Key Points
PAI-1 was associated with venous thromboembolism in pancreatic cancer patients.
Mice bearing human pancreatic tumors expressing PAI-1 have impaired thrombus resolution.
Abstract
Pancreatic cancer patients have a high risk of venous thromboembolism (VTE). Plasminogen activator inhibitor 1 (PAI-1) inhibits plasminogen activators and increases the risk of thrombosis. PAI-1 is expressed by pancreatic tumors and human pancreatic cell lines. However, to date, there are no studies analyzing the association of active PAI-1 and VTE in pancreatic cancer patients. We investigated the association of active PAI-1 in plasma and VTE in pancreatic cancer patients. In addition, we determined if the presence of human pancreatic tumors expressing PAI-1 impairs venous thrombus resolution in mice. Plasma levels of active PAI-1 in patients with pancreatic cancer and mice bearing human tumors were determined by enzyme-linked immunosorbent assay. We measured PAI-1 expression in 5 different human pancreatic cancer cell lines and found that PANC-1 cells expressed the highest level. PANC-1 tumors were grown in nude mice. Venous thrombosis was induced by complete ligation of the inferior vena cava (IVC). Levels of active PAI-1 were independently associated with increased risk of VTE in patients with pancreatic cancer (subdistribution hazard ratio per doubling of levels: 1.39 [95% confidence interval, 1.09-1.78], P = .007). Mice bearing PANC-1 tumors had increased levels of both active human and active mouse PAI-1 and decreased levels of plasmin activity. Importantly, mice bearing PANC-1 tumors exhibited impaired venous thrombus resolution 8 days after IVC stasis compared with nontumor controls. Our results suggest that PAI-1 contributes to VTE in pancreatic cancer.
Introduction
Cancer patients have an increased risk of both venous thromboembolism (VTE; 0%-26%) and arterial thromboembolism (0%-3%).1-6 The rate of VTE varies in different types of cancer, with pancreatic cancer and brain cancer having the highest rates.7,8 This suggests that there may be cancer type-specific pathways that enhance VTE.9
Many studies have identified biomarkers that are associated with VTE in cancer patients that can be used to improve risk assessment scores.10 One of the most reliable biomarkers to predict VTE in the general cancer population is the fibrin degradation product D-dimer, which is part of the Vienna risk assessment score.11-13 Recently, some cancer type-specific biomarkers have been reported. For instance, extracellular vesicle tissue factor (TF) activity was associated with VTE in patients with pancreatic cancer.14-20 More recently, we found that plasma levels of citrullinated histone H3, which is a biomarker of formation of neutrophil extracellular traps, was associated with VTE in pancreatic and lung cancer patients.21
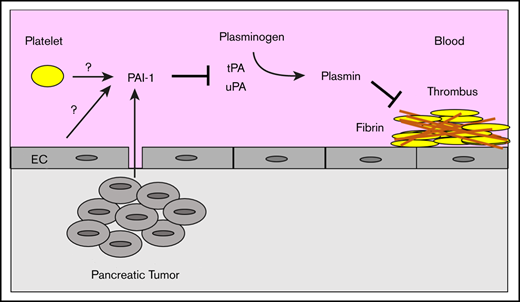
Plasminogen activator inhibitor 1 (PAI-1) is associated with thrombosis in a variety of diseases, including obesity, diabetes, and metabolic syndrome.22 23 PAI-1 reduces the generation of plasmin by inhibiting the plasminogen activators tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA). PAI-1 circulates in blood in 3 different forms: active; latent inactive; or complexed with either tPA, uPA, or vitronectin.24,25 Different assays can be used to measure either total or active PAI-1 in plasma. Importantly, active PAI-1 but not PAI-1 antigen is a potential marker of VTE.26
Pancreatic tumors express high levels of PAI-1 (The Human Protein Atlas, www.proteinatlas.org). Similarly, pancreatic cancer cell lines also express high levels of PAI-1.27,28 However, there are only 2 studies that have measured plasma levels of PAI-1 in pancreatic cancer patients. One study observed elevated plasma levels of total PAI-1 antigen and PAI-1 activity in 27 patients with pancreatic cancer compared with healthy controls.29 Another study found that there was no difference in total PAI-1 levels between pancreatic cancer patients with and without VTE.30
The role of PAI-1 in venous thrombosis has been studied in a mouse model of venous stasis induced by ligation of the inferior vena cava (IVC). PAI-1-deficient mice exhibited more rapid venous thrombus resolution that resulted in significantly smaller thrombi 8 and 21 days after IVC ligation.31 Conversely, mice overexpressing PAI-1 exhibit impaired thrombus resolution that led to larger thrombi 6 and 14 days after IVC ligation.32 Importantly, PAI-1-deficient mice and mice overexpressing PAI-1 have normal thrombogenesis because the size of venous thrombi 2 days after IVC ligation is not different from that in control mice.31,32
Mouse models have been developed to investigate the mechanisms of cancer-associated thrombosis.33 Most of these studies have used mouse and human pancreatic or breast tumors. We showed that tumor-derived human TF contributed to the increased thrombus weight in mice bearing human pancreatic tumors.34 In addition, we and others found that neutrophils and neutrophil extracellular traps contribute to thrombosis in mice bearing breast or pancreatic tumors.35-37
In the present study, we investigated the role of PAI-1 in VTE in pancreatic cancer using samples from patients and mice bearing human pancreatic tumors. We measured levels of active PAI-1 in pancreatic cancer patients who were prospectively observed during the disease with regards to the occurrence of VTE. In addition, we measured the weight of venous thrombi in mice with or without human pancreatic tumors that express PAI-1. Our results suggest that PAI-1 contributes to VTE in pancreatic cancer.
