Key Points
IDH1/2-inhibitor–based combinations conferred significant clinical responses in patients with IDH1/2-mutated post–MPN AML.
Complete remission was achieved in 3/7 patients (1 attaining MRD–) with new IDH1/2-mutated post–MPN AML treated with IDH1/2-i combinations.
Introduction
Leukemic transformation of myeloproliferative neoplasms (MPNs) to post–MPN acute myeloid leukemia (AML) occurs in ∼20% to 25% of cases and carries a dismal prognosis, with a median survival of ∼6 months.1-3 Only allogeneic stem cell transplantation (alloSCT) has the potential to provide long-term survival, but this modality is feasible in a limited number of patients.4,5
Mutations in the isocitrate dehydrogenase 1/2 (IDH1/2) genes result in accumulation of the oncometabolite 2-hydroxyglutarate, elicit DNA and histone hypermethylation, impair myeloid differentiation, and promote leukemogenesis.6,7 The occurrence of IDH1/2 mutations increases from ∼1% to 4% in chronic-phase MPN (MPN-CP) to ∼22% in blast-phase MPN (MPN-BP or post-MPN AML), strongly evidencing their role in MPN progression.8 Indeed, co-occurring JAK2 V617F, the most common "driver" mutation in MPN patients, and IDH1/2 mutations have been reported to induce a more profound MPN phenotype with increased stem/myeloid progenitor cell populations and altered cell functions, likely facilitating disease transformation to AML.8,9
The oral, targeted, small-molecule isocitrate dehydrogenase inhibitors enasidenib (formerly AG-221)10-12 and ivosidenib (formerly AG-120)13 were approved for treatment of patients with IDH2- and IDH1-mutated relapsed/refractory (R/R) AML, respectively. Furthermore, ivosidenib was recently approved for treatment of newly diagnosed IDH1-mutated AML patients aged ≥75 years or considered unfit for intensive chemotherapy.14 However, the efficacy of IDH-inhibitor–based therapies in patients with IDH1/2-mutated post–MPN AML is largely unknown, because few patients were enrolled in the original studies evaluating IDH1/2 inhibitors. Herein, we analyze the clinical characteristics and outcomes of IDH1/2-mutated post–MPN AML patients treated with IDH1/2 inhibitors as monotherapies or in combination with ruxolitinib (JAK1/2 inhibitor), venetoclax (BCL-2 inhibitor), hypomethylating agents (azacitidine, decitabine), or intensive chemotherapy.
Methods
We searched the Leukemia Department database at the MD Anderson Cancer Center for patients with post–MPN AML (≥20% blasts in the bone marrow or peripheral blood) who harbored IDH1/2 mutations and were treated with IDH1/2 inhibitors.
Responses to AML treatment were assessed according to the 2017 European LeukemiaNet (ELN) criteria.15 Next-generation sequencing (NGS) of 28, 53, or 81 gene panels (depending on the year of presentation) was performed using an Illumina MiSeq platform.16 The variant allele frequency (VAF) detection limit for IDH1/2 and JAK2 was ∼2%.
Responses and clinical data were analyzed using descriptive statistics. Overall survival (OS) and duration of responses (both calculated from the start of initial IDH1/2-inhibitor–based treatment) were estimated using Kaplan-Meier curves. GraphPad Prism v.8 (San Diego, CA) and SPSS v.23 (IBM Corp., New York, NY) were used for all the analyses. This study was approved by the Institutional Review Board and was in accordance with the Declaration of Helsinki.
Results and discussion
Among 107 patients with post–MPN AML, we identified 12 who harbored IDH1 (n = 7) or IDH2 (n = 5) and received IDH1/2-inhibitor–based treatments. Antecedent MPN diagnoses included primary myelofibrosis (PMF) in 7 patients, polycythemia vera (PV) in 2 patients, and post–PV myelofibrosis (MF), essential thrombocythemia (ET), and post–ET MF in 1 patient each (Figure 1A). Median time to AML from PMF, post–PV MF, and post–ET MF was 24 months (range, 5-92), and median time to AML from ET or PV was 108 months (range, 48-264).
