Key Points
Thiotepa at 50 mg/m2 was safely incorporated into TIER immunochemotherapy.
Despite a clinically meaningful treatment response rate, long-term survival was seen only with ASCT consolidation.
Abstract
Relapsed or refractory primary central nervous system lymphoma (rrPCNSL) confers a poor prognosis with no accepted standard of care. Very few prospective studies have been conducted in this patient group. This study was a multicenter phase 1/2 study that investigated thiotepa in combination with ifosfamide, etoposide, and rituximab (TIER) for the treatment of PCNSL relapsed or refractory to high-dose methotrexate-based chemotherapy. A 3 + 3 design investigated the recommended phase 2 dose of thiotepa for a single-stage phase 2 cohort by assessing the activity of 2 cycles of TIER against rrPCNSL. The primary outcome was overall response rate. The dose-finding study demonstrated that 50 mg/m2 of thiotepa could be safely delivered within the TIER regimen. No dose-limiting toxicities were encountered in phase 1, and TIER was well-tolerated by the 27 patients treated in phase 2. The most common grade 3 to 4 toxicities were neutropenia (56% of patients) and thrombocytopenia (39%). An overall response was confirmed in 14 patients (52%), which met the prespecified threshold for clinically relevant activity. The median progression-free survival was 3 months (95% confidence interval [CI], 2 to 6 months) and overall survival 5 months (95% CI, 3 to 9 months). Exploratory analyses suggest a greater benefit for thiotepa-naïve patients. Six patients successfully completed autologous stem cell transplantation (ASCT) consolidation, with 4 experiencing durable remissions after a median follow-up of 50 months. The TIER regimen can be delivered safely and is active against rrPCNSL. When it is followed by ASCT, it can provide durable remission and long-term survival. However, for the majority of patients, prognosis remains poor, and novel treatment strategies are urgently needed. This trial was registered at https://www.clinicaltrialsregister.eu/ctr-search/search as EudraCT 2014-000227-24 and ISRCTN 12857473.
Introduction
Primary central nervous system lymphoma (PCNSL) is a rare and aggressive malignancy with an annual incidence of 4 to 5 per million population.1 Histopathologically, PCNSL is diffuse large B-cell lymphoma (DLBCL) restricted to the CNS or vitreoretinal compartment, although a number of pathobiological and clinical features distinguish it from systemic DLBCL.2 The anatomical localization presents unique challenges, including neurocognitive disability and impaired performance status because of the tumor, the impact of the blood-brain barrier on treatment delivery, and the vulnerability of surrounding brain tissue to toxicity from therapy.3
Multiagent, methotrexate-based induction regimens followed by consolidation have significantly improved outcomes for patients with PCNSL: complete response (CR) rates of 43% to 66% and 2-year survival rates of 60% to 90% are now reported.4–7 However, notwithstanding intensive first-line treatment protocols that incorporate autologous stem cell transplantation (ASCT), a significant proportion of patients experience relapse, and the majority of patients with relapsed or refractory PCNSL (rrPCNSL) will die as a result of their disease.
There is currently a paucity of prospective trial data, and no standard of care for patients with rrPCNSL for whom prognosis is poor; population-based data show a median survival of 3.5 months.8 The concept of using chemotherapy regimens that are not cross-resistant for rrPCNSL is intuitive, and it is an established approach for systemic DLBCL. Previous studies have demonstrated that for patients with rrPCNSL who undergo ASCT consolidation, durable responses are achievable with 5-year survival rates of >50%.9,10
A retrospective study of patients treated with salvage rituximab, ifosfamide, and etoposide (R-IE) described encouraging rates of response in a high-risk cohort of rrPCNSL patients. The R-IE regimen was well tolerated, the overall response rate (ORR) was 41%, and 18% of patients proceeded to ASCT.11 We hypothesized that adding another CNS-penetrating agent with minimal nonhematologic adverse effects (AEs) to the R-IE regimen could improve outcomes without significantly increasing toxicity. Thiotepa is a highly lipophilic, polyfunctional alkylator with a steep dose-response curve and highly efficient CNS penetration. Thiotepa is an established component of ASCT conditioning regimens for CNS lymphoma, it has recently been incorporated into multiagent chemotherapy regimens,7 although the dose used in the MATRix regimen (30 mg/m2) was empirically adopted without formal dose-finding studies.
