Key Points
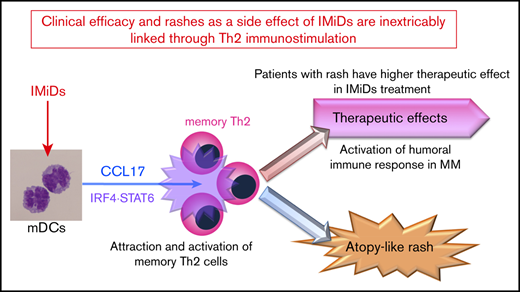
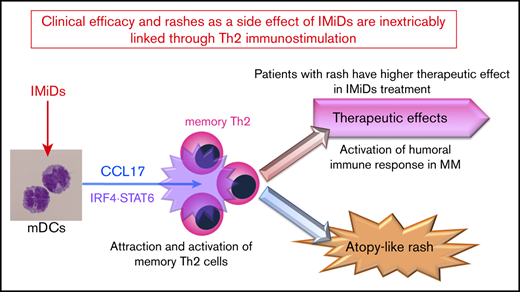
IMiDs potentially enhance DC-mediated allergic Th2 responses (CCL17 secretion and memory Th2 response) through upregulated STAT6 and IRF4.
Serum CCL17 levels at the onset of lenalidomide-associated rash are higher and correlate with clinical outcome of lenalidomide treatment.
Abstract
Immunomodulatory drugs (IMiDs), lenalidomide and pomalidomide, are widely used treatments for multiple myeloma; however, they occasionally lead to episodes of itchy skin and rashes. Here, we analyzed the effects of IMiDs on human myeloid dendritic cells (mDCs) as major regulators of Th1 or Th2 responses and the role they play in allergy. We found that lenalidomide and pomalidomide used at clinical concentrations did not affect the survival or CD86 and OX40-ligand expression of blood mDCs in response to lipopolysaccharide (LPS) and thymic stromal lymphopoietin (TSLP) stimulation. Both lenalidomide and pomalidomide dose-dependently inhibited interleukin-12 (IL-12) and TNF production and STAT4 expression, and enhanced IL-10 production in response to LPS. When stimulated with TSLP, both IMiDs significantly enhanced CCL17 production and STAT6 and IRF4 expression and promoted memory Th2-cell responses. In 46 myeloma patients, serum CCL17 levels at the onset of lenalidomide-associated rash were significantly higher than those without rashes during lenalidomide treatment and those before treatment. Furthermore, serum CCL17 levels in patients who achieved a very good partial response (VGPR) were significantly higher compared with a less than VGPR during lenalidomide treatment. The median time to next treatment was significantly longer in lenalidomide-treated patients with rashes than those without. Collectively, IMiDs suppressed the Th1-inducing capacity of DCs, instead promoting a Th2 response. Thus, the lenalidomide-associated rashes might be a result of an allergic response driven by Th2-axis activation. Our findings suggest clinical efficacy and rashes as a side effect of IMiDs are inextricably linked through immunostimulation.
Introduction
Multiple myeloma (MM) is a multistep clonal B-cell malignancy characterized by the aberrant accumulation of plasma cells within bone marrow or an extramedullary site, leading to bone destruction, renal dysfunction, and marrow failure. This disease is generally regarded as incurable. However, treatment of MM has evolved with the introduction of new drugs, including immunomodulatory drugs (IMiDs), proteasome inhibitors, and antibody drugs; thus, the 5-year survival rate has gradually increased due to new drug development over the past decade.
