Key Points
In this phase 1 study, the JAK1 inhibitor itacitinib was well tolerated and demonstrated preliminary efficacy in patients with aGVHD.
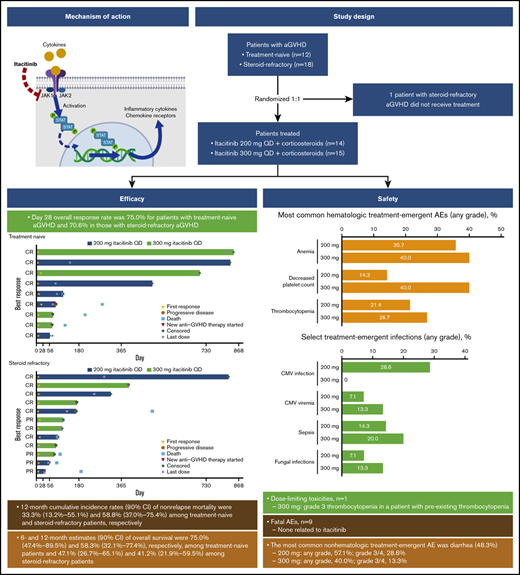
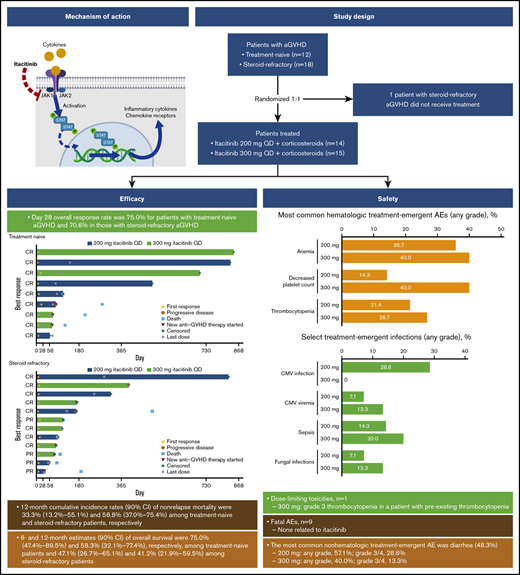
Overall responses were observed in 75% and 71% of patients with treatment-naive and treatment-refractory aGVHD, respectively, at day 28.
Abstract
Acute graft-versus-host disease (aGVHD) following allogeneic hematopoietic cell transplantation (HCT) is a primary cause of nonrelapse mortality and a major barrier to successful transplant outcomes. Itacitinib is a Janus kinase (JAK)1–selective inhibitor that has demonstrated efficacy in preclinical models of aGVHD. We report results from the first registered study of a JAK inhibitor in patients with aGVHD. This was an open-label phase 1 study enrolling patients aged ≥18 years with first HCT from any source who developed grade IIB to IVD aGVHD. Patients with steroid-naive or steroid-refractory aGVHD were randomized 1:1 to itacitinib 200 mg or 300 mg once daily plus corticosteroids. The primary endpoint was safety and tolerability; day 28 overall response rate (ORR) was the main secondary endpoint. Twenty-nine patients (200 mg, n = 14; 300 mg, n = 15) received ≥1 dose of itacitinib and were included in safety and efficacy assessments. One dose-limiting toxicity was reported (grade 3 thrombocytopenia attributed to GVHD progression in a patient receiving 300 mg itacitinib with preexisting thrombocytopenia). The most common nonhematologic treatment-emergent adverse event was diarrhea (48.3%, n = 14); anemia occurred in 11 patients (38%). ORR on day 28 for all patients in the 200-mg and 300-mg groups was 78.6% and 66.7%, respectively. Day 28 ORR was 75.0% for patients with treatment-naive aGVHD and 70.6% in those with steroid-refractory aGVHD. All patients receiving itacitinib decreased corticosteroid use over time. In summary, itacitinib was well tolerated and demonstrated encouraging efficacy in patients with steroid-naive or steroid-refractory aGVHD, warranting continued clinical investigations. This trial was registered at www.clinicaltrials.gov as #NCT02614612.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) offers a potentially curative treatment option for a variety of malignant and nonmalignant hematologic conditions.1 However, many patients subsequently develop acute graft-versus-host disease (aGVHD), a serious complication of HCT that manifests primarily in the skin, liver, and gastrointestinal (GI) tract.2 aGVHD occurs in 50% to 70% of patients depending on the degree of human leukocyte antigen match, type of prophylaxis employed, donor tissue source, and donor relation3-7 and accounts for ∼10% of deaths in patients after HCT.1 The presence and intensity of aGVHD also increase the risk of chronic GVHD (cGVHD), escalates hospitalization burden, erodes functional status posttransplant, and affects the overall economic burden of HCT.6,8,9
Corticosteroids are the accepted first-line systemic therapy for aGVHD,10 producing responses in 40% to 60% of patients depending on disease severity.11-13 A number of agents have been studied in combination with corticosteroids as both first-line treatment14-19 and as treatment of corticosteroid-refractory aGVHD.10,20-22 The combination therapies tried to date have yielded modest or no benefit over corticosteroids alone.14-19,23 At the time this study was initiated, there were no Food and Drug Administration–approved therapies for steroid-refractory aGVHD. After this study was completed, ruxolitinib, an oral selective Janus kinase (JAK)1/JAK2 inhibitor, was US Food and Drug Administration approved based on meeting the primary endpoint of day 28 response rate in a phase 2 trial.24
The pathogenesis of aGVHD involves dysregulation of inflammatory cytokine and chemokine signaling caused by tissue injury from transplant-preparative regimens, which may be modulated by JAK inhibition as initially demonstrated in preclinical studies.25 JAK1 and JAK2 activation play important roles in transducing inflammatory cytokine signaling,26 as normal JAK activity is essential for the expression of several chemokines and chemokine receptors.25,27-30 In preclinical models, JAK inhibition hampers the production of various cytokines27,30-32 and, consequently, the differentiation, proliferation, and trafficking of T cells implicated in the pathogenesis of aGVHD.25,27,30-33 Specific targeting of JAK1 may abrogate cytokine signaling involved in GVHD pathogenesis without inducing cytopenias caused by coinhibition of JAK2 signaling.34,35