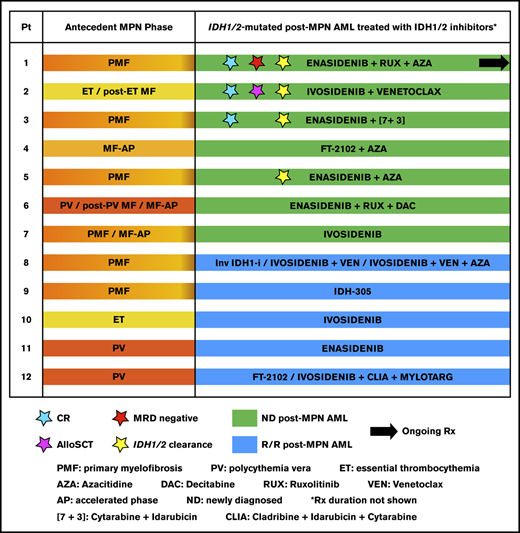
Timelines and treatments (IDH1/2-inhibitor–based regimens and others) during MPN in chronic- or accelerated-phase (MPN-CP/AP), post-MPN acute leukemia (for patients 8 through 12), and IDH1/2-mutated post-MPN AML for the patients in this study (N = 12). (A) Type of antecedent MPN, duration of disease, and treatment(s) are depicted for each patient; horizontal bar colors are coded according to the type of MPN (left panel). Acute leukemia that evolved from MPN (AML in patients 8-11 and ALL in patient 12), duration, and treatment of disease at other institutions (right panel). (B) Continued timelines from (A) for patients 1 through 12 with IDH1/2-mutated post–MPN AML, treated with IDH1/2 inhibitors as monotherapies or combinations, at the MD Anderson Cancer Center. Patients 1 through 7 had newly diagnosed post–MPN AML (depicted with green horizontal bars), and patients 8 through 12 had R/R post–MPN AML (depicted with blue horizontal bars); horizontal bar length indicates the duration of each treatment. Treatments based on IDH1/2 inhibitors are shown on the green and blue horizontal bars. IDH1/2 inhibitors were named according to their clinical use: enasidenib for the IDH2 inhibitor AG-221 if it was used off clinical study, and AG-221 if it was used in a clinical study; and ivosidenib for the IDH1 inhibitor AG-120 if it was used off clinical study, and AG-120 if it was used in a clinical study. FT-2102 and IDH-305 are IDH1 inhibitors (IDH-305 is no longer in clinical development). ACE-011, LCL161, inhibitor of bromodomain containing protein 4 (BRD4i), and HM43239 are investigational agents. IDH1/2 clearance is defined as undetectable VAF by NGS. AL, acute leukemia; ALL, acute lymphoblastic leukemia; Ara-C, cytarabine; AZA, azacitidine; [7+3], 7-day continuous infusion of cytarabine on days 1 to 7 and idarubicin on days 1 to 3; BIDFA, combination chemotherapy comprising fludarabine and idarubicin twice daily; BLINA, blinatumomab; CLIA, combination chemotherapy with cladribine, high-dose cytarabine, and idarubicin; CLOF, clofarabine; DAC, decitabine; EWALL, regimen comprising dasatinib in combination with chemotherapy, used in the European Working Group on Adult Acute Lymphoblastic Leukemia study for treatment of patients with ALL; FLAG-IDA, combination chemotherapy regimen comprising fludarabine, cytarabine, idarubicin and granulocyte colony-stimulating factor (G-CSF); GO, gemtuzumab ozogamicin (Mylotarg); HU, hydroxyurea; Inv IDH1-i, investigational IDH1 inhibitor; IDH2-i Rx, IDH2-inhibitor–based treatment; IVO, ivosidenib; LEN, lenalidomide; MF-AP, myelofibrosis in accelerated phase (≥10%-19% blasts in the bone marrow or peripheral blood); MRD, measurable residual disease; ND, newly diagnosed; Pt, patient; R/R, relapsed/refractory; RUX, ruxolitinib; SOR, sorafenib; VEN, venetoclax.