Materials and methods
Patients
This study included pancreatic cancer patients (n = 139) who were recruited into the Vienna Cancer and Thrombosis Study, an ongoing prospective and observational cohort study.38 The study was approved by the Medical University of Vienna Ethics Committee. Details on design, inclusion, and exclusion criteria have been published previously.38 In brief, adult patients with newly diagnosed or recurrent/progressive cancer were eligible for inclusion. Key exclusion criteria include continuous anticoagulation therapy, diagnosis of VTE within 3 months, chemotherapy within 3 months, and radiotherapy or surgery within 2 weeks before inclusion.11 Each patient gave a written informed consent. Before initiation of chemotherapy, a baseline blood sample was collected, and patients were prospectively followed for a maximum of 2 years. Blood samples were centrifuged at 1500g for 15 minutes to obtain platelet-poor plasma and aliquots were stored at −80°C until testing was performed in series. The primary outcome of Cancer and Thrombosis Study is occurrence of objectively confirmed, independently adjudicated VTE.
Cell lines
Human pancreatic cancer cell lines BxPC-3, HPAF-II, MIA PaCa-2, and PANC-1 were obtained from American Type Culture Collection (Manassas, VA). AsPC-1 was obtained from Bruce A. Sullenger (Duke University, Durham, NC). Cells were cultured in the recommended medium containing 10% fetal bovine serum (Omega Scientific, Tarzana, CA) and 1% antibiotics-antimycotic (Thermo Fisher Scientific, Waltham, MA) in a 5% CO2 incubator at 37°C. Cancer cells (1.0 × 105 cells/well) were cultured in fetal bovine serum-free recommended medium in a 12-well plate for 24 hours. The culture medium was centrifuged at 3000g for 15 minutes and supernatant was collected. Culture supernatant was frozen and stored at −20°C until use.
Generation of luciferase-expressing PANC-1 cells
Luciferase-expressing PANC-1 cells were generated using the same strategy as described previously.34
Mouse tumor model
All animal studies were approved by University of North Carolina at Chapel Hill Animal Care and Use Committee and complied with National Institutes of Health guidelines. Crl:NU-Foxn1nu male mice (nude mice) aged 3 to 4 weeks were purchased from the animal service core facility in the University of North Carolina at Chapel Hill. Luciferase expressing PANC-1 cells (2 × 106 in phosphate-buffered saline and Matrigel, Corning, Corning, NY) were injected into the pancreas. PANC-1 tumors were grown for 12 to 19 weeks. Luciferase expression was measured weekly with the IVIS Kinetic In Vivo Imaging System (Caliper Life Sciences, Waltham, MA). When the tumors gave a signal count of ≥1.0 × 105, mice were scheduled for a thrombosis experiment. For biomarker studies, we used mice with tumors weighing from 0.9 to 4.0 g. For thrombosis experiments, we used mice with tumors weighing 2.1 to 2.9 g. We observed 20% of PANC-1 tumor-bearing mice have up to 5 g of ascites. In addition, we observed 13% of PANC-1 tumor-bearing mice have metastasis mainly found in liver. We did not use mice with either ascites or liver metastasis. Blood was collected as described,39 and plasma was prepared by centrifuging the blood at 4500g for 15 minutes. Plasma samples were frozen and stored at −80°C until use.