Itacitinib (INCB039110; Incyte Corporation, Wilmington, DE), a selective JAK1 inhibitor, showed preclinical activity in aGVHD models, providing the rationale for testing this drug in patients. In a major histocompatibility complex–mismatched mouse model of aGVHD, itacitinib prophylactic and therapeutic dosing regimens significantly inhibited weight loss and improved GVHD scores without detrimental effects on engraftment of donor leukocytes.36 In addition, itacitinib modulated levels of helper T-cell 1 and helper T-cell 2 relevant cytokines important in the pathophysiology of aGVHD.36 Itacitinib also improved survival relative to vehicle in a murine model of aGVHD.30,37
We report the results of the first registered prospective study of JAK inhibition to treat aGVHD with the longest follow-up to date for trials evaluating JAK inhibitors in steroid-refractory aGVHD. This open-label, phase 1 trial evaluated the safety, efficacy, and pharmacokinetics (PK) of itacitinib in combination with corticosteroids in patients with treatment-naive or steroid-refractory aGVHD.
Methods
Patients
Patients ≥18 years old were eligible for the study if they had their first HCT from any donor source using bone marrow, peripheral blood stem cells, or cord blood for a hematologic malignancy and developed grade IIB to IVD aGVHD (per modified Minnesota Center for International Blood and Marrow Transplant Research [MN-CIBMTR] criteria38 ). Other eligibility criteria included ≤2 prior treatments for aGVHD, evidence of myeloid engraftment (eg, absolute neutrophil count >1.0 × 109/L for 3 consecutive days), Eastern Cooperative Oncology Group performance status (ECOG PS) 0 to 3, no recurrent primary disease, no active uncontrolled infection, adequate renal and cardiac function, and no previous JAK inhibitor therapy for any indication. The study was performed in accordance with the Declaration of Helsinki; each participating site’s institutional review board reviewed and approved the study, and all patients provided written informed consent.
Study design
This was an open-label, parallel-cohort, multicenter phase 1 trial of itacitinib combined with corticosteroids for the treatment of grades IIB to IVD aGVHD. Patients were randomized 1:1 to treatment with corticosteroids plus oral itacitinib at 200 mg or 300 mg once daily and were stratified based on prior GVHD treatment as treatment naive (no prior systemic therapy for aGVHD except ≤3 days of methylprednisolone 2 mg/kg per day or prednisone equivalent) or steroid refractory (progressive disease after 3 days of treatment with methylprednisolone 2 mg/kg per day or equivalent, or grade ≥II aGVHD that did not improve after 7 days of treatment with methylprednisolone 2 mg/kg per day or equivalent). Corticosteroids were administered at a starting dose of 2 mg/kg per day methylprednisolone or prednisone equivalent and tapered per institutional guidelines by day 56; patients who began the study on a different dose were permitted to remain on that dose if clinically appropriate. Corticosteroid dose was increased as needed per investigator discretion to control GVHD flares. Itacitinib dose could be reduced or interrupted to manage toxicity. Anti-infection medications, GVHD prophylaxis medications (including calcineurin inhibitors), transfusion support, nonabsorbable steroids, and topical steroid therapy were permitted. Patients received study treatment until treatment failure (GVHD progression, no response, or requirement for additional systemic therapy), unacceptable toxicity, or death for as long as clinical benefit was being derived per the opinion of the treating physician. After treatment discontinuation, patients were followed for safety (30 to 35 days) and survival (until death or study withdrawal).
The primary endpoint was safety and tolerability. The main secondary endpoints were overall response rate (ORR), defined as the proportion of patients with a complete response (CR), very good partial response (VGPR), or partial response (PR)39 at day 28, and population PK parameters. Exploratory endpoints included nonrelapse-related mortality (NRM) at 6 months, relapse rate, cumulative corticosteroid dose, incidence of cGVHD, and immunophenotyping and biomarker analyses.
Assessments
Dose-limiting toxicities (DLTs; see supplemental Methods for definition) observed in the first 28 days of study treatment were used to inform the recommended phase 2 dose. Clinical chemistry and hematology assessments occurred at screening, weekly (±3 days) through day 56, every 28 days until day 100, on day 365, and at end of treatment.
