Key Points
PD-L1 structural variants are recurrent in mycosis fungoides with large cell transformation.
PD-L1 structural variants in relapsed/refractory mycosis fungoides should prompt consideration of treatment with PD-1 inhibitors.
Introduction
Treatment of advanced mycosis fungoides (MF) and Sézary syndrome (SS) remains a clinical challenge. Despite the addition of new therapeutic options, meaningful clinical responses are achieved in only a minority of unselected patients.1-5 The heterogeneity in clinical response to therapies may reflect the molecular heterogeneity of MF/SS.6,7 Personalized treatment based on genomic features may improve clinical outcomes. Identification of clinically relevant genomic biomarkers is needed to help prioritize treatment selections.
Genetic disruptions of the PD1/PD-L1 pathway have been identified in cutaneous T-cell lymphomas (CTCL). Among these events are structural variants (SVs) involving CD274, the gene encoding PD-LI, which was previously described in 3 patients.8,9 Similar recurrent alterations causing disruption of the 3′ untranslated region (UTR) of PD ligands have been identified in other T-cell lymphoma subtypes, most notably virus-associated lymphomas including adult T-cell leukemia lymphoma and extranodal natural killer/T-cell lymphomas.10,11 The 3′ UTR disruption interferes with the binding site for downregulatory microRNAs and consequently enhances PD-L1 protein expression.11
Immune checkpoint blockade with the PD-1 inhibitor pembrolizumab demonstrated a 38% response rate in patients with relapsed/refractory MF/SS in a phase 2 trial.12 Although some patients experience a remarkably durable response to pembrolizumab, no predictive biomarkers have yet been identified to select patients that are likely to benefit. We hypothesized that genomic alterations of PD-L1 may predict response to PD-1 targeting therapy in CTCL. We applied a targeted next-generation sequencing (NGS) panel to identify PD-L1 SVs in patients with CTCL and assessed their response to pembrolizumab-based therapy. Here, we report the outcome of 3 patients with CTCL and PD-L1 SVs treated with pembrolizumab.
Methods
We identified patients from the Stanford Multidisciplinary Cutaneous Lymphoma clinic between August 2018 and April 2020 who had skin biopsies or blood analyzed by the Stanford Actionable Mutation Panel for Hematopoietic and Lymphoid Neoplasms, which is a Clinical Laboratory Improvement Amendments–certified NGS panel. This panel includes all CD274 coding exons and continuous coverage of exon 5 through the 3′ UTR. SVs were detected by Fusion And Chromosomal Translocation Enumeration and Recovery Algorithm (FACTERA).13 Four patients with PD-L1 SVs were identified, and no other coding exonic mutations were found. All variants were validated by polymerase chain reaction and Sanger sequencing of the known breakpoints. Of these 4 patients, 3 were treated with pembrolizumab-based therapy. Clinical response was assessed according to the international consensus criteria.14
This study was approved by the institutional review board at Stanford University.
PD-L1 structural variants
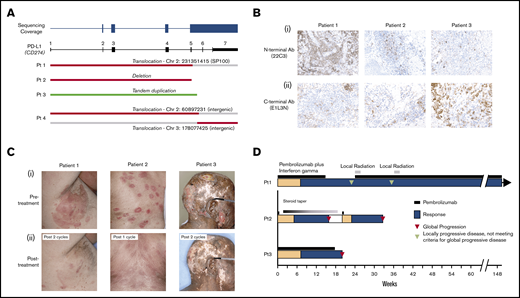
Skin biopsies and/or peripheral blood from 69 patients with MF or SS and 12 patients with other subtypes of CTCL were sequenced. Of 35 patients with MF, 11 had large cell transformation (LCT). A total of 4 patients with PD-L1 SVs were identified, and all 4 had a diagnosis of MF with LCT. Breakpoints from the 4 patients clustered within exon 5 and intron 5 and all resulted in disruption of exons 6 to 7 and the 3′ UTR (Figure 1A). There were 2 interchromosomal translocations including a complex rearrangement involving chromosomes 2 and 3, 1 deletion, and 1 tandem duplication. All evaluated patients expressed PD-L1 as assessed by immunohistochemistry (Figure 1B). Of note, in 1 patient, anti–PD-L1 antibody targeting the N terminus (22C3) revealed strikingly higher expression compared with the C terminus antibody (E1L3N).
Pembrolizumab treatment of mycosis fungoides with PD-L1 structural variants. (A) Depiction of discovered PD-L1 structural variants. (B) Immunohistochemical staining of PD-L1 using 22C3 (i) and E1L3N (ii) clones for 3 pembrolizumab-treated patients. (C) Photographs of 3 patients with MF and LCT treated with pembrolizumab-based therapy before (i) and after (ii) treatment. (D) A swimmer’s plot representation of the responses to pembrolizumab-based therapy.