The TIER trial is a prospective, dose-finding, multicenter phase 1/2 study of thiotepa in combination with ifosfamide, etoposide, and rituximab for the treatment of rrPCNSL. The aims of this study were to establish the recommended phase 2 dose (RP2D) and safety of adding thiotepa to ifosfamide, etoposide, and rituximab (the TIER regimen), and to investigate the activity of this regimen in the treatment of rrPCNSL after failure of methotrexate-based therapy.
Methods
Participants
Adults with rrPCNSL (defined as CD20+ DLBCL confined to the CNS) were eligible for this study. Relapsed or refractory disease was defined as recurrence after CR, unconfirmed CR (CRu), partial response (PR), or failure to achieve at least PR after 1 or 2 previous lines of chemotherapy that included at least 1 regimen containing high-dose methotrexate ≥1 g/m2. Although histologic confirmation of relapse was recommended, patients were eligible if initial diagnostic tissue was available and magnetic resonance imaging (MRI) scans were consistent with PCNSL. Further inclusion criteria were performance status of 0 to 2 (or 3 if attributed to lymphoma), adequate organ function, and the ability to undergo MRI scanning. Key exclusion criteria included evidence of systemic lymphoma, HIV seropositivity, recent treatment of lymphoma before registration (chemotherapy within 4 weeks, whole-brain radiation therapy [WBRT] within 6 months, thiotepa-based ASCT within 12 months, or previous R-IE), active infection, breastfeeding, or risk of pregnancy.
The TIER study was approved by the United Kingdom Research Ethics Committee (reference 14/LO/1568) and the local institutional review boards of participating sites. Patients gave informed consent to enter the trial. For patients who were unable because of their lymphoma to provide informed consent (as clinically assessed by investigators), informed consent was provided by a legal representative who was independent from the trial and could represent the patient’s presumed will, according to United Kingdom law.
Patients were recruited through the Blood Cancer UK and Cure Leukaemia charity-funded Trials Acceleration Program (TAP). This network of United Kingdom hemato-oncology centers provides patients with access to novel treatments through early-phase clinical trials with a catchment population of >20 million people.
Trial design
The phase 1 component of the TIER trial followed a conventional dose-finding 3 + 3 trial design12 to establish the RP2D of thiotepa in combination with ifosfamide (2 g/m2 per day, days 2 to 4), etoposide (250 mg/m2, day 2), and rituximab (375 mg/m2 per day, days 1 and 2). The dose levels of thiotepa studied were 20 mg/m2, 30 mg/m2 (starting dose), 40 mg/m2, and 50 mg/m2, given intravenously over 1 hour on day 5 of a 21-day cycle. The full treatment regimen, delivered as a 21-day cycle for 2 cycles, is shown in supplemental Figure 1. Antimicrobial prophylaxis was recommended according to local guidelines; aciclovir was suggested for herpes, co-trimoxazole was suggested for infection with Pneumocystis, and quinolone antibiotics were suggested for use during neutropenic periods. Granulocyte colony-stimulating factor was mandated until neutrophil recovery or for stem cell mobilization. Corticosteroids were tapered down and discontinued before response assessment after cycle 2 (supplemental Figure 1). Protocol-defined dose-limiting toxicities (DLTs) were independently assessed after 1 cycle by the Trial Steering Committee. Definition of DLTs included treatment delays of >14 days attributable to thiotepa, grade 4 thrombocytopenia or neutropenia causing a treatment delay of >14 days, or any clinically significant grade 3 or 4 nonhaematological toxicity attributable to thiotepa.
Once the RP2D was identified, patients were enrolled to the phase 2 component in a single-stage study based on A’Hern’s design.13 A prespecified clinically relevant ORR of ≥50% was chosen as a basis for further investigation, whereas an ORR of ≤30% indicated that further investigation was not warranted. To detect a meaningful effect with 90% power and a 20% risk of adopting treatment with an ORR of 30%, 28 patients were required in phase 2. Eleven patients with a radiologic response, as determined by central MRI review, would be required to meet the efficacy threshold. Patients treated at the RP2D in phase 1 were analyzed together with those treated in phase 2.
After 2 cycles of TIER, patients were treated at the discretion of investigators according to individual patient characteristics, patient preference, and treatment history. For eligible patients who responded to TIER, consolidation with thiotepa-based ASCT was recommended but not mandated. Alternative approaches for consolidation therapy included an additional 2 cycles of thiotepa, ifosfamide, and etoposide (TIE), or WBRT, which reflected the available treatment options in the United Kingdom during the conduct of the study. Similarly, re-treatment with a second ASCT was not commissioned within the United Kingdom National Health Service (NHS). Investigators were required to state at trial entry which consolidative strategy was planned.