Timelines and treatments (IDH1/2-inhibitor–based regimens and others) during MPN in chronic- or accelerated-phase (MPN-CP/AP), post-MPN acute leukemia (for patients 8 through 12), and IDH1/2-mutated post-MPN AML for the patients in this study (N = 12). (A) Type of antecedent MPN, duration of disease, and treatment(s) are depicted for each patient; horizontal bar colors are coded according to the type of MPN (left panel). Acute leukemia that evolved from MPN (AML in patients 8-11 and ALL in patient 12), duration, and treatment of disease at other institutions (right panel). (B) Continued timelines from (A) for patients 1 through 12 with IDH1/2-mutated post–MPN AML, treated with IDH1/2 inhibitors as monotherapies or combinations, at the MD Anderson Cancer Center. Patients 1 through 7 had newly diagnosed post–MPN AML (depicted with green horizontal bars), and patients 8 through 12 had R/R post–MPN AML (depicted with blue horizontal bars); horizontal bar length indicates the duration of each treatment. Treatments based on IDH1/2 inhibitors are shown on the green and blue horizontal bars. IDH1/2 inhibitors were named according to their clinical use: enasidenib for the IDH2 inhibitor AG-221 if it was used off clinical study, and AG-221 if it was used in a clinical study; and ivosidenib for the IDH1 inhibitor AG-120 if it was used off clinical study, and AG-120 if it was used in a clinical study. FT-2102 and IDH-305 are IDH1 inhibitors (IDH-305 is no longer in clinical development). ACE-011, LCL161, inhibitor of bromodomain containing protein 4 (BRD4i), and HM43239 are investigational agents. IDH1/2 clearance is defined as undetectable VAF by NGS. AL, acute leukemia; ALL, acute lymphoblastic leukemia; Ara-C, cytarabine; AZA, azacitidine; [7+3], 7-day continuous infusion of cytarabine on days 1 to 7 and idarubicin on days 1 to 3; BIDFA, combination chemotherapy comprising fludarabine and idarubicin twice daily; BLINA, blinatumomab; CLIA, combination chemotherapy with cladribine, high-dose cytarabine, and idarubicin; CLOF, clofarabine; DAC, decitabine; EWALL, regimen comprising dasatinib in combination with chemotherapy, used in the European Working Group on Adult Acute Lymphoblastic Leukemia study for treatment of patients with ALL; FLAG-IDA, combination chemotherapy regimen comprising fludarabine, cytarabine, idarubicin and granulocyte colony-stimulating factor (G-CSF); GO, gemtuzumab ozogamicin (Mylotarg); HU, hydroxyurea; Inv IDH1-i, investigational IDH1 inhibitor; IDH2-i Rx, IDH2-inhibitor–based treatment; IVO, ivosidenib; LEN, lenalidomide; MF-AP, myelofibrosis in accelerated phase (≥10%-19% blasts in the bone marrow or peripheral blood); MRD, measurable residual disease; ND, newly diagnosed; Pt, patient; R/R, relapsed/refractory; RUX, ruxolitinib; SOR, sorafenib; VEN, venetoclax.
Demographics and clinical characteristics of the patients are detailed in Tables 1 and 2. Median age was 67 years (range, 47-82). Sixty-six percent of the patients had anemia (hemoglobin <10 g/dL) or thrombocytopenia (platelet count <100 × 109/L) at baseline. Prior to IDH1/2-inhibitor–based treatment, seven and 5 patients harbored IDH1 (R132C in 6 and R132H in 1) and IDH2 R140Q mutations, respectively (Tables 1 and 2). The majority of the patients (10/12) harbored JAK2 V617F at baseline. Very high-risk/unfavorable-risk cytogenetics were detected in 75% of the patients (Table 1).