Pembrolizumab treatment of mycosis fungoides with PD-L1 structural variants. (A) Depiction of discovered PD-L1 structural variants. (B) Immunohistochemical staining of PD-L1 using 22C3 (i) and E1L3N (ii) clones for 3 pembrolizumab-treated patients. (C) Photographs of 3 patients with MF and LCT treated with pembrolizumab-based therapy before (i) and after (ii) treatment. (D) A swimmer’s plot representation of the responses to pembrolizumab-based therapy.
Treatment responses
Three of the 4 patients with PD-L1 SVs received treatment with pembrolizumab-based therapy (Table 1). One patient had a history of an orthotopic cardiac transplant for a hypoplastic left heart and therefore was not treated with pembrolizumab. One patient was enrolled and treated in a phase 2 clinical trial of pembrolizumab combined with interferon γ (IFNγ; NCT03063632). The remaining 2 patients were ineligible for the clinical trial and were treated with pembrolizumab monotherapy.
All 3 patients treated with pembrolizumab experienced rapid clinical responses (Figure 1B-C). Patient 1, who was treated with pembrolizumab and IFNγ, subsequently experienced localized progression of skin disease after 6 cycles of treatment. He discontinued the clinical trial and received radiation to 2 sites of localized skin disease progression. Because of durable clinical benefit in other areas, pembrolizumab monotherapy was continued in addition to involved-site radiation therapy. He has since received >110 weeks of pembrolizumab monotherapy without the need for additional radiation or other systemic treatments.
Patient 2 had relapsed MF after an allogeneic stem cell transplant. Because of limited treatment options, he was treated with pembrolizumab monotherapy. He received a single dose of pembrolizumab with rapid clinical response. However, his course was complicated by pneumonitis that resolved with a prolonged steroid taper. Four months later, his disease relapsed, prompting a second single treatment with pembrolizumab. He again experienced an excellent partial response to this single dose of treatment. Because of a recurrent pneumonitis, pembrolizumab was permanently discontinued.
The third patient attained a near-complete remission of his disease with resolution of prior ulcerative lesions. He subsequently progressed with new ulcerative skin lesions, prompting treatment termination after 18 weeks.
Results and discussion
Our findings suggest that PD-L1 SVs may identify CTCL patients susceptible to anti–PD-1–based immunotherapy. The only other reported case of MF with a PD-L1 SV treated with the PD-1 inhibitor nivolumab resulted in a durable response lasting almost a year before treatment discontinuation because of adverse events.9 Two patients in this study developed progression within 6 months of starting treatment, suggesting that patients with PD-L1 SVs may benefit from combinatorial treatments to prolong durability of response. Radiation therapy to progressing lesions did result in a subsequent prolonged response lasting over 2 years for 1 patient, and thus the addition of radiotherapy may be considered to prolong responses. The mechanism of acquired resistance to pembrolizumab remains unknown, but this does not appear to be caused by loss of PD-L1 SVs, because biopsies at the time of progression for 2 patients in this study continued to demonstrate their known PD-L1 SVs and continued PD-L1 expression.
All 4 cases in our study showed histopathologic features of LCT. The other 3 reported cases of CTCL with PD-L1 SVs also were found in patients with LCT.8,9 Patients with LCT appear to have higher total mutational burden,8,12 which has been associated with improved responses to immune checkpoint therapy.15 Thus, MF/SS with LCT may be inherently immunogenic and upregulate PD-L1 to avoid immune clearance.
The breakpoints of the PD-L1 SVs identified here involve a cluster targeting intron 5 of the PD-L1 gene, leading to loss of exons 6 and 7 and the 3′ UTR, consistent with previous findings in CTCL8 and other T-cell lymphomas. Although structural variations in PD-L1 have only been detected rarely in prior NGS-based studies of CTCL,7,8,16-19 their detection by NGS methodologies is dependent on coverage of the key intronic regions typically excluded in prior studies. Although PD-L1 alterations appear to be uncommon across CTCL, we detected PD-L1 SVs in 4 of 11 (36%) patients with MF and LCT in our cohort. We did not identify PD-L1 SVs in CTCL patients without LCT and therefore would recommend consideration of testing for PD-L1 SVs to be focused on patients with LCT.
In summary, our findings highlight PD-L1 SVs as potential genomic biomarker of response to PD-1 axis inhibition in CTCL. The predictive value of described PD-L1 alterations will need to be further explored in ongoing studies of immune checkpoint blockade.
For data, please contact the corresponding author at mkhodado@stanford.edu.
Authorship
Contribution: S.B., S.F.-P., G.D., and M.S.K. designed and performed research and data analysis. E.B.W., W.-K.W., Y.H.K., and M.S.K. contributed to clinical evaluation of patients, specimen acquisition, and clinical data analysis. N.R., S.P.F., M.A.C., Y.H.K., and M.S.K. developed the protocol and conducted the clinical trial of pembrolizumab + interferon γ (NCT03063632). H.S., J.L.Z., and M.S.K. developed the next-generation sequencing panel and contributed to interpretation of the genomic data. S.B. and M.S.K. wrote the manuscript with contributions from all the coauthors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael S. Khodadoust, 300 Pasteur Dr, S169A Grant Building, Stanford University, Stanford, CA 94305; e-mail: mkhodado@stanford.edu.