Outcome measures
The primary outcome of the phase 1 dose escalation was the RP2D of thiotepa within the multidrug TIER regimen. The primary outcome of the phase 2 component was ORR (the proportion of patients with CR, CRu, or PR) by independent central neuroradiology review after 2 cycles of TIER treatment. Response was evaluated with gadolinium-enhanced MRI according to the International Primary CNS Lymphoma Collaborative Group criteria14 on an intention-to-treat basis. Secondary outcomes were CR rate after 2 cycles of TIER, overall survival (OS, time from registration to death as a result of any cause), progression-free survival (PFS, time from registration to disease progression or death as a result of any cause), event-free survival (EFS, time from registration to death as a result of any cause, disease progression, treatment toxicity, neurologic deterioration after treatment, or trial withdrawal), rate of successful stem cell harvest, proportion of patients proceeding to ASCT, and toxicity of the TIER regimen according to the Common Terminology Criteria for Adverse Events version 4.0. Relative dose intensity was calculated as a percentage of the unadjusted protocol-defined dose. Patients from both phases of the trial are included in the safety and consolidation outcomes. Patients received follow-up for a minimum of 2 years.
Additional details on the trial methods and procedures can be found in the trial protocol (supplemental Data). Trial data were analyzed by C.P.F., A.S.A., G.M., C.M.T., and A.E.J. All authors had access to the primary clinical data. All trial data will be uploaded to the EudraCT database after the end of trial declaration for publication at https://www.clinicaltrialsregister.eu/ctr-search/search.
Results
Patients
Between June 2015 and April 2019, 36 patients were recruited from 13 United Kingdom TAP centers, a network for hemato-oncology early-phase trials that encompasses a catchment population of >20 million people. Median age was 62 years, and 47% had an Eastern Cooperative Oncology Group (ECOG) performance status of 2 to 3 at study entry. Patient characteristics and details of previous treatments are summarized in Table 1. Notably, 11 patients had previously received thiotepa within the MATRix regimen (high-dose methotrexate, cytarabine, thiotepa 30 mg/m2, and rituximab),7 and 2 patients had undergone ASCT before trial entry, all of whom were enrolled into the phase 2 part of the trial. Six patients (17%) lacked the capacity to consent because of PCNSL-related cognitive impairment, so their informed consent was provided by a legal representative.
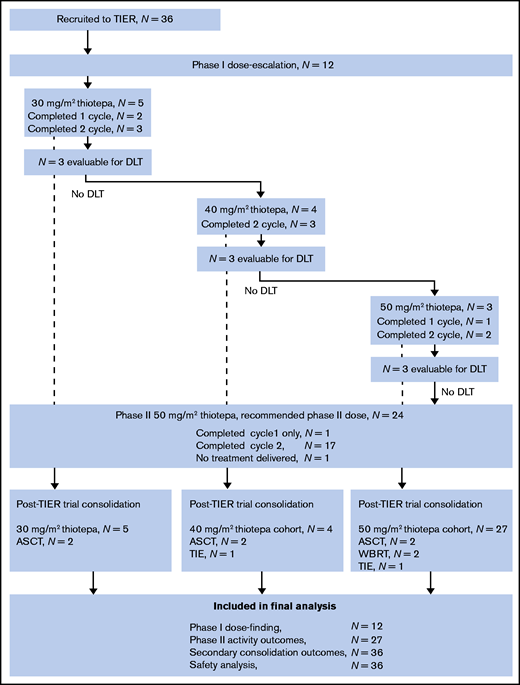
Sixty-one cycles of TIER were delivered within both phases of the trial, 45 of which were at the RP2D of thiotepa (83% of 54 planned doses in 27 patients). The Trial Management Group and Safety Committee recommended stopping enrollment after reaching a sample of 27 patients at RP2D (of 28 planned) because preplanned interim monitoring indicated that the primary end point had been reached. The flow of patients through the phase 1 and 2 components of the study and their inclusion in analyses is shown in Figure 1.
Patient flow diagram. Shown are TIER treatments across both phases of the trial, consolidation, and inclusion in the final analyses. ASCT, autologous stem cell transplantation; DLT, dose-limiting toxicity; TIE, thiotepa, ifosfamide and etoposide; WBRT, whole brain radiotherapy.