Patients were treated with ivosidenib, FT-2102 (olutasidenib),17 IDH-305,17 an investigational IDH1 inhibitor, or enasidenib in the frontline setting (n = 7; first 7 patients in Table 2 and Figure 1B) or in the salvage setting (n = 5; patients 8 through 12 in Table 2 and Figure 1B). Two patients received >1 regimen containing IDH1 inhibitors (patients 8 and 12 in Figure 1B and Table 2), both in the salvage setting. Median follow-up since post–MPN AML diagnosis was 11 months (range, 1 to >22+).
Three patients achieved complete remission (CR); all were treated with IDH1/2-inhibitor–based combinations in the frontline setting (Tables 1 and 2; Figure 1B). These 3 patients also achieved undetectable IDH1/2 mutations by NGS, and patient 1 achieved measurable residual disease (MRD) negativity by flow cytometry. Two of the 3 patients had ongoing responses as of data cutoff date. The first case involves the extraordinary outcome of an 82-year-old patient who has been in sustained CR (both hematologic and molecular with undetectable IDH2 and JAK2 V617F) and achieved MRD negativity, with ongoing IDH2-inhibitor-based combination treatment (enasidenib/ruxolitinib/azacitidine) for more than 2 years (patient 1 in Figure 1B). At the time of progression from PMF to AML, which occurred after 4 years on combination treatment with ruxolitinib and azacitidine for PMF (50 cycles),18 the mutation IDH2 R140Q was detected in patient 1, and enasidenib was added to the regimen. The second case pertains to a 47-year old man (patient 2 in Figure 1B) who achieved CR (MRD 0.18%) with 1 cycle of ivosidenib/venetoclax, followed by alloSCT (ongoing CR for 9+ months). The third patient achieved CR (MRD 0.3%) for 7 months while on treatment with enasidenib and intensive chemotherapy but ultimately relapsed (patient 3 in Figure 1B). Median duration of response for the 3 responders was 17.5 months (range, 3-22+). Two additional patients treated with frontline IDH1/2-inhibitor–based combinations achieved stable disease (SD) with a ≥50% blast reduction that was maintained for 4 to 8 months (patients 4 and 5 in Figure 1B and Table 2).
Median OS of all patients and those in CR (n = 3, all were treated in the frontline setting) was 10 months (range, 1-22+) and 19 months (range, 9-22+) after initiation of IDH1/2 inhibitors, respectively. Median OS of the patients with newly diagnosed post–MPN AML and R/R post–MPN AML was 11 and 7 months, respectively. Among 8 patients with initial red cell transfusion dependency, 4 became transfusion independent for ≥56 days during therapy (median duration 9 months; range, 4-18).
Treatments were well tolerated, with only 1 patient discontinuing therapy because of gastrointestinal complaints (nausea/vomiting). Five patients experienced differentiation syndrome (1 grade 3); all responded to oral corticosteroids. Two newly diagnosed and 3 R/R patients experienced febrile neutropenia, with 4 patients requiring IV antibiotics. These results are in line with the overall good tolerability of these agents, as previously reported.11-14,17,19
The present study showed that IDH1/2-inhibitor–based treatments in patients with IDH1/2-mutated post–MPN AML were well tolerated and effective, especially in those with newly diagnosed post–MPN AML. It should be noted that our results are in agreement with the overall better efficacy of combination regimens over monotherapies in IDH1/2-mutated AML without an antecedent MPN. The synergism of IDH1/2-inhibitors with ruxolitinib, hypomethylating agents, venetoclax, and intensive chemotherapy was previously shown in preclinical studies, and recent clinical trials.9,19-26 Among 6 patients with newly diagnosed post–MPN AML that were treated with combination regimens, 3 achieved CR with deep molecular responses, namely: undetectable IDH1/2 mutations (patients 1 through 3 in Figure 1B and Table 2). Patients 4 and 5 who were treated with a combination of an IDH1/2 inhibitor and azacitidine did not achieve CR but had a significant decrease in blasts (from 86% to 7% and from 79% to 9%, respectively), and patient 5 achieved undetectable IDH2 by NGS (Table 2; Figure 1B). In contrast, complete responses were not noted in any of the newly diagnosed or R/R post–MPN AML patients treated with monotherapy.