Lenalidomide (LEN) and pomalidomide (POM) are analogs of thalidomide and integral backbone drugs for the treatment of MM. At present, the standard of care for patients with newly diagnosed or relapsed/refractory MM is to administer LEN combined with dexamethasone (LEN-DEX). POM is considered a treatment option in patients with LEN-refractory MM. Despite the benefits of IMiDs, recent clinical investigations have determined their toxicity profile and found that skin rashes are a frequent side effect. In a randomized trial for newly diagnosed MM patients, rashes of any grade were observed in 26.1% to 28% of patients administered LEN-DEX as a first-line treatment1 and in 50% of Japanese patients.2 Similarly, any grade rashes have been reported for 11.3% in LEN-treated patients and 20.6% in POM-treated patients with relapsed or refractory MM.3-5 Furthermore, 20% to 31.7% of patients receiving LEN maintenance therapy after autologous stem-cell transplantation experienced rashes.1,6
IMiDs have an immunomodulatory effect in addition to a direct tumoricidal effect. LEN promotes the proliferation of some immune effector cells in vivo and the total percentage of proliferating CD4+ T cells, CD8+ T cells, and natural killer (NK) cells progressively increased after LEN-DEX administration in high-risk smoldering MM patients.7 Additionally, LEN induces qualitative activation of NK cells and cytotoxic lymphocyte,8-13 and inhibits the proliferation and function of regulatory T cells.14,15 POM also has immunostimulatory activity, expanding T cells and NK cells in LEN-refractory MM patients, which leads to improved clinical responses.16 Thus, IMiDs have a potent immunostimulatory effect, facilitating the attack of MM cells by activated immune effectors. However, the cellular and molecular mechanisms underlying their immunomodulatory effects remain largely unclear.
There is evidence of immunomodulatory activity of IMiDs on mouse dendritic cells (DCs)13,17 and a synergistic effect with DC vaccination in model of murine MM18-20 or colon cancer,21 however, only a few reports have examined its effects on human DC subsets.22-25 DCs are capable of inducing Th1 or Th2 responses. Myeloid DCs (mDCs) are the major inducers of Th2 responses and play an important role in allergy by their dysregulated Th2-inducing function in diseases such as atopic dermatitis and asthma.26,27 Epithelial cell-derived thymic stromal lymphopoietin (TSLP) is a key cytokine in the communication between epithelial cells and mDCs such as Langerhans cells (LCs) at the interface of allergic inflammation.28,29 TSLP is highly expressed by keratinocytes in atopic dermatitis patients, and TSLP expression is associated with LC migration and activation.30 Overexpression of TSLP in mice leads to the development of atopic dermatitis, confirming the link between TSLP and atopic dermatitis.31-33 TSLP-stimulated mDCs can induce naïve CD4+ T cells to differentiate into Th2 cells.30,34 Furthermore, TSLP equips mDCs with the capacity to produce TARC/CCL17,35 which attracts memory Th2 cells, the principal cells responsible for the maintenance of allergic inflammation and the relapse of allergic inflammation upon reexposure to allergens.36,37
Therefore, to assess the immunomodulatory effects of IMiDs, we focused on the actions of LEN and POM on human mDC function. We elucidated an additional cellular target of IMiDs and reported here that IMiDs inhibited DC-mediated Th1 immune responses but enhanced DC-mediated Th2 memory responses. Furthermore, serum levels of CCL17 expression were associated with skin rashes caused by LEN in MM patients, which indicate an improved response to LEN treatment.
Material and Methods
Reagents
Media used in our experiments is shown in supplemental Methods. TSLP (R&D Systems), polyinosinic:polycytidylic (poly[I:C]; InvivoGen), R848 (InvivoGen) and lipopolysaccharide (LPS; from Escherichia coli 0111:B4, InvivoGen) were used at 15 ng/mL, 25 μg/mL, 1 μg/mL, and 1 μg/mL, respectively, and dexamethasone (Sigma-Aldrich) was used at 10-8 to 10-6 M. Lenalidomide (SelleckChem), pomalidomide (Sigma), and bortezomib (LC Laboratories) were dissolved in 100% dimethyl sulfoxide before further dilution in cell culture media. Dimethyl sulfoxide was diluted in parallel to be used as vehicle controls.
Isolation of blood DCs
All studies involving human samples were performed under institutional review board-approved protocols at Kansai Medical University. All subjects provided written informed consent in accordance with the recommendations of the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki.
Human peripheral blood DC subsets were isolated from the peripheral blood mononuclear cells of healthy adult donors, as described previously.38,39
Complete methods and a full list of antibodies are provided in supplemental Methods. mDCs as CD11c+/lineage−/BDCA4−/CD4+ fraction was sorted (supplemental Figure 1). Purified mDCs were seeded at a density of 5 × 104 cells per 200 μL media in flat-bottomed 96-well plates.