Acute GVHD was graded by the investigator weekly for the first 8 weeks after randomization and every 28 days thereafter using MN-CIBMTR criteria.38 Response was assessed per modified CIBMTR severity index (supplemental Table 1).40 Additional assessments were performed on days 100, 180, 365, and at end of treatment. Duration of response was defined as the interval between the first response and progression. Patients were assessed for signs and symptoms of cGVHD at days 100, 180, 365, and end of treatment per the National Institutes of Health consensus guidelines for cGVHD.41 Infections were monitored according to institutional practice guidelines.
Pharmacokinetic assessments were conducted on days 1 and 7. Following collection of a predose blood sample, study treatment was administered, and serial blood samples were taken at 1 hour (±15 minutes), 2 hours (±30 minutes), and between 4 and 8 hours. Concentrations of itacitinib in plasma were compared with the concentration needed to inhibit interleukin-6 (IL-6)– or thrombopoietin-induced signal transducer and activator of transcription (STAT) protein phosphorylation by 50%, as determined by an ex vivo whole blood assay.
Immunophenotyping
Immunophenotyping was performed using 21-color flow cytometry. Peripheral blood mononuclear cells were isolated before the first dose of itacitinib on days 1, 7, 14, 28, 56, 100, 180, and at end of treatment. Single-panel white blood cell analyses were conducted according to the National Institutes of Health Human Immunology Project.42
Biomarker analysis
Circulating levels of regenerating islet-derived protein 3-α precursor (REG3A), suppression of tumorigenicity 2 (ST2), tumor necrosis factor receptor-1 (TNFR1), and trappin-2 (elafin) were measured in plasma using the Simple Plex (ProteinSimple, San Jose, CA) multiplex platform according to the manufacturer’s instructions and as previously described.43
Statistical analyses
The data cutoff date was 18 March 2019. The safety and efficacy analyses described in this report included patients who were enrolled in the study and received ≥1 dose of study drug. Safety data were summarized using descriptive statistics. Continuous monitoring for DLTs from day 1 to day 28 was performed using a Bayesian approach.44-46 ORR was estimated with a 90% confidence interval (CI). The PK-evaluable population was defined as patients who received ≥1 dose of study drug and provided ≥1 measurable PK sample after study drug administration (see supplemental Methods for full details).
Results
Patients
Thirty patients were enrolled in the study: 14 were randomized to the 200-mg itacitinib group (treatment naive, n = 6; steroid refractory, n = 8) and 16 to the 300-mg group (treatment naive, n = 6; steroid refractory, n = 10). One patient with steroid-refractory aGVHD randomized to the 300-mg group withdrew before treatment and was not included in the analysis. Most patients were male (69%) and white (82.8%) and had an ECOG PS of 1 or 2 (89.7%). Median (range) age was 51.0 (18 to 71) years (Table 1). Most patients had grade II (34.5%) or III (55.2%) aGVHD per Minnesota grading criteria, and grade B (37.9%) or C (48.3%) per CIBMTR criteria. Organ involvement was primarily lower GI (58.6%) followed by skin (44.8%), upper GI (31.0%), and liver (17.2%). Among the 17 patients with steroid-refractory aGVHD who received itacitinib, the median (range) duration of prior corticosteroid treatment was 18 (5 to 407) days.
Median (range) treatment duration was 76 (5 to 291) days for the 200-mg dose group and 61 (5 to 817) days for the 300-mg dose group. Rates of and reasons for treatment discontinuations were similar between the 2 dosage groups (supplemental Table 2). The most common reasons for treatment discontinuation were adverse events (AEs; 200-mg group, n = 8; 300-mg group, n = 7) and physician decision (200-mg group, n = 3; 300-mg group, n = 5). All patients discontinued from the study with most common reasons being death (200-mg group, n = 9; 300-mg group, n = 8) and study termination by the sponsor (200-mg group, n = 3; 300-mg group, n = 7).
Safety
One DLT was reported for a patient in the 300-mg group who had preexisting thrombocytopenia and dose-limiting grade 3 thrombocytopenia that was attributed to GVHD progression. The most common nonhematologic treatment-emergent adverse event (TEAE) was diarrhea (48.3%, n = 14). Grade 3/4 diarrhea was reported in 4 patients in the 200-mg group and 2 patients in the 300-mg group (Table 2); 79% (11/14) of patients with diarrhea events had GI GVHD at baseline. GI hemorrhage was reported in 3 patients receiving itacitinib 200 mg (grade 3/4, n = 3) and 2 patients receiving 300 mg (grade 3/4, n = 1). None of the patients with GI hemorrhage had ulcers; 1 patient had 2 cytomegalovirus (CMV) infections. The most commonly reported hematologic TEAEs (all grades) were anemia (37.9%, n = 11), decreased platelet count (27.6%, n = 8), and thrombocytopenia (24.1%, n = 7). Grade 3/4 thrombocytopenia was reported in 2 and 3 patients in the 200-mg and 300-mg dose groups, respectively. Decreased platelet count was reported in 2 and 6 patients, respectively. Sepsis (all grade 3/4) was the most common infectious AE, occurring in 2 patients in the 200-mg group and 3 patients in the 300-mg group. Four patients (all 200-mg itacitinib) had a CMV infection, and 3 patients had CMV viremia (200 mg, n = 1; 300 mg, n = 2). Two patients (1 each in 200- and 300-mg groups) had a Candida infection, and 1 patient (300-mg group) had a fungal skin infection.