Although none of the 5 patients with R/R AML (patients 8 through 12 in Figure 1B) achieved CR, 3 had SD with a ≥50% reduction in blasts, with a clinical benefit lasting >8 months. Patient 8 was treated with 3 IDH1 inhibitors (salvage 3 to 5) and survived >12 months (since treatment with the first IDH1 inhibitor). This is a considerably long survival for a patient with refractory post–MPN AML who had failed a hypomethylating agent and high-intensity chemotherapy.
Our results are noteworthy given that the cohort included older (median age 67 years) post–MPN AML patients; and the majority of them had unfavorable-risk/very high-risk cytogenetics27 and high molecular risk/adverse mutations12,15,19,28,29 (eg, ASXL1, RUNX1, and RAS-pathway; Table 1), which have been associated with inferior OS and resistance to treatments, including IDH1/2 inhibitors.7,11,17 Well tolerated IDH1/2-inhibitor–based therapies further emphasize their favorable clinical impact in this IDH1/2-mutated post–MPN AML population. Median OS of 10 months for all patients and 19 months in responders compare favorably with the historically modest or poor survivals reported for post-MPN AML.1-3 For example, the median overall survivals of two cohorts with post-MPN AML that were treated with the combination of ruxolitinib and decitabine in phase 1 and 1/2 trials, conducted by Rampal et al30 and Bose et al,31 were 7.2 and 8.4 months, respectively. In this study, the overall responses in newly diagnosed IDH1/2-mutated post–MPN AML were comparable to those reported for IDH1/2-inhibitor–treated AML without an antecedent MPN.11,14
During the time that our manuscript was in revision, Patel et al reported another retrospective study showing long-lasting responses in a small cohort of 8 patients who had IDH2-mutated MPN in accelerated or blast phase (MPN-AP/BP) and were treated with enasidenib monotherapy or enasidenib/azacitidine (in 1 case).32 Both the aforementioned study and the present one demonstrate the notable benefit that treatment with IDH1/2 inhibitors confers to patients with IDH1/2-mutated advanced-phase MPN and indicate that there is merit in pursuing clinical trials with IDH1/2 inhibitors in this population. For example, a phase 2 clinical trial, evaluating the combination of enasidenib with ruxolitinib in patients with IDH2-mutated MPN-AP/BP or chronic-phase MF (4%-9% circulating blasts), is planned for the near future (NCT04281498), given that preclinical studies demonstrated synergism of enasidenib with ruxolitinib in patient-derived IDH2/JAK2-mutated MPN and MPN-BP cells.9 The 2 studies in discussion complement each other given the differences in the 2 cohorts of patients, their clinical and molecular characteristics, and the IDH1/2-inhibitor–based treatments they received. All the patients in this study had MPN-BP, whereas the patients in the study reported by Patel et al had MPN-AP/BP32 ; typically, the median OS of patients with MPN-AP is longer than that of patients with MPN-BP.30,33 Furthermore, in the study by Patel et al, all the patients harbored IDH2, and the majority of them were treated with enasidenib monotherapy in the frontline setting.32 Our cohort included IDH1/2-mutated MPN-BP patients who were treated in the frontline or R/R (salvage ≥2 for all patients) setting, and the majority of the patients (8/12) received combination treatments of IDH1/2 inhibitors with other drugs. Recently, Choe et al34 reported high response rates in a small group of patients, with IDH1-mutated MPN-BP and JAK2 V617F at baseline; the patients were treated with ivosidenib monotherapy in the R/R setting, in a multicenter phase 1 study, conducted by DiNardo et al.13 Collectively, all the aforementioned results indicate that timely detection of the high molecular risk mutations IDH1/235,36 and early initiation of IDH1/2-inhibitor–based treatment may result in superior outcomes in patients with advanced-phase MPN.