Measurement of PAI-1 and thrombin-antithrombin complexes
Levels of active PAI-1 in plasma from pancreatic cancer patients were measured with the TECHNOZYM PAI-1 Actibind enzyme-linked immunosorbent assay (ELISA) (#TC16075; Technoclone, Vienna, Austria). This ELISA plate is coated with tPA and detects only active PAI-1. Levels of human total PAI-1 in the culture supernatant of cancer cells were measured using a commercial ELISA (#HPAIKT-TOT; Molecular Innovations, Novi, MI). This ELISA detects all forms of PAI-1, including free, latent, and complexed PAI-1. Commercial ELISAs were used to measure levels of active mouse PAI-1 (#MPAIKT; Molecular Innovations) and active human PAI-1 (#HPAIKT; Molecular Innovations) in plasma from mice with or without tumors. These ELISA plates are coated with uPA and detect free PAI-1, but not latent or complexed PAI-1. Plasma levels of thrombin-antithrombin (TAT) complexes (#OWMG15; Siemens, Munich, Germany) in mice were measured using a commercial assay.
Measurement of circulating neutrophils in mice
Neutrophils, platelets, and red blood cells were counted in whole blood using a Hemavet HV950FS (Drew Scientific, Miami Lakes, FL).
Thrombosis model
We used the IVC stasis venous thrombosis model as described previously.34 We harvested thrombieither 2 days or 8 days after IVC ligation. Thrombi were fixed with 10% formalin and then stored in 70% ethanol.
Microscopic analysis of thrombi
The fixed thrombi were embedded in paraffin, and 4-µm-thick sections were cut and stained with either hematoxylin and eosin or Martius Scarlet Blue by the Animal Histology and Laboratory Medicine Core Facility at the University of North Carolina at Chapel Hill.
Macrophage staining
Paraffin-embedded thrombi sections were deparaffinized with antigen retrieval. Slides were blocked with 5% nonfat skim milk (AppliChem, Council Bluffs, IA) in phosphate-buffered saline (Thermo Fisher Scientific) for 1 hour at room temperature. Endogenous avidin and biotin were blocked with avidin solution and biotin solution, respectively (Vector Laboratories, Burlingame, CA). Sections were incubated overnight at 4°C with 0.4 μg/mL rabbit anti-mouse Iba1 antibody (#019-19741; FUJIFILM Wako Chemicals, Osaka, Japan). After washing, sections were incubated with biotinylated goat anti-rabbit antibody (#BA-1000; Vector Laboratories) for 1 hour at room temperature. After adding avidin-peroxidase complex solution (#PK-6100; Vector Laboratories), Iba-1 staining (brown) was detected using the DAB substrate (#K3468; Agilent Technology, Santa Clara, CA), which was added for 10 minutes. Sections were counterstaining with hematoxylin (Thermo Fisher Scientific) for 1 minute. Stained sections were evaluated with a 1 to 5 scale by 2 blinded investigators.
Statistics
Cumulative incidence functions of VTE over time were estimated in competing risk analysis, accounting for all-cause mortality as competing outcome event to avoid overestimating VTE risk in this high mortality cohort.40 Accordingly, uni- and multivariable modeling of the association between PAI-1 per doubling of levels and risk of VTE was studied within a competing risk regression model according to Fine and Gray,40,41 adjusting for potential confounders (age, sex, body mass index, disease stage, cancer resection as time-dependent covariable). For visualization, levels of PAI-1 were dichotomized at the median of distribution. The proportional subhazards assumption was confirmed by fitting an interaction between predictor variables and follow-up time and by plotting Schoenfeld-like residuals against follow-up time. These statistical analyses were performed using STATA 15.0 (Stata Corp, Houston, TX).
For in vitro and mouse studies, the Shapiro-Wilk test was used for normality test and the F-test was used to compare variances. All data were normally distributed and parametric tests were used for the all data. For the 2-group comparisons, the unpaired 2-tailed Student t test or the Welch t test were used depending on the variances. Data are shown as mean ± standard deviation. All statistical analyses for mouse experiments were performed with PRISM version 7.03 (GraphPad Software, La Jolla, CA). Values of P < .05 were considered statistically significant for all experiments.