The most common itacitinib-related TEAEs were anemia and decreased platelet counts, which occurred in more patients in the 300-mg group (supplemental Table 3). Mean platelet counts initially decreased and then increased to above the lower limit of normal for both doses (supplemental Figure 1A). Mean absolute neutrophil count at the end of the study was similar to baseline in both groups (supplemental Figure 1B). Seven patients (24.1%; 200-mg group, n = 4; 300-mg group, n = 3) discontinued itacitinib because of TEAEs deemed related to itacitinib by the investigator. Fatal AEs occurred in 9 patients (supplemental Table 4); no deaths were deemed related to itacitinib.
Efficacy
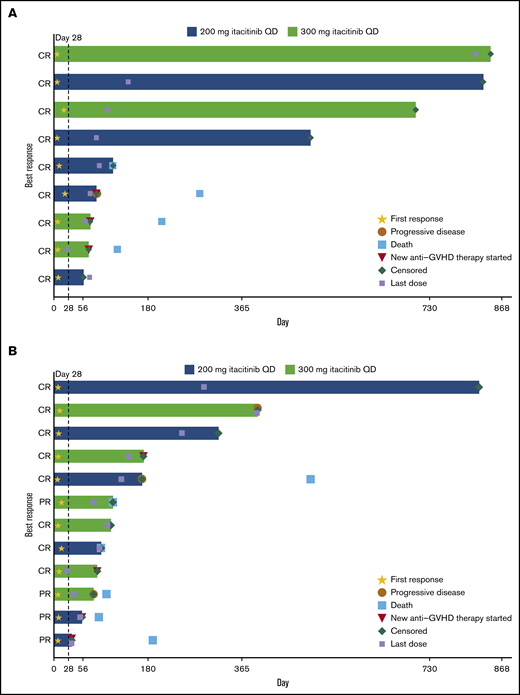
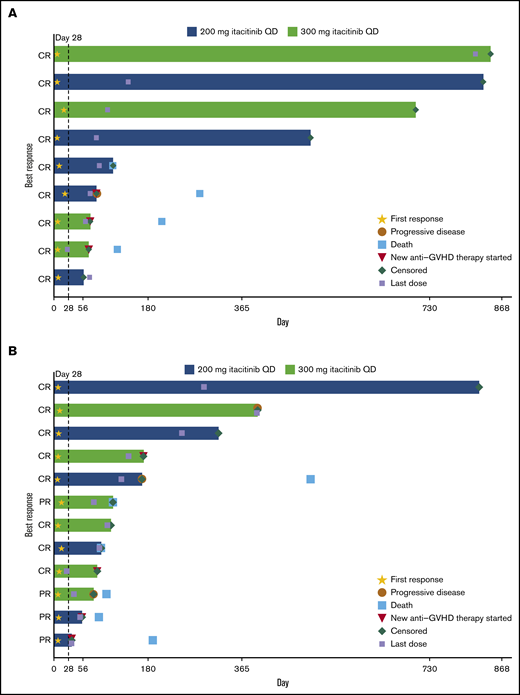
The day 28 ORR was 75.0% for patients with treatment-naive aGVHD and 70.6% for patients with steroid-refractory aGVHD (Table 3). At a median (range) follow-up time of 492 (50 to 841) days for the treatment-naive group and 104 (29 to 818) days for the steroid-refractory group, median duration of response was not reached (95% CI lower limit, 63 days) for patients with treatment-naive aGVHD and was 386 days (95% CI, 71 to not reached) for patients with steroid-refractory aGVHD (Figure 1). Responses were observed across involved organs (supplemental Table 5). ORR was 90.9% (10/11) for patients with standard-risk GVHD and 61.1% (11/18) for patients with high-risk GVHD. The day 28 ORR for all patients in the 200- and 300-mg groups was 78.6% and 66.7%, respectively. Twelve patients (41.4%) had aGVHD flares through 100 days (treatment naive, n = 5 [41.7%]; steroid refractory, n = 7 [41.2%]).
Duration of response. Duration of response is shown for individual patients with steroid-naive (n = 9 of 12 treated) (A) and steroid-refractory (n = 12 of 17 treated) (B) aGVHD at baseline.
Duration of response. Duration of response is shown for individual patients with steroid-naive (n = 9 of 12 treated) (A) and steroid-refractory (n = 12 of 17 treated) (B) aGVHD at baseline.