Patient flow diagram. Shown are TIER treatments across both phases of the trial, consolidation, and inclusion in the final analyses. ASCT, autologous stem cell transplantation; DLT, dose-limiting toxicity; TIE, thiotepa, ifosfamide and etoposide; WBRT, whole brain radiotherapy.
Treatment tolerability
Twenty-six patients received both of the planned 21-day cycles of TIER, 11 of whom experienced a delayed start to cycle 2; the median delay was 2 days (interquartile range, 1-4 days; range, 1-17 days). The commonest reason for delay was administrative or organizational; only 2 treatment cycles were delayed to allow platelet recovery to the mandated count of >80 × 109/L. Four patients (11%) required dose reductions in any of the TIER regimen drugs. Across both cycles of TIER, the median relative dose intensity was 100% (range, 73%-100%) for thiotepa, 100% (range, 67%-100%) for ifosfamide, and 100% (range 50%-100%) for etoposide.
During the phase 1 study, there were no DLTs among patients at the start of thiotepa dosing (at 30 mg/m2, there were 5 patients, 3 of whom were evaluable for DLTs), first dose escalation (at 40 mg/m2, there were 4 patients, 3 of whom were evaluable for DLTs), or final escalation (at 50 mg/m2, there were 3 patients, all evaluable) doses of thiotepa. Thus, the RP2D of TIER was established as 50 mg/m2.
Across both phases, 574 AEs were reported in 33 patients, of which 312 AEs (54%) reported in 27 patients were grade 3 to 4 (Table 2). Hematologic and neurologic events and electrolyte disturbance accounted for 95% of grade 3 to 4 AEs. There was a similar pattern of toxicity when considering AEs of all grades that affected >5% of patients (supplemental Figure 2). Of the 22 patients with available data, performance status improved in 1 patient or was maintained in 15 patients after 2 cycles of TIER, whereas it deteriorated in 6 patients (supplemental Figure 3).
Seventeen serious AEs (SAEs) were experienced by 12 patients, of which 13 AEs in 11 patients were judged to be related to TIER. The treatment-related SAEs were infection (n = 5), hematologic toxicity (n = 4), and neurologic toxicity (n = 4) (supplemental Table 1). A single unexpected serious adverse reaction was reported (serotonin syndrome). One patient death as a result of pneumococcal sepsis and neurologic deterioration was attributed to the trial treatment and underlying PCNSL; all other deaths were a result of disease.
Response to treatment
After 2 cycles of TIER, 18 patients were evaluable for objective imaging response at the RP2D. In an intention-to-treat analysis, 14 (52%) of 27 patients allocated to the RP2D had an objective response: CR (n = 4), CRu (n = 5), or PR (n = 5). This exceeded the prespecified threshold of clinical interest. The secondary outcome of CR (including CRu) was observed in 9 (33%) of 27 patients. The remaining 4 patients were confirmed to have progressive disease on MRI after 2 cycles of TIER. Nine patients were not evaluable because of progressive disease (n = 1), death as a result of PCNSL (n = 4), discontinuation because of toxicity (n = 2; 1 prolonged infectious episode and 1 suspected serotonin syndrome), and withdrawal from the trial (n = 2) before the MRI time point after cycle 2. Of the 14 patients who responded to TIER, 10 subsequently developed progressive disease, of whom 8 are known to have died; 1 other patient died as a result of unknown causes. Median duration of response was 5 months (95% confidence interval [CI], 2-9 months). After a median follow-up of 21 months, 22 (81%) of 27 patients treated at the RP2D had died: 19 because of underlying PCNSL and 3 as a result of unknown causes. The median OS was 5 months (95% CI, 3-9 months; Figure 2). The median PFS was 3 months (95% CI, 2-6 months; Figure 2) and the median EFS was 2 months (95% CI, 1-3 months). Patients who achieved CR or CRu had a median OS of 11 months (95% CI, 6 months to not calculable) and a median PFS of 6 months (95% CI, 4-11 months).
Consolidation
At trial registration, 34 (94%) of 36 enrolled patients were planned to subsequently receive some form of consolidation therapy after 2 cycles of TIER. The consolidation treatments planned at study entry and those ultimately delivered are summarized in Figure 3. Neither patient who previously underwent ASCT was planned for a second transplant. Overall, 17 (47%) of 36 patients received consolidation therapy: ASCT (n = 6), WBRT (n = 2), and further cycles of TIE chemotherapy only (n = 9).