Results
Association of active PAI-1 with VTE in pancreatic cancer patients
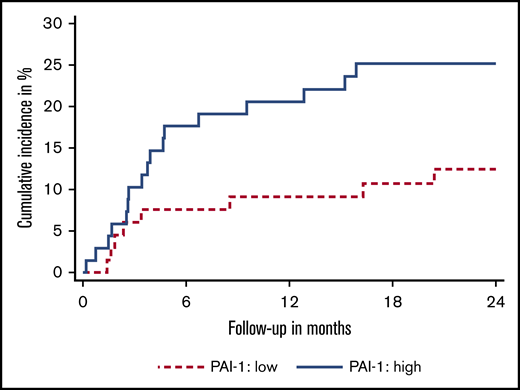
To determine if there was an association between active PAI-1 and VTE in pancreatic cancer, we measured the level of active PAI-1 in 139 pancreatic cancer patients (Table 1). The median observation time was 10.8 months (interquartile range, 4.9-20.5). During this time, 25 patients developed VTE (cumulative incidence, 19.0%) (supplemental Figure 1; supplemental Table 2) and 98 patients died (70.5%). We found an association between plasma levels of active PAI-1 and risk of VTE subdistribution hazard ratio (sHR) per doubling of PAI-1 levels: 1.39 (95% confidence interval [CI], 1.09-1.79, P = .009). This association prevailed upon multivariable adjustment for sex, age, body mass index, disease stage, and cancer resection (sHR, 1.40; 95% CI, 1.11-1.76; P = .004; model #1 in supplemental Table 1), and upon adjustment for baseline levels of D-dimer, coagulation factor VIII, and soluble P-selectin (sHR: 1.39; 95% CI, 1.04-1.86; P = .023; model #2 in supplemental Table 1). The cumulative 2-year incidence of VTE in patients with PAI-1 levels at or above the median was 25.1% (95% CI, 15.6-35.9) compared with only 12.4% (95% CI, 5.8-21.7) in those with levels below the median (Figure 1).
Cumulative incidence of venous thromboembolism of pancreatic cancer patients with different levels of active plasminogen activator inhibitor 1. Low levels (dashed line) defined as patients with active PAI-1 levels below the median; high levels (solid line) defined as active PAI-1 levels at or above the median.
Cumulative incidence of venous thromboembolism of pancreatic cancer patients with different levels of active plasminogen activator inhibitor 1. Low levels (dashed line) defined as patients with active PAI-1 levels below the median; high levels (solid line) defined as active PAI-1 levels at or above the median.
PAI-1 expression in human pancreatic cancer cell lines
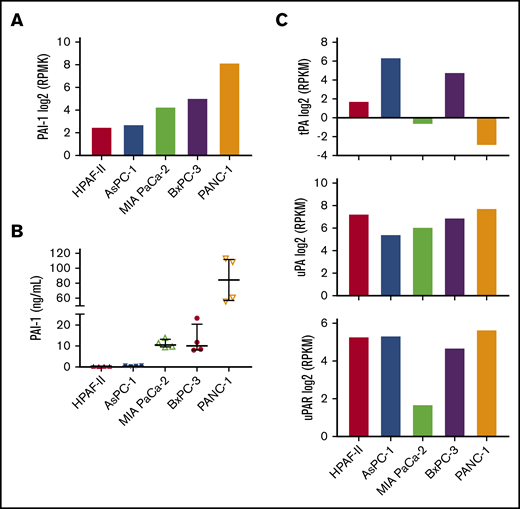
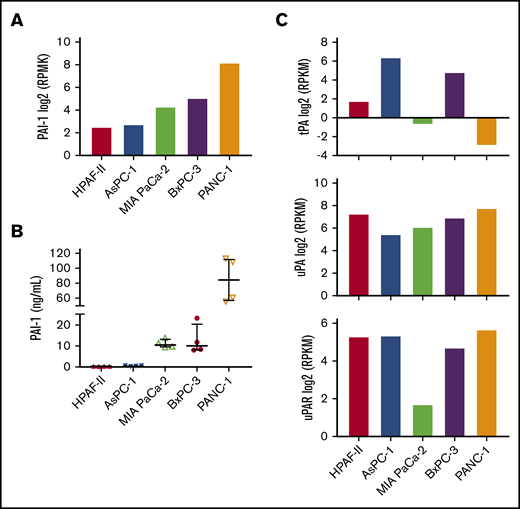
To establish a mouse model to study the effect of tumor-derived PAI-1 on venous thrombosis, we first evaluated PAI-1 expression in human pancreatic cancer cell lines. We used the Cancer Cell Line Encyclopedia database (https://portals.broadinstitute.org/ccle) to select cell lines that expressed low, medium, and high levels of PAI-1 (Figure 2A). Next, we measured levels of total PAI-1 in the culture supernatant. Consistent with the levels of PAI-1 messenger RNA (mRNA) expression, PANC-1 cells expressed the highest level of PAI-1, BxPC-3, and MIA PaCa-2 cells expressed medium levels and HPAF-II and AsPC-1 cells expressed very low levels of total PAI-1 (Figure 2B). Previous studies have also reported that PANC-1 cells express a high level of PAI-1.27,28 In addition, we used the Cancer Cell Line Encyclopedia database to evaluate tPA, uPA, and urokinase-type plasminogen activator receptor (uPAR) mRNA expression (Figure 2C). PANC-1 cells expressed high uPA and uPAR but low tPA (Figure 2C). Importantly, we have previously shown that PANC-1 cells express very low levels of TF.42,43 Therefore, PANC-1 cells can be used to study the role of PAI-1 in thrombosis with minimal interference from tumor-derived TF expression.