The NRM rate for all patients was 48.3% and was similar in the 200-mg (7/14, 50%) and 300-mg (7/15, 46.7%) groups. Two patients (6.9%) in the 200-mg group had a relapse of their underlying malignancy. Two patients in the 300-mg group developed cGVHD after day 365. Among patients with treatment-naive aGVHD (n = 12), 4 patients (33%) died of causes other than their underlying malignancy; 6- and 12-month cumulative incidence rates (90% CI) of NRM were 16.7% (3.9% to 37.1%) and 33.3% (13.2% to 55.1%), respectively. Among patients with steroid-refractory aGVHD (n = 17), 10 patients (58.8%) died of causes other than their underlying malignancy; 6- and 12-month NRM rates (90% CI) in these patients were 52.9% (31.7% to 70.3%) and 58.8% (37.0% to 75.4%), respectively. Determined causes of death are shown in supplemental Table 4. Six- and 12-month estimates (90% CI) of overall survival were 58.6% (42.2% to 71.9%) and 48.3% (32.5% to 62.4%), respectively, for all patients (treatment naive, 75.0% [47.4% to 89.5%] and 58.3% [32.1% to 77.4%]; steroid refractory, 47.1% [26.7% to 65.1%] and 41.2% [21.9% to 59.5%]; supplemental Figure 2).
Corticosteroid and immunosuppressive treatment
The average corticosteroid dose decreased at each visit for patients receiving itacitinib (supplemental Figure 3). By day 56, all patients either discontinued corticosteroid treatment or received a reduced dose. Five patients were receiving <20 mg daily prednisone, and 5 patients had discontinued corticosteroids by day 56. Of 27 patients who were treated with immunosuppressive medications (eg, calcineurin inhibitors, sulfonamides) at any time during the study treatment period, 16 (59.3%) discontinued all immunosuppressive therapy by the end of study treatment.
PK
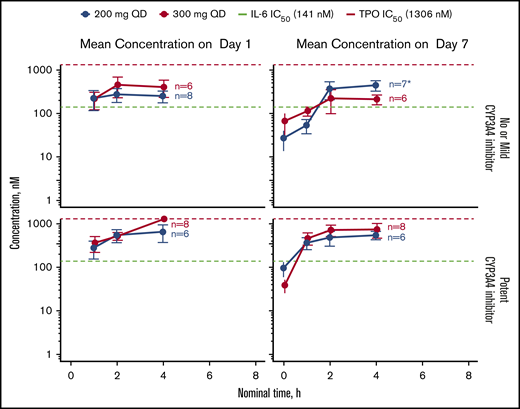
Mean plasma concentrations of itacitinib over time on days 1 and 7 in 28 patients are shown in Figure 2. Plasma concentrations across the 200- and 300-mg dose groups approached the ex vivo half-maximal inhibitory concentration for the inhibition of IL-6–induced STAT phosphorylation. Median time to maximum concentration (Cmax) occurred 2 to 4 hours after itacitinib treatment. A large overlap in steady-state PK exposures (Cmax and area under the curve [AUC]) was observed between itacitinib 200 mg and 300 mg (n = 24 evaluable). The geometric mean itacitinib Cmax and exposure at steady state (AUC0-τ) was 534 nM and 4700 nM·h, respectively, for the 200-mg group and 484 nM and 4110 nM·h, respectively, for the 300-mg group.
PK of itacitinib on day 1 and day 7 stratified by concomitant CYP3A4 inhibitor use in relation to the concentration needed to inhibit IL-6- or TPO-induced STAT phosphorylation by 50%. Data are shown as mean ± standard error of the mean. *Only 6 patient samples were available for analysis at the first time point (baseline). IC50, half maximal inhibitory concentration; TPO, thrombopoietin.
PK of itacitinib on day 1 and day 7 stratified by concomitant CYP3A4 inhibitor use in relation to the concentration needed to inhibit IL-6- or TPO-induced STAT phosphorylation by 50%. Data are shown as mean ± standard error of the mean. *Only 6 patient samples were available for analysis at the first time point (baseline). IC50, half maximal inhibitory concentration; TPO, thrombopoietin.
At steady state, 50% of patients (200-mg group, n = 5; 300-mg group, n = 7) were taking ≥1 potent CYP3A4 inhibitor, nearly all of whom (11/12) were receiving posaconazole. The geometric mean AUC0-τ was higher in patients receiving potent CYP3A4 inhibitors vs those not receiving CYP3A4 inhibitors or taking a mild inhibitor for both dose groups. Exposures were similar regardless of GVHD organ involvement (supplemental Figures 4 to 6; supplemental Table 6), including between patients with or without lower GI involvement and less advanced disease (stage <4; supplemental Figure 4). There were no differences in exposure between responders and nonresponders.
Biomarker analysis
Plasma samples from 27 patients were available for biomarker analysis, including 19 responders (CR, VGPR, PR) and 8 nonresponders (mixed response, progressive disease/death). Mean (standard error of the mean) baseline levels were significantly higher in nonresponders compared with responders for TNFR1 (3.8 [0.6] vs 2.6 [0.3] ng/mL; P = .037; supplemental Table 7). REG3A was elevated in nonresponders compared with responders; however, the difference did not reach significance (85.5 [51.4] vs 17.4 [6.0] ng/mL, P = .055; supplemental Figure 7A). Levels of ST2 (supplemental Figure 7B) and trappin-2 (elafin) were not significantly different between response cohorts. Although elevated levels of REG3A and ST2 have previously been reported to be associated with higher-risk aGVHD using a validated algorithm,47 the assay used to measure these levels in our study and the heterogeneous nature of these patients (treatment naive and steroid refractory) prevent us from reliably calculating a biomarker risk score for these patients.