Diagram showing the post-TIER consolidation planned at baseline and the treatment ultimately delivered. ASCT, autologous stem cell transplantations; PBSC, peripheral blood stem cell; TIE, thiotepa, ifosfamide and etoposide; WBRT, whole brain radiotherapy.
Diagram showing the post-TIER consolidation planned at baseline and the treatment ultimately delivered. ASCT, autologous stem cell transplantations; PBSC, peripheral blood stem cell; TIE, thiotepa, ifosfamide and etoposide; WBRT, whole brain radiotherapy.
Of the 25 patients for whom ASCT consolidation was planned at study entry, 11 patients received at attempt at stem cell mobilization after 2 cycles of TIER (1 CR, 6 CRu, and 4 PR): 5 harvests were unsuccessful, and the 6 patients for whom stem cells were successfully collected underwent ASCT. All patients undergoing ASCT were TIER responders (3 patients each with CRu and PR), and 2 patients were treated with a bridging cycle of TIE before proceeding to ASCT. Eight patients in CR or CRu did not proceed to ASCT, 4 because of failed stem cell harvest, and 3 because of progressive disease. One patient in CR had previously received ASCT and underwent WBRT as planned. ASCT was planned in 8 of 11 patients previously treated with MATRix, and stem cell mobilization was attempted for 3 of them, resulting in successful ASCT in 1.
OS was longest in 6 patients who underwent ASCT. After a median follow-up of 50 months, 2 patients relapsed and died 14 and 27 months after trial entry. The 4 surviving patients are in sustained remission 24, 36, 50, and 52 months after the start of the trial.
Two patients received WBRT consolidation, 1 as planned (who later died as a result of relapse at 10 months) and 1 after failed stem cell harvest (ongoing remission at 37 months). Nine patients received TIE chemotherapy consolidation, including 1 patient as planned and 4 after a failed stem cell harvest; 6 patients died, 2 were lost to follow-up (at 4 and 12 months), and 1 patient is alive at 25 months.
Subgroup analyses
Prespecified subgroup analyses explored the response rate to TIER and survival times according to previous treatment regimen. Thiotepa-naïve patients had a superior ORR of 10 (63%) of 16; 11 patients had previously been treated with thiotepa as part of the MATRix protocol, achieving an ORR of 4 (36%) of 11. Moreover, longer median OS was seen in thiotepa-naïve patients (5 months; 95% CI, 2-14 months) compared with those previously treated with MATRix (3 months; 95% CI, 2-9 months), although the number of patients in each subgroup was small.
We also analyzed whether the duration of treatment response before entering the study was associated with response and survival after treatment with TIER. The ORR to TIER for patients with >12 months from the start of their previous treatment to relapse was 10 (67%) of 15 compared with 4 (33%) of 12 for patients who were refractory or who relapsed within 12 months of starting their previous therapy. Consistent with this observation, the median OS times for these subgroups were 6 months (95% CI, 4-14 months) and 3 months (95% CI, 2-7 months), respectively. No formal tests of statistical significance were performed between subgroups because of the small number of patients per group.
Discussion
Improved therapies are urgently required for rrPCNSL; dismal survival outcomes clearly highlight this group of patients with unmet clinical need.8 Conducting prospective clinical trials in this patient group is extremely challenging; there are currently no licensed therapies for rrPCNSL and no accepted standard of care. Although thiotepa has been widely adopted for PCNSL therapy, TIER is the first thiotepa dose-finding study of its kind and one of the few dose-finding trials of any agent in rrPCNSL.15–18
TIER successfully met its primary end points for both phase 1 and phase 2 components. Thiotepa 50 mg/m2 was established as a safe RP2D to be incorporated into the R-IE regimen for the treatment of rrPCNSL. No DLTs were encountered during the phase 1 study, and TIER continued to be well-tolerated and deliverable throughout phase 2. As expected, the most frequent toxicities were hematologic, infectious, and neurologic, which also accounted for all 13 SAEs experienced by less than one-third of the patients. This represents a manageable safety profile in a group of patients whose age, neurocognitive impairment, performance status, and treatment history place them at significant risk of treatment-related toxicity.
