shown to be associated with progression to LRD in other studies.3,5,25 In addition, it is possible that defining the pretransplant period as up to 90 days may have interfered with precise differentiation of patients into the RVI vs asymptomatic control group in some cases, due to the potential for underreporting of early RVIs. This study shows that URI due to any respiratory virus is not associated with poor transplant outcomes in general, including HRV and endemic HCoVs. For the 5 major respiratory viruses, current recommendations to delay HCT, even with URI alone, likely contribute to the lack of impact on posttransplant outcomes. We were unable to assess the impact of transplant delay directly on mortality and length of hospitalization, given the diverse clinical scenarios that determine decisions to proceed with transplantation.
In conclusion, we found that the presence of pretransplant HRV LRD was associated with negative HCT outcomes, especially among allogeneic recipients with myeloablative conditioning, whereas HRV and endemic HCoV URI were not. LRD manifestations with all viruses continue to represent a clinical problem. Our study provides data to develop rational management strategies, which may include transplant delays and less toxic conditioning regimens when possible until effective antiviral agents become available.
Acknowledgments
The authors thank Rachel Blazevic, Elizabeth Nguyen, Jennifer Schaeffer, Lisa Chung, Sonia Goyal, Larry Mose, and Erika Lovas for assistance with chart review, and Ashley Sherrid for editorial assistance.
Nonauthor contributors included the following: Chris Davis, Zach Stednick, E. Lisa Chung, and Sonia Goyal, all from the Fred Hutchinson Cancer Center.
This work was supported by the National Institutes of Health (National Heart, Lung, and Blood Institute, K24 HL093294, M.B.; National Institute of Allergy and Infectious Diseases, K23 AI114844 [A.W.] and K23 AI139385 [C.O.]), and institutional support from the Seattle Cancer Care Alliance.
Authorship
Contribution: Y.-J.K. and A.W. performed the research, collected data, analyzed data, and wrote the manuscript; H.X. and W.M.L. analyzed data and critically reviewed the manuscript; C.O. collected data and critically reviewed the manuscript; L.H., S.A.P., K.R.J., A.P.C., and J.A.E. critically reviewed the manuscript; M.B. designed and performed the research, analyzed data, provided resources, and wrote the manuscript; and all authors had access to the data.
Conflict-of-interest disclosure: Y.-J.K. reports research funding from Ansun Biopharma and Merck. A.W. reports research funding from Ansun Biopharma, AlloVir, Amazon, Inc., and VB Tech; and serves on the advisory board for Kyorin Pharmaceuticals, all outside of the submitted work. L.H. reports research funding from Seattle Genetics, Sanofi, Millennium-Takeda, Bristol Myers Squibb, Merck, and Janssen, all outside of the submitted work. S.A.P. reports research funding from Global Life Technologies, Inc.; and has participated in research trials with Chimerix, Inc., and Merck. S.A.P. also participated in a clinical trial sponsored by the National Institute of Allergy and Infectious Diseases (U01-AI132004); vaccines for this trial are provided by Sanofi-Aventis. All are outside of the submitted work. J.A.E. reports research funding from Merck, AstraZeneca, GlaxoSmithKline, and Pfizer; and is a consultant for Sanofi Pasteur and Meissa Vaccines, all outside of the submitted work. M.B. reports research funding from Ansun Biopharma, Janssen, Amazon, Inc., VB Tech, Gilead Sciences, Vir Biotechnology, and Merck; and received consulting fees from ReViral, Janssen, Gilead, Symbio, Kyorin Pharmaceuticals, and Merck, all outside of the submitted work. The remaining authors declare no competing financial interests.
The current affiliation for A.P.C. is Centers for Disease Control and Prevention, Atlanta, GA.
The current affiliation for C.O. is Pediatric Infectious Diseases, National Center for Child Health and Development, Tokyo, Japan.
The current affiliation for Y.-J.K. is Division of Infectious Diseases, Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University, Seoul, Republic of Korea.
Correspondence: Alpana Waghmare, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, 1100 Fairview Ave N, Seattle, WA 98109; e-mail: awaghmar@fredhutch.org.
References
Author notes
Y.-J.K. and A.W. contributed equally to this study.
Presented in part at the American Society of Blood and Marrow Transplant Tandem Meeting, Orlando, FL, 22-26 February 2017.
Requests for original data may be submitted to the corresponding author (Alpana Waghmare; e-mail: awaghmar@fredhutch.org).
The full-text version of this article contains a data supplement.