Plasminogen activator inhibitor 1 and fibrinolytic factors expression in human pancreatic cancer cell lines. We analyzed PAI-1 expression in 5 human pancreatic cancer cell lines. (A) Levels of PAI-1 mRNA in the different cell lines were from the Cancer Cell Line Encyclopedia database. The y-axis represents reads per kilobase of transcript per million mapped reads (RPKM) of RNA sequencing data. Data are shown as median ± interquartile range. (B) Levels of total PAI-1 in culture supernatant were determined by a commercial ELISA (n = 4). (C) Levels of tPA (top), uPA (middle), and uPAR (bottom) mRNA were from the Cancer Cell Line Encyclopedia database.
Plasminogen activator inhibitor 1 and fibrinolytic factors expression in human pancreatic cancer cell lines. We analyzed PAI-1 expression in 5 human pancreatic cancer cell lines. (A) Levels of PAI-1 mRNA in the different cell lines were from the Cancer Cell Line Encyclopedia database. The y-axis represents reads per kilobase of transcript per million mapped reads (RPKM) of RNA sequencing data. Data are shown as median ± interquartile range. (B) Levels of total PAI-1 in culture supernatant were determined by a commercial ELISA (n = 4). (C) Levels of tPA (top), uPA (middle), and uPAR (bottom) mRNA were from the Cancer Cell Line Encyclopedia database.
Establishment of a human pancreatic tumor model expressing PAI-1 in mice
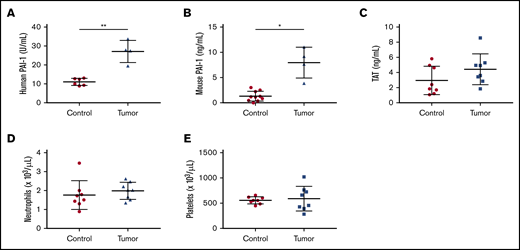
For the mouse tumor model, we used PANC-1 cells to form tumors because they express high levels of PAI-1. We measured levels of active human and mouse PAI-1 in the plasma of mice bearing PANC-1 tumors using species-specific ELISAs. Mice bearing PANC-1 tumors had increased plasma levels of both active human and mouse PAI-1 (Figure 3A-B).
Measurement of circulating cells and biomarkers in tumor-bearing mice. Plasma levels of active human (A, n = 6 and 4) and active mouse (B, n = 10 and 4) plasminogen activator inhibitor 1 (PAI-1), and thrombin-antithrombin (TAT) complexes (C, n = 8 and 8) were measured using commercial assays. Levels of neutrophils (D, n = 8 and 8) and platelets (E, n = 8 and 8) are shown. Data are shown as mean ± standard deviation. Data were analyzed with either the Welch t test (A-B) or the unpaired, 2-tailed Student t test (C-E) depending on the variances (*P < .05, **P < .01).
Measurement of circulating cells and biomarkers in tumor-bearing mice. Plasma levels of active human (A, n = 6 and 4) and active mouse (B, n = 10 and 4) plasminogen activator inhibitor 1 (PAI-1), and thrombin-antithrombin (TAT) complexes (C, n = 8 and 8) were measured using commercial assays. Levels of neutrophils (D, n = 8 and 8) and platelets (E, n = 8 and 8) are shown. Data are shown as mean ± standard deviation. Data were analyzed with either the Welch t test (A-B) or the unpaired, 2-tailed Student t test (C-E) depending on the variances (*P < .05, **P < .01).