TIER also met the prespecified threshold for clinical activity, achieving an ORR of 52% when thiotepa was given at the RP2D. This response rate was in the context of a patient group with a poor baseline performance status (47% with performance status ≥2), with adverse risk disease (72% intermediate or high risk), who had previously received intensive multidrug treatment (including 31% exposed to MATRix). A previously published retrospective study of R-IE salvage for PCNSL with a cohort that featured more favorable PS and disease risk profile achieved an ORR of 41% (95% CI, 41%-61%).11 However, despite the relatively high response rates to TIER, this activity did not translate into durable PFS or OS. The 2-year OS after treatment with TIER is similar to the 25% reported previously with R-IE,11 which highlights a very challenging group of patients for whom enrollment in clinical studies of new treatment approaches represents the most desirable therapeutic option.
Impairment of neurocognitive function is a common feature of PCNSL, in many cases limiting patients’ capacity to consent for treatment and inherently limiting such patients’ ability to participate in trials of new therapies. Importantly, 17% of patients enrolled in the TIER study lacked independent capacity to consent but were successfully enrolled in the study by virtue of Schedule 1 provisions of the United Kingdom’s Medicines for Human Use (Clinical Trials) Regulations 2004. With a clearly defined consent process within a legal framework, patients could be included in their best interest and according to their presumed will. This is an important outcome of our trial, demonstrating that disease-related and potentially reversible loss of capacity should not preclude access to clinical trials of novel treatment options. Moreover, inclusive enrollment into this PCNSL trial strengthens its generalizability to the real-world setting.
The first patients were recruited to the TIER trial when the standard first-line therapy for PCNSL was high-dose methotrexate plus cytarabine, which was demonstrably superior to methotrexate alone.19 During the course of the TIER trial, MATRix emerged as a superior first-line treatment option and has been widely adopted in the United Kingdom, Europe, and internationally.7,20,21 Consequently, 41% of patients recruited to the phase 2 component of the TIER trial were exposed to thiotepa. Exploratory analyses suggest greater benefit of TIER among the subgroup of patients who had not been exposed to thiotepa. Moreover, the rate of ASCT was relatively low among patients who had previously been treated with the MATRix regimen: only 1 of the 8 patients for whom the approach was intended completed this consolidation. This could be explained by a more biologically aggressive relapse after MATRix treatment or by a lower efficacy of TIER in patients exposed to thiotepa. Although the trial met its primary end point overall, there is likely to be a greater benefit in settings that do not include thiotepa as part of first-line treatment for PCNSL. For those refractory to or relapsing after first-line MATRix, the benefit of TIER was less convincing; such patients are likely to require novel nonchemotherapy approaches.
The manageable toxicity profile of TIER allowed it to be delivered with few treatment delays or dose reductions, and most patients who completed treatment retained their performance status. Despite the overall poor baseline characteristics of patients and the use of an intensive multidrug immunochemotherapy regimen, it is encouraging that stem cell mobilization was attempted in 11 patients, which resulted in successful ASCT in 6 patients. The most common reason for not proceeding to stem cell mobilization was early disease relapse, which emphasizes the high risk of this cohort. Nonetheless, the frequency of unsuccessful stem cell harvest was higher than expected and higher than that in previous studies.22 This may be a reflection of the higher intensity of first-line treatment in the TIER cohort. Two patients remained fit enough for WBRT and 9 additional patients received TIE consolidation. Some patients did not receive consolidation therapy, and this was primarily the result of a lack of sustained disease control. Those patients who went on to receive ASCT consolidation benefited from prolonged survival, and most were alive at the end of 24 months of follow-up. The durable OS in these patients reflects that seen previously in patients with rrPCNSL.9,10 TIER is therefore a valid immunochemotherapy option for re-inducing remission in advance of consolidation ASCT. When necessary, interim consolidation with TIE chemotherapy may provide a bridge to ASCT, although it is clear that quickly proceeding to ASCT is crucial for long-term survival.