Analysis of DCs
mDCs were stained with FITC-labeled anti-CD86 (2331), anti-CD80 (L307.4), or anti-CD83 (HB15e) antibodies (all from BD Biosciences) after 24-hour culture and phycoerythrin (PE)-labeled anti-OX40L (ANC10G1: Ancell) after 48-hour culture, and then analyzed using a FACS Calibur (BD Biosciences). Viable cells were counted in triplicate using trypan-blue exclusion to identify dead cells and were also evaluated using an annexin-V-FITC Apoptosis Detection kit (Sigma-Aldrich). The production of interleukin-10 (IL-10), IL-12p40, IL-12p70, tumor necrosis factor (TNF), CCL17, IL-27 (kits from R&D Systems), and interferon-λ1 (IFN-λ1) (eBioscience) in the culture supernatants was determined by enzyme-linked immunosorbent assay (ELISA).
Purification of CD4 T-cell subsets
CD4+ T cells were isolated using CD4+ T cell Isolation Kit II (Miltenyi Biotec). Naïve CD4+ T cells were sorted into fractions of CD4+CD45RA+ of the stained total CD4+ T cells with a fluorescein isothiocyanate (FITC)-labeled lineage cocktail (CD8, CD14, CD16, CD19, CD56, BDCA2, TCRγ/δ, and glycophorin-A), PE-Cy5.5-labeled anti-CD4, and APC-labeled anti-CD45RA (BD Biosciences). CRTH2+CD4+ memory T cells were isolated using a CD294 (CRTH2) MicroBead Kit (Miltenyi Biotec), followed by cell sorting using PE-labeled anti-CD294 (BM-16: Miltenyi Biotec) and PE-Cy5.5-labeled anti-CD4.
Analyses of T-cell cytokine production
Purified allogenic 1 × 104 CD4+ T cells were cocultured with cultured mDCs (DC-to-T cell ratio, 1:2) in 96-well round-bottom culture plates. After 7 days of DC-T cell coculture, primed CD4+ T cells were collected and restimulated for 24 hours with immobilized anti-CD3 (OKT3, 5 μg/mL) and soluble anti-CD28 (1 μg/mL) at a concentration of 106 cells/mL. The levels of IL-4, IL-5, IL-13, and IFN-γ in the supernatant were each measured by CBA (BD Biosciences).
Real-time PCR and microarray analysis
For real-time polymerase chain reaction (PCR) analysis, purified mDCs were stimulated with LPS or TSLP in the presence of LEN or POM at 0.3 μM for 4 hours. The cells were collected for complementary DNA synthesis using the QuantiTect. Additional experimental procedures of PCR method are detailed in the supplemental Methods.
For microarray gene expression analysis, mDCs from 5 donors were individually stimulated with LPS or TSLP with or without 0.3 μM LEN or 0.3 μM POM for 8 hours. Total RNA was directly extracted from DCs in each treatment group. Then, for each treatment group, samples from the 5 donors were pooled and used for hybridization by a single run microarray. The synthesis of target complementary RNA probes and hybridization were performed using a Low RNA Input Linear Amplification kit (Agilent Technologies) according to the manufacturer’s instructions. Additional experimental procedures and bioinformatics are detailed in the supplemental Methods.
Patient and treatment in MM patients
We analyzed 46 cases of newly diagnosed or relapsed/refractory MM receiving LEN-DEX along with 28 cases treated with bortezomib and DEX without discontinuation because of adverse events between January 2013 and December 2017 (supplemental Table 1). Patients received oral LEN 5 to 25 mg per day (depending on the judgment of attending physicians in clinical practice) on days 1 through 21 of each 28-day cycle, or patients received intravenous or intracutaneous bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11. All patients received 12 to 40 mg oral or intravenous DEX on days 1, 8, 15, and 22 of each 28-day cycle until disease progression. Best response rate based on the International Myeloma Working Group criteria40 was evaluated during the 6 cycles of treatments retrospectively. Onset and grade of skin rashes were evaluated using the Common Terminology Criteria for Adverse Events, version 4.0. Fourteen of the 46 patients had skin rashes after LEN-DEX treatment (supplemental Table 2), and the treatment was continued by reduction or transient discontinuation of LEN with simultaneous administration of steroid and/or antihistamine drugs in clinical practice. One patient developed grade 1 skin rash after administration of bortezomib with DEX, but bortezomib treatment was continued in this patient. Serum samples were collected from patients with written informed consent at the onset of skin rashes on patients or after the 2 cycles of the treatment without skin rash on patients together with blood examination in clinical practice. Serum CCL17/TARC levels were analyzed using ELISA kits (R&D Systems). Time to the next treatment was defined as the time from LEN-DEX administration to the date of next antimyeloma treatment or death as recorded in the medical records. Patients who did not receive subsequent therapy were censored at the date of their last follow-up visit. The median follow-up period of 46 patients was 20.3 months (range, 2.0-55.6 months).