Longitudinal analysis demonstrated that plasma biomarker levels generally decreased over time in both responders and nonresponders (supplemental Table 7). Biomarker levels were generally higher among nonresponders than responders during the course of treatment, but only comparison of day 7 TNFR1 and REG3A reached significance.
Immunophenotyping analysis
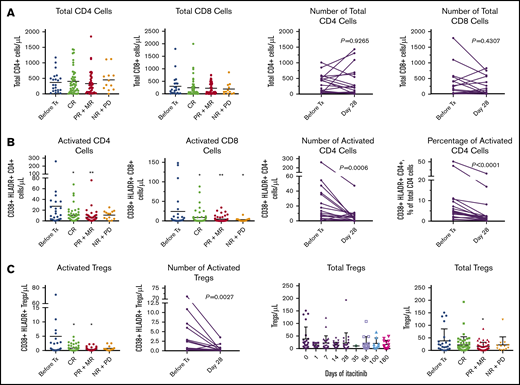
Itacitinib treatment was associated with decreased levels of markers for T-cell and regulatory T-cell (Treg) activation (Figure 3A-B). Importantly, the change in overall Treg levels in peripheral blood was minimal (Figure 3C), although migration of Tregs to inflammation sites is possible and was not measured. Total T-cell levels did not differ upon treatment. CR to itacitinib was associated with reduced levels of myeloid-derived suppressor cells (MDSCs; supplemental Figure 8A), elevated levels of natural killer (NK) cells (supplemental Figure 8B), and an NK:MDSC ratio of >0.31 (sensitivity, 63%; specificity, 81% of CR; supplemental Figure 8C). Among complete responders, STAT-5 phosphorylation, which is critical for Treg expansion,48 doubled from baseline to day 28, but the change did not reach statistical significance (P = .08; supplemental Figure 8D).
Effects of itacitinib on T cells. Peripheral blood samples from serial time points from baseline through day 180 were analyzed (total samples: before treatment (Tx), n = 23; CR, n = 48; PR+MR, n = 47; NR+PD, n = 13). Patients who had both baseline and day 28 samples were evaluable for analysis of absolute change from baseline to day 28 (n = 18). Total CD4 count was not available for 1 patient at day 28, although data for activated CD4 cells were available for this patient. Only 2 nonresponders had evaluable samples at day 28. (A) Absolute peripheral blood CD4 and CD8 T-cell numbers following itacitinib treatment through day 180 by response and change over time at day 28. (B) CD4 and CD8 T-cell activation levels following itacitinib treatment through day 180 by response and change over time at day 28. (C) Overall and activated peripheral blood Treg levels following itacitinib treatment through day 180 by response and change over time at day 28. CR includes CR and VGPR. Pairwise comparison was assessed by Wilcoxon rank test. *P < .05 and **P < .01 vs before Tx, analysis of variance with Dunnett post hoc. HLADR, human leukocyte antigen D related; MR, mixed response; NR, no response; PD, progressive disease.
Effects of itacitinib on T cells. Peripheral blood samples from serial time points from baseline through day 180 were analyzed (total samples: before treatment (Tx), n = 23; CR, n = 48; PR+MR, n = 47; NR+PD, n = 13). Patients who had both baseline and day 28 samples were evaluable for analysis of absolute change from baseline to day 28 (n = 18). Total CD4 count was not available for 1 patient at day 28, although data for activated CD4 cells were available for this patient. Only 2 nonresponders had evaluable samples at day 28. (A) Absolute peripheral blood CD4 and CD8 T-cell numbers following itacitinib treatment through day 180 by response and change over time at day 28. (B) CD4 and CD8 T-cell activation levels following itacitinib treatment through day 180 by response and change over time at day 28. (C) Overall and activated peripheral blood Treg levels following itacitinib treatment through day 180 by response and change over time at day 28. CR includes CR and VGPR. Pairwise comparison was assessed by Wilcoxon rank test. *P < .05 and **P < .01 vs before Tx, analysis of variance with Dunnett post hoc. HLADR, human leukocyte antigen D related; MR, mixed response; NR, no response; PD, progressive disease.
Discussion
In this phase 1 study, itacitinib was well tolerated by patients with steroid-naive and steroid-refractory aGVHD. TEAEs were consistent with those reported in patients with aGVHD49 and those in patients with myelofibrosis treated with itacitinib.50 The rates of infections (sepsis, 17.2%; fungal infections, 10.3%) and CMV events (infection, 13.8%; viremia, 10.3%) were comparable to those previously reported for patients with aGVHD.51-53 Hematologic AEs associated with itacitinib were consistent with events reported for patients with myelofibrosis.50 Mean platelet counts decreased initially but returned to above-normal levels by the end of treatment. Itacitinib-related hematologic AEs occurred in both dose groups and were numerically higher in the 300- vs 200-mg group, including anemia (n = 4 vs n = 2), decreased platelet count (n = 4 vs n = 2), and thrombocytopenia (n = 3 vs n = 2). However, it is worth noting that cytopenias may result from underlying GVHD rather than study treatment, so doses of itacitinib that alleviate GVHD could potentially lead to improvements in cytopenias over time. Because of the increased incidence of hematologic AEs in the 300-mg group and similar efficacy and PK profiles observed across doses, the 200-mg dose was selected for future itacitinib studies in patients with aGVHD.