We have previously shown that mice bearing human pancreatic tumors with high TF expression (HPAF-II, HPAC, and BxPC-3) but not those expressing low levels of TF (MIA PaCa-2 and PANC-1) have increased levels of circulating tumor-derived human TF and TAT complexes, which are markers of activation of coagulation.34,42,44 Here, we observed a slightly elevated level of TAT complexes in mice bearing PANC-1 tumors; this was not statistically significant (Figure 3C). We have also observed increased levels of neutrophils in mice bearing BxPC-3 tumors.34,37 In contrast, PANC-1 tumor-bearing mice did not have increased numbers of neutrophils compared with controls (Figure 3D). Similarly, PANC-1 tumor-bearing mice did not have increased number of platelets and red blood cells compared with controls (Figure 3E; data not shown).
Tumor-bearing mice have impaired venous thrombus resolution in the IVC stasis model
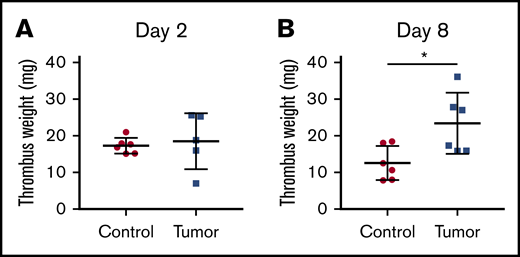
We measured thrombus weight both 2 and 8 days after IVC ligation to study thrombogenesis and thrombus resolution, respectively. We did not observe a difference in thrombus weight 2 days after IVC ligation in mice with or without tumors (Figure 4A). In contrast, mice bearing PANC-1 tumors had significantly larger venous thrombi 8 days after IVC stasis compared with the weight of thrombi formed in control mice (Figure 4B), which suggested an impairment of thrombus resolution
Thrombus weight in control and tumor-bearing mice. Venous thrombosis was induced in control and PANC-1 tumor-bearing mice by ligation of IVC. Thrombi were harvested 2 days (A, n = 6 and 5) or 8 days (B, n = 6 and 6) after IVC stasis and weighed. Mice with tumors between 2.1 and 2.9 g were used. Data are shown as mean ± standard deviation. Data were analyzed with either the Welch t test (A) or the unpaired, 2-tailed Student t test (B) depending on the variances (*P < .05).
Thrombus weight in control and tumor-bearing mice. Venous thrombosis was induced in control and PANC-1 tumor-bearing mice by ligation of IVC. Thrombi were harvested 2 days (A, n = 6 and 5) or 8 days (B, n = 6 and 6) after IVC stasis and weighed. Mice with tumors between 2.1 and 2.9 g were used. Data are shown as mean ± standard deviation. Data were analyzed with either the Welch t test (A) or the unpaired, 2-tailed Student t test (B) depending on the variances (*P < .05).
Characterization of thrombi from tumor-bearing mice and controls
We stained thrombi from control and tumor-bearing mice with hematoxylin and eosin (Figure 5A). With this stain, red blood cell areas are red, platelet areas are pink, and inflammatory cell areas are purple. In addition, we stained the thrombi with Martius Scarlet Blue (Figure 5B). With this stain, red blood cell areas are yellow, platelet areas are gray, and fibrin areas are red. However, no gross differences in composition were observed between thrombi from control (n = 5) and tumor-bearing (n = 6) mice (Figure 5A-B).
Characterization of thrombi from control and tumor-bearing mice. Thrombi were embedded in paraffin, sectioned longitudinally, and stained with hematoxylin and eosin (A) or Martius Scarlet Blue (B). With hematoxylin and eosin, red blood cell areas are red, platelet areas are pink, and inflammatory cell areas are purple. With Martius Scarlet Blue staining, red blood cell areas are yellow, platelet areas are gray, and fibrin areas are red. Representative pictures from 5 thrombi from controls and 6 thrombi from PANC-1 tumor-bearing mice. Scale bars, 700 µm. (C) Macrophages in thrombi from control and tumor-bearing mice were stained with an anti-Iba-1 antibody. Scale bars, 200 µm.