Notwithstanding the challenge of treating patients with post–MPN AML, especially R/R, and the heterogeneity of treatments and the small size of our cohort, this study demonstrated that treatment with IDH1/2 inhibitors provides an efficacious option for patients who are older or unfit for intensive chemotherapy and alloSCT. Moreover, IDH1/2-inhibitor–based treatments can serve as a bridge to alloSCT in young and/or fit patients; alloSCT should be considered in patients with post–MPN AML upon achievement of CR or return to MPN-CP.4,5,37-41
In summary, we noted encouraging clinical efficacy and safety in our cohort of patients with IDH1/2-mutated post–MPN AML treated with IDH1/2-inhibitor–based regimens. Three patients who were treated with IDH1/2-inhibitor–based combinations in the frontline setting achieved CR (1 MRD negative) with deep IDH1/2 molecular remissions and reached a median OS of 19 months; these results compare favorably with the historically poor survivals of post–MPN AML patients who do not undergo alloSCT. Our results suggest that timely identification of actionable (targetable) IDH1/2 mutations at initial diagnosis or relapse and treatment with IDH1/2 inhibitors, alone or in rational combinations, should be considered for patients with IDH1/2-mutated post–MPN AML.
Data sharing requests should be sent to Srdan Verstovsek (sverstov@mdanderson.org).
Acknowledgments
This research was supported, in part, by the MD Anderson Cancer Center Support Grant P30 CA016672 from the National Cancer Institute, National Institutes of Health. C.D.D. is a recipient of the Lloyd Family/V Foundation Clinical Scholar Award.
Authorship
Contribution: L.M. and S.V. designed the study; H.T.C., L.M., M.A., and S.V. analyzed data; K.P.P. provided molecular data and analyses; L.M., C.D.D., N.D., Y.A., E.J., M.K., H.M.K. and S.V. treated patients and provided study material; H.T.C., L.M., and S.V. wrote the manuscript; C.D.D., N.D., E.J. and M.K. reviewed the manuscript and made insightful clinical points; and all authors participated in the discussion, reviewed and approved the final version of the article, and had access to all the data.
Conflict-of-interest disclosure: C.D.D. has acted as a consultant/advisor for AbbVie, Agios, Celgene, Daiichi Sankyo, and Notable Labs; and has received research funds from AbbVie, Agios, Celgene, and Daiichi Sankyo. N.D. has acted as a consultant/advisor for Daiichi-Sankyo, Bristol Myers Squibb, Astellas, AbbVie, Genentech, Immunogen, Pfizer, Amgen, Forty Seven, and Novartis; and has received research funds from Bristol Myers Squibb, Pfizer, Forty Seven, Genentech, AbbVie, Astellas, Daiichi-Sankyo, Incyte, Novimmune, and Immunogen. E.J. has acted as a consultant/advisor for Adaptive Biotechnologies, AbbVie, Amgen, Bristol Myers Squibb, Daiichi Sanko, Astellas, Pfizer and Takeda; and has received research funds from Adaptive Biotechnologies, AbbVie, Amgen, Pfizer, and Takeda. H.M.K. has acted as a consultant/advisor for AbbVie, Actinium (Advisory Board), Agios, Amgen, Immunogen, Pfizer, and Takeda; and has received research funds from AbbVie, Agios, Amgen, ARIAD Pharmaceuticals, Astex, Bristol Myers Squibb, Cyclacel Pharmaceuticals, Daiichi-Sankyo, Immunogen, Jazz Pharma, Novartis, and Pfizer. The remaining authors declare no competing financial interests.
The current affiliation for M.A. is Department of Hematology, King Fahad Medical City, Riyadh, Saudi Arabia.
Correspondence: Srdan Verstovsek, Department of Leukemia, MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: sverstov@mdanderson.org.
References
Author notes
H.T.C. and L.M. contributed equally to this work and share first authorship.