PCNSL relapsing after first-line immunochemotherapy represents a substantial clinical challenge. For patients experiencing a late relapse after first-line MATRix (with a PFS of at least 2 years), re-challenge with a high-dose methotrexate-based protocol should be considered.20 Alternative regimens that are not cross-resistant could also be considered such as platinum-containing regimens with efficacy in PCNSL23,24 and secondary CNS involvement of systemic lymphoma.25 The potential role for TIER in secondary CNS lymphoma would require a dedicated study. The overall performance of investigational agents in early-phase trials for rrPCNSL has been disappointing for both conventional cytotoxics and novel agents. Targeted or immunomodulatory agents have been widely investigated in rrPCNSL, including lenalidomide, ibrutinib, tirabrutinib, and temsirolimus.17,26–29 Despite promising response rates, PFS duration in these studies was also short (2.1 months with temsirolimus, 2.9 months with tirabrutinib, 4.6 months with ibrutinib, and 7.8 months with rituximab plus lenalidomide) and was similar to that observed in our study. Therapeutic progress for rrPCNSL is likely to be achieved only by rationally designed protocols focused on appropriate cohorts. The group of rrPCNSL patients with the greatest unmet clinical need are those who experience refractory disease or early relapse after intensive first-line protocols; for such patients, clinical studies of novel agents informed by pathobiological data represent an attractive alternative. Nevertheless, there remains a role for conventional therapy, including the judicious use of thiotepa-based ASCT in selected patients with rrPCNSL.
The low incidence of PCNSL, together with the significant neurocognitive morbidity and impaired performance status associated with relapsed or refractory disease, translate into challenges for clinical trial recruitment. An important feature of the TIER trial was the concurrent collection of biological samples and advanced imaging data for correlative science. Ongoing analyses of diagnostic tissues samples, sequential blood samples, and advanced MRI scans from the TIER cohort will provide new insights into PCNSL pathobiology.
The TIER regimen is an immunochemotherapy option that is not cross-resistant for sufficiently fit patients whose PCNSL has relapsed after treatment with high-dose methotrexate. In meeting its prespecified end point, this trial demonstrates that thiotepa can be safely incorporated into a multidrug regimen and can produce a meaningful response rate worthy of further clinical development. For example, novel agents that are not myelotoxic could be combined with a TIER backbone with the aim of improving the rate and durability of response. However, in the TIER study, durable remissions were predominantly seen in those patients undergoing ASCT consolidation, and this remains the goal of therapy in fit and ASCT-naïve patients. For patients who are ineligible for transplantation or those previously treated with combination immunochemotherapy, relapsed or refractory disease represents a substantial clinical challenge for which novel approaches are urgently needed.
Acknowledgments
This work (the TIER trial) was supported by the Blood Cancer UK (reference number 13069) and Cure Leukaemia Trials Acceleration Programme, by the facilities funded through Birmingham Science City Translational Medicine Clinical Research Infrastructure and Trials Platform, and by Advantage West Midlands (part of the Science City University of Warwick and University of Birmingham Research Alliance). Thiotepa was provided free of charge by Adienne.
S.T. receives research funding from the Department of Health National Institute for Health Research (NIHR) Biomedical Research Centre (to University College London Hospital). G.P.C. is supported by the NIHR Oxford Biomedical Research Centre and Cancer Research UK Experimental Cancer Medicines Centre.
Authorship
Contribution: C.P.F., A.S.A., G.M., S.T., N.M.-C., A.E.J., L.M.H., C.M.T., S.K., J.W., S.C., J.S., I.C., D.C., K.M.L., G.P.C., A.J.M.F., D.L., A.J.D., R.J., D.P.A., and K.C. made substantial contributions to the trial design, data acquisition, or data interpretation of this study, were involved in drafting and revising the manuscript, gave approval of the final version, and are accountable for the accuracy and integrity of the work.
Conflict-of-interest disclosure: C.P.F. received consultant fees and research funding from Roche and Adienne. J.S. received funding to attend EHA 2018 and ICML 2019 from AbbVie and Janssen. G.P.C. received consultant fees from Roche. A.J.M.F. received speaker’s fees from Adienne; received research grants from Bristol Myers Squibb, BeiGene, Pharmacyclics, Hutchison MediPharma, Amgen, Genmab, ADC Therapeutics, Gilead, Novartis, and Pfizer; participated in advisory boards for Gilead, Novartis, Juno, and PletixaPharm; and holds patents on NGR-human tumor necrosis factor-a in brain tumours and NGR-hTNF/R-CHOP in relapsed or refractory PCNSL and SNGR-hTNF in brain tumors. K.C. received consultant fees and research funding from Roche and Adienne. The remaining authors declare no competing financial interests.
Correspondence: Christopher P. Fox, Clinical Haematology, Nottingham University Hospitals (City Campus), Hucknall Road, Nottingham, NG5 1PB, United Kingdom; e-mail: christopher.fox@nhs.net.
References
Author notes
The full-text version of this article contains a data supplement.