Characterization of thrombi from control and tumor-bearing mice. Thrombi were embedded in paraffin, sectioned longitudinally, and stained with hematoxylin and eosin (A) or Martius Scarlet Blue (B). With hematoxylin and eosin, red blood cell areas are red, platelet areas are pink, and inflammatory cell areas are purple. With Martius Scarlet Blue staining, red blood cell areas are yellow, platelet areas are gray, and fibrin areas are red. Representative pictures from 5 thrombi from controls and 6 thrombi from PANC-1 tumor-bearing mice. Scale bars, 700 µm. (C) Macrophages in thrombi from control and tumor-bearing mice were stained with an anti-Iba-1 antibody. Scale bars, 200 µm.
A previous study showed that mice overexpressing PAI-1 had significantly reduced numbers of macrophages in thrombi compared with controls.32 Therefore, we measured the numbers of macrophages in thrombi in control and tumor-bearing mice. Interestingly, macrophages were distributed around the edge of thrombi from both controls and tumor-bearing mice (Figure 5C). However, there was no difference of numbers of macrophages in thrombi from controls and tumor-bearing mice (assessment of investigator 1: control vs tumor-bearing mice [mean ± standard deviation] = 2.0 ± 1.4 vs 2.7 ± 1.4 [arbitrary unit, P = .40], assessment of investigator 2: 2.4 ± 1.1 vs 2.5 ± 0.5 [P = .82]).
Discussion
In the present study, we found an association between plasma levels of active PAI-1 and VTE in patients with pancreatic cancer (∼40% increase per doubling of PAI1-1 levels). In contrast to our result, Kondo and colleagues did not observe an association between total PAI-1 and VTE in patients with pancreatic cancer.30 However, these 2 studies measured different forms of PAI-1 (active vs total PAI-1) and have different observation times. We believe that levels of active PAI-1 are a better measure of thrombotic risk than levels of total PAI-1 because the latter measures both active and inactive PAI-1. A previous study showed that active PAI-1 but not PAI-1 antigen is a potential marker of VTE.26 In addition, our study was specifically designed to investigate biomarkers for VTE in patients with cancer. Another study observed increased plasma levels of total PAI-1 protein and activity in pancreatic cancer patients compared with healthy controls, but no difference in patients with or without a prior deep vein thrombosis.29 However, this study observed intermittent peaks in both PAI-1 antigen and activity over time in the pancreatic cancer patients, which suggests that they may have transient hypercoagulable episodes.29
We established a new mouse model to study venous thrombosis in mice bearing human pancreatic tumors expressing PAI-1. The important features of our mouse model are that PANC-1 tumors grown in the pancreas release human PAI-1 into the circulation and that tumor-bearing mice have impaired thrombus resolution 8 days after IVC ligation. PANC-1 tumors express low levels of TF; mice bearing PANC-1 tumors do not have an activated coagulation system. These data are consistent with our previous study that showed no difference in plasma TAT complexes in mice bearing PANC-1 tumors and control mice.42
Human PAI-1 inhibits mouse tPA, albeit with reduced inhibitory activity (∼40%) compared with mouse PAI-1.45 Therefore, human PAI-1 derived from the PANC-1 tumors can inhibit the fibrinolytic system in mice. The presence of the PANC-1 tumors was also associated with an increase in levels of mouse PAI-1 in the plasma. At present, we do not know the source of active mouse PAI-1 in tumor-bearing mice. PAI-1 is expressed in various types of cells, including endothelial cells and platelets.25,46-49 PANC-1 tumor-bearing mice have ∼8 ng/mL of active mouse PAI-1 and ∼30 U/mL of active human PAI-1, which is equivalent to 40 ng/mL of active human PAI-1 (1 U = 1.34 ng: according to the manufacturer’s instruction). Taken together, we calculated that the net ratio of antifibrinolytic activity of mouse PAI-1 (8 ng/mL) to human PAI-1 (16 ng/mL [40% of 40 ng/mL]) in PANC-1 tumor-bearing mice is 1 to 2. This suggests that both tumor-derived and host PAI-1 may impair fibrinolysis in PANC-1 tumor-bearing mice.