Day 28 response was chosen as a key efficacy endpoint because previous studies demonstrated that response at day 28 is predictive of longer-term outcomes.54 Overall, 75.0% of patients with treatment-naive aGVHD and 70.6% of those with steroid-refractory aGVHD responded to itacitinib treatment at day 28. Results were comparable to recent findings from other prospective clinical trials of investigational therapies for aGVHD, in which day 28 ORR ranged from 55% to 83%.55-58 Within this class of agents, the REACH1 phase 2 study of the JAK1/JAK2 inhibitor ruxolitinib showed a day 28 ORR of 55% in patients with steroid-refractory aGVHD.55 The high overall NRM rate of 48% observed in this study was largely driven by the subset of patients with steroid-refractory aGVHD and is consistent with expectations for this patient population.53 NRM and survival outcomes are key endpoints in an ongoing randomized phase 3 study (GRAVITAS-301 [#NCT03139604]).
As long-term use of corticosteroids is associated with complications such as diabetes, hypertension, myopathy, osteoporosis, and increased risk of infection,59,60 safe and effective treatment options that enable corticosteroid taper are desirable. All patients who received itacitinib in the present study discontinued or reduced corticosteroid use by day 56.
The PK analysis demonstrated no difference in steady-state PK exposures between 200- and 300-mg dosing. Approximately half of the patients in this study were receiving ≥1 potent CYP3A4 inhibitor. The fold-increase in plasma itacitinib exposure with potent CYP3A4 inhibitors was 1.7-fold at the 200-mg dose and 2.9-fold at the 300-mg dose. The increased itacitinib exposure in this small patient population was not associated with an altered risk-benefit profile. Therefore, the results of this study indicate that up to a threefold increase in itacitinib exposures may occur with potent CYP3A4 inhibitors, but a dose adjustment is not needed. These findings are consistent with those from a previous study of itacitinib PK when coadministered with itraconazole, a potent CYP3A4 inhibitor, in healthy volunteers.61 The PK analysis has 2 important limitations. First, sparse PK sampling was used in this study, which should be considered before comparing PK parameters derived in this study with other populations. Second, the sample population was heterogeneous and small (24 patients evaluable for final steady-state PK). Therefore, the effect of GVHD organ staging on exposure may be difficult to interpret, particularly in cases where patients were on concomitant CYP3A4 inhibitors. As such, inferences from this study should be drawn cautiously.
Biomarker analysis demonstrated significantly higher levels of TNFR1 at baseline in patients who did not respond to itacitinib treatment compared with responders. Levels of ST2 significantly decreased over time with itacitinib treatment in responders, whereas nonsignificant reductions in levels of TNFR1, REG3A, and trappin-2 (elafin) were observed over time in both responders and nonresponders. Previous studies suggest that these biomarkers may have predictive value for GVHD outcomes and treatment response. Biomarker algorithms based on levels of TNFR1, ST2, and REG3A predicted risk of NRM independent of clinical symptoms and risk factors in patients with GHVD.47,62 Another study demonstrated that a 6-biomarker panel that included TNFR1, REG3A, and elafin predicted day 28 nonresponse and day 180 mortality in patients with aGVHD receiving corticosteroids.63
Exploratory immunophenotyping data suggest that an elevated NK:MDSC ratio may be predictive of itacitinib response. NK cells may protect against GVHD through cytotoxic depletion of antigen-presenting cells and activated alloreactive T cells.64 Elevated NK cell levels in itacitinib responders, along with decreased T-cell activation during treatment with itacitinib, support this hypothesis, but further study is needed to confirm these findings given this small cohort and confounding effects of steroids. The role of MDSCs in aGVHD is complicated by contradictory data and the lack of a clear phenotype to define these functionally suppressive cells in humans.65-67 Possibly, the key inflammatory signal of interferon-γ, which signals through JAK1/JAK2, is required for the maturation and function of these cells and is being blocked by itacitinib, resulting in decreasing numbers in responders.68 A limitation of immunophenotyping immune cell subsets from the peripheral blood of patients with aGVHD is the limited number of cells and events that can be analyzed by multiparameter flow cytometry and the potential lack of correlation with effects at the tissue level.
This study is the first to test JAK inhibition for the treatment of steroid-refractory or steroid-naive aGVHD. The findings from this study are limited by small sample size and no comparator group. Despite these limitations, our results support future studies of itacitinib for aGVHD, including use in the prophylactic setting. Based on our understanding of JAK/STAT inhibition in mouse HCT models,30 itacitinib may disrupt T-cell trafficking to GVHD organs, suggesting that clinical benefit may be greatest if itacitinib is introduced in first-line or prophylactic settings. Such studies are underway (GRAVITAS-301 and GRAVITAS-119 [#NCT03320642]).
In conclusion, results from this phase 1 study demonstrate that itacitinib, an oral, selective JAK1 inhibitor, was effective and well tolerated by patients with steroid-naive or steroid-refractory aGVHD. No unexpected TEAEs were observed in either dosage group, but thrombocytopenia was observed more commonly in the 300-mg dosage group. CMV events were observed in both dose groups and should be monitored in future itacitinib studies. Overall responses at day 28 were achieved in 75% and 71% of patients with steroid-naive and steroid-refractory aGVHD, respectively. All patients were able to decrease steroid use during the course of the study; 50% of patients receiving immunosuppressive medications at baseline discontinued corticosteroids or were receiving <20 mg prednisone by day 56. Data from the ongoing clinical trial program with itacitinib should provide additional evidence about the clinical benefits observed when used earlier in the treatment paradigm.