![Timelines and treatments (IDH1/2-inhibitor–based regimens and others) during MPN in chronic- or accelerated-phase (MPN-CP/AP), post-MPN acute leukemia (for patients 8 through 12), and IDH1/2-mutated post-MPN AML for the patients in this study (N = 12). (A) Type of antecedent MPN, duration of disease, and treatment(s) are depicted for each patient; horizontal bar colors are coded according to the type of MPN (left panel). Acute leukemia that evolved from MPN (AML in patients 8-11 and ALL in patient 12), duration, and treatment of disease at other institutions (right panel). (B) Continued timelines from (A) for patients 1 through 12 with IDH1/2-mutated post–MPN AML, treated with IDH1/2 inhibitors as monotherapies or combinations, at the MD Anderson Cancer Center. Patients 1 through 7 had newly diagnosed post–MPN AML (depicted with green horizontal bars), and patients 8 through 12 had R/R post–MPN AML (depicted with blue horizontal bars); horizontal bar length indicates the duration of each treatment. Treatments based on IDH1/2 inhibitors are shown on the green and blue horizontal bars. IDH1/2 inhibitors were named according to their clinical use: enasidenib for the IDH2 inhibitor AG-221 if it was used off clinical study, and AG-221 if it was used in a clinical study; and ivosidenib for the IDH1 inhibitor AG-120 if it was used off clinical study, and AG-120 if it was used in a clinical study. FT-2102 and IDH-305 are IDH1 inhibitors (IDH-305 is no longer in clinical development). ACE-011, LCL161, inhibitor of bromodomain containing protein 4 (BRD4i), and HM43239 are investigational agents. IDH1/2 clearance is defined as undetectable VAF by NGS. AL, acute leukemia; ALL, acute lymphoblastic leukemia; Ara-C, cytarabine; AZA, azacitidine; [7+3], 7-day continuous infusion of cytarabine on days 1 to 7 and idarubicin on days 1 to 3; BIDFA, combination chemotherapy comprising fludarabine and idarubicin twice daily; BLINA, blinatumomab; CLIA, combination chemotherapy with cladribine, high-dose cytarabine, and idarubicin; CLOF, clofarabine; DAC, decitabine; EWALL, regimen comprising dasatinib in combination with chemotherapy, used in the European Working Group on Adult Acute Lymphoblastic Leukemia study for treatment of patients with ALL; FLAG-IDA, combination chemotherapy regimen comprising fludarabine, cytarabine, idarubicin and granulocyte colony-stimulating factor (G-CSF); GO, gemtuzumab ozogamicin (Mylotarg); HU, hydroxyurea; Inv IDH1-i, investigational IDH1 inhibitor; IDH2-i Rx, IDH2-inhibitor–based treatment; IVO, ivosidenib; LEN, lenalidomide; MF-AP, myelofibrosis in accelerated phase (≥10%-19% blasts in the bone marrow or peripheral blood); MRD, measurable residual disease; ND, newly diagnosed; Pt, patient; R/R, relapsed/refractory; RUX, ruxolitinib; SOR, sorafenib; VEN, venetoclax.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/4/21/10.1182_bloodadvances.2020001528/3/m_advancesadv2020001528f1.png?Expires=1768480068&Signature=qB97m-QqqE17plGGHbLt98T4~AO~4blg6jYwPsi3pNMQLmX-j7QifCwSqj-wObm3lXwPzZQOfht2snmonF5B20snwVWvJZRySnOMjwGOiiSqR0ogq46xRmwQMJvoh8HZ7jfeP-Mwv6MySKVPhK5cXGAB7g5TUGwt4b8lqrEk03Fri5ya2TCTjisxviyJcnIHBHgyuzVDbLqmUpx1-LW8ZSCdt0r63k0exSLrB9CXYG5PW1NGRe975oNGU25qN8qsSlq1~ABv8NoctOw9rhp-47Bp4Oje3sHvAp7fVj8NGGn7wF~hI5Mln3CMSnhG88NVgqYJ3CHpQAxO6Fp7kLgmbg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