We analyzed the weight of thrombi in mice bearing PANC-1 tumors and controls both 2 and 8 days after IVC ligation to study thrombogenesis and thrombus resolution, respectively. We have shown that tumor-derived TF and elevated levels of circulating neutrophils in mice bearing BxPC-3 tumors both enhance thrombogenesis 2 days after IVC ligation.34,37 In contrast to these studies with mice bearing BxPC-3 tumors, we did not observe an increase in thrombogenesis in mice bearing PANC-1 tumors 2 days after IVC ligation. This result is consistent with previous studies with PAI-1-deficient mice and mice overexpressing PAI-1, showing that PAI-1 does not affect thrombogenesis in this model.31,32 Furthermore, mice bearing PANC-1 tumors do not increase circulating neutrophils. Importantly, mice bearing PANC-1 tumors had significantly larger venous thrombi 8 days after IVC ligation indicating impaired thrombus resolution. This result is consistent with studies with PAI-1-deficient mice and mice overexpressing PAI-1, which showed a role for PAI-1 in thrombus resolution in this model.31,32 Therefore, mice bearing human PANC-1 tumors expressing PAI-1 have impaired thrombus resolution that results in larger thrombi 8 days after IVC ligation. We did not observe any difference in the composition of thrombi from controls and tumor-bearing mice. These data are expected because we did not observe a difference in the numbers of circulating red blood cells, platelets, and neutrophils in control and tumor-bearing mice. Mice overexpressing PAI-1 had significantly reduced numbers of macrophages in thrombi compared with controls.32 In contrast, we did not observe difference of macrophage infiltration in thrombi from controls and tumor-bearing mice. This suggests that the primary mechanism by which the elevated levels of PAI-1 in tumor-bearing mice impair thrombus resolution is by inhibiting plasmin generation.
A recent study showed that mice bearing human lung A549 tumors, which express PAI-1, had larger venous thrombi in an IVC stenosis model.50 However, thrombus weight was evaluated 3 hours after IVC ligation, which would imply an effect on thrombogenesis. Furthermore, the role of tumor-derived PAI-1 in the increase in thrombus weight was not investigated. Interestingly, treatment of mice with the vascular endothelial growth factor inhibitor bevacizumab increased tumor-derived human PAI-1 expression in A549 tumors and thrombus weight.50 In addition, administration of an oral PAI-1 inhibitor PAI-039 reduced the thrombus weight to baseline.50 However, PAI-039 inhibits both human and mouse PAI-151,52 and does not inhibit vitronectin-bound PAI-1.53
A limitation of the current study is that we used platelet-poor plasma. Because platelets are one of the major sources of PAI-1, we cannot exclude the possibility that a low level of contaminating platelets in the plasma could affect the measured levels of PAI-1.
In summary, we have demonstrated an association of active PAI-1 levels and VTE in pancreatic cancer. Mice bearing human pancreatic tumors have increased levels of tumor-derived, human PAI-1 and host-derived, mouse PAI-1 that is associated with impaired thrombus resolution. Our data suggest that PAI-1 is a risk marker of VTE in pancreatic cancer.
Requests for data sharing should be e-mailed to the corresponding author, Nigel Mackman (nigel_mackman@med.unc.edu).
Acknowledgments
The authors acknowledge Charlene M. Santos and Sherie Quillen in the Animal Service Core facility at the University of North Carolina (UNC) and Bentley R. Midkiff in the Translational Pathology Laboratory at UNC. The Animal Surgery Core Laboratory of the McAllister Heart Institute at UNC performed the thrombosis experiments. The authors also thank Alisa S. Wolberg, Silvio Antoniak, Nigel Key, Vaibhav Kumar, and Sara R. Abrahams for helpful comments and technical support.
This work was supported by grants from the Japanese Society on Thrombosis and Hemostasis (Y.H.), National Institutes of Health/National Heart, Lung, and Blood Institute (T32 HL007149 [Y.H.] and R01 HL147149 [N.M.]), American Heart Association (S.P.G.), SENSHIN Medical Research Foundation (T.K.), the John C. Parker Professorship (N.M.), and the Austrian Science Fund (Special Research Program [SFB] 54) (I.P. and C.A.).
Authorship
Contribution: Y.H., J.E., M.J.F., I.P., N.M., and C.A. designed the study; Y.H., K.B.G., A.M., S.P.G., T.K., B.C.C., and C.A. performed the experiments; Y.H., T.K., F.M., N.M., and C.A. analyzed the data; Y.H., F.M., N.M., and C.A. wrote the manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nigel Mackman, UNC Blood Research Center, Department of Medicine, University of North Carolina at Chapel Hill, 8004 Mary Ellen Jones Building, CB#7035, 116 Manning Dr, Chapel Hill, NC 27599; e-mail: nigel_mackman@med.unc.edu; and Cihan Ay, Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Medical University of Vienna, Waehringer Guertel 18-20, A-1090 Vienna, Austria; e-mail: cihan.ay@meduniwien.ac.at.
References
Author notes
N.M. and C.A. are joint senior authors.
The full-text version of this article contains a data supplement.