Presented in abstract form at the 58th annual meeting of the American Society of Hematology, San Diego, CA, 3-6 December 2016 (Abstract 390) and the 22nd Congress of the European Hematology Association, Madrid, Spain, 22-25 June 2017 (Abstract S794).
Access to individual patient-level data is not available for this study. For original data requests, please contact skanthala@incyte.com.
Acknowledgments
The authors thank Michael Howell, at Incyte Corporation, for assistance with biomarker and translational assessments. Writing assistance was provided by Jane Kovalevich, at Complete Healthcare Communications, LLC (North Wales, PA), a CHC Group company, and funded by Incyte Corporation. The authors acknowledge the participating study sites and all participating patients.
This study was funded by Incyte Corporation and received support from National Institutes of Health, National Cancer Institute grants P30-CA9184201 (J.F.D.), SPORE P50-CA171963-01 (J.F.D.), R35 1R35-CA210084 (J.F.D.), the Amy Strelzer Manasevit Research Program (Be The Match Foundation and the National Marrow Donor Program) (J.C.), and the Alvin J. Siteman Cancer Center Siteman Investment Program (Foundation for Barnes-Jewish Hospital Cancer Frontier Fund, National Cancer Institute Cancer Center Support Grant, P30 CA091842, and Barnard Trust) (J.C.).
The authors thank H. Jean Khoury for his substantial contributions to the development of this study and countless others, as well as for his mentorship, friendship, and legacy of fostering the love of medicine and scientific investigation in all those he met.
Authorship
Contribution: M.A.S. contributed to study design, protocol writing and development, data acquisition, data interpretation, and manuscript writing; H.J.K. contributed to study design; M.J. contributed to data acquisition, data interpretation, and manuscript writing; H.A. contributed to data acquisition, data analysis, data interpretation, and manuscript writing; G.J.S. contributed to study design, data acquisition, data interpretation, and manuscript writing; K.S. contributed to data acquisition, data analysis, and data interpretation related to correlative studies, and manuscript writing; J.C. contributed to data acquisition, data analysis, and data interpretation related to correlative studies, and manuscript writing; L.G. contributed to data acquisition, data analysis, and data interpretation related to correlative studies, and manuscript writing; M.C.A. contributed to study design, development of the study protocol, data analysis, data interpretation, and manuscript writing; Y.Y. contributed to development of the statistical analysis plan, data analysis, and manuscript writing; P.L. contributed to study design, data analysis, data interpretation, and manuscript writing; N.S. contributed to data acquisition, data analysis, data interpretation, and manuscript writing; M.P. contributed to study design, data acquisition, data analysis, and data interpretation related to the biomarker analyses, and manuscript writing; M.-A.P. contributed to study design, data analysis, data interpretation, and manuscript writing; Y.-B.C. contributed to study design, data acquisition, data analysis, data interpretation, and manuscript writing; G.M. contributed to data acquisition and manuscript writing; and J.F.D. contributed to study design, data acquisition, data analysis, data interpretation, correlative laboratory studies, and manuscript writing.
Conflict-of-interest disclosure: M.A.S. received honoraria and research funding from Incyte Corporation. H.J.K. served on an advisory board and received research funding from Incyte Corporation. M.J. received research funding from Therakos, Janssen, and Incyte Corporation. H.A. and G.J.S. received research funding from Incyte Corporation. K.S. served as a consultant for RiverVest and Kinetic River and is a coinventor on a patent related to CART therapy. J.C. received honorarium from Incyte Corporation, received research funding from Mallinckrodt Pharmaceuticals, and served as a consultant for Daewoong Pharmaceutical. M.C.A., Y.Y., P.L., N.S., and M.P. are employees and stockholders of Incyte Corporation. M.-A.P. received honoraria from AbbVie, Bellicum, Bristol-Myers Squibb, Incyte Corporation, Merck, Novartis, Nektar Therapeutics, Omeros, and Takeda; served on data and safety monitoring boards for Servier and Medigene; served on scientific advisory boards for MolMed and NexImmune; and has received research support for clinical trials from Incyte Corporation, Kite/Gilead, and Miltenyl Biotec. Y.-B.C. served as a consultant for Incyte Corporation and Takeda and received research funding from Incyte Corporation and Novartis Pharmaceuticals. J.F.D. served on advisory boards for Incyte Corporation, CellWorks, Macrogenics, Amphivena, Arch, Rivervest, and Bioline and is a cofounder of Magenta Therapeutics and WUGen. The remaining authors declare no competing financial interests.
H. Jean Khoury died on 22 May 2017.
Correspondence: Mark A. Schroeder, Division of Oncology, Section of Bone Marrow Transplantation and Leukemia, Washington University School of Medicine, 4590 Children’s Pl, St Louis, MO 63110; e-mail: markschroeder@wustl.edu.
References
Author notes
The full-text version of this article contains a data supplement.