Statistical analysis
Data of the in vitro experiments were analyzed using paired Student t test. Data of serum CCL17 levels in patients were analyzed using Wilcoxon matched-pairs signed-rank test for paired samples or Mann-Whitney U tests for unpaired samples. Data from 2 groups (supplemental Table 2) were compared by 2-way contingency-table analysis using Fisher’s exact tests. For the time to the next treatment analyses in Figure 7E used the Kaplan-Meier method and log-rank test for comparison between the groups. A value of P < .05 was considered statistically significant. Data analysis was carried out using Prism (GraphPad Software).
Results
Clinical concentration of LEN and POM do not affect human mDC survival and maturation
LEN and POM, as well as bortezomib, have direct tumoricidal effects against MM cells41,42 ; therefore, we first analyzed the viability of human mDCs upon activation in the presence of LEN and POM. Clinical pharmacokinetics shows that clinical peak plasma LEN concentrations in MM patients administered with 10 and 25 mg oral LEN are 1.2 μM (311 ng/mL) and 2.7 μM (714 ng/mL), respectively,43 and 4 mg oral POM yields 0.27 μM (75 ng/mL) in the plasma.5
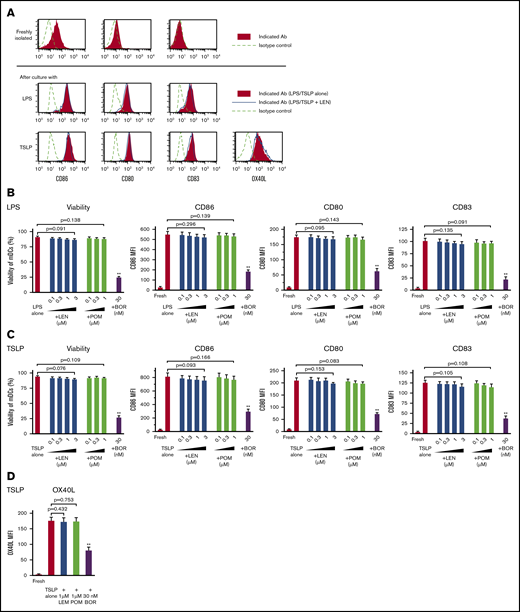
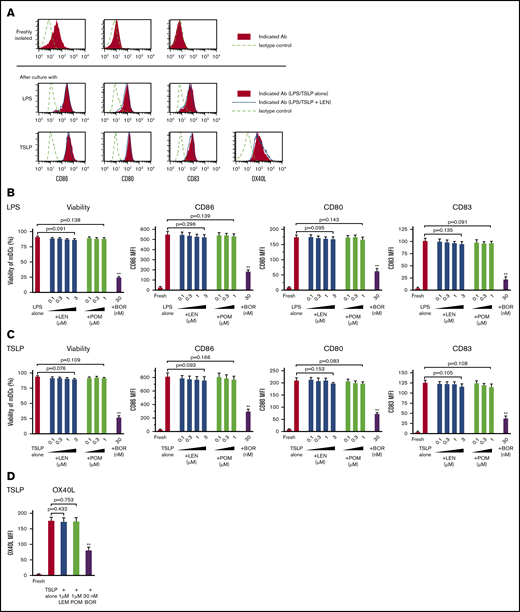
Purified blood CD11c+ mDCs (supplemental Figure 1) were stimulated with Toll-like receptor (TLR)-ligand LPS or TSLP for 24 hours in the presence of 0.1 to 3 μM LEN and 0.1 to 1 μM POM (covering the clinical plasma concentration range of oral LEN and POM administration) or 30 nM bortezomib (clinical peak plasma concentration of subcutaneous bortezomib administration; 53 nM [20.4 ng/mL]44 ). Analysis of annexin-V, CD86, CD80, and CD83 staining showed that 0.1 to 3 μM LEN and 0.1 to 1 μM POM did not affect the expression of these markers on mDCs in response to LPS and TSLP (Figure 1A-C). Conversely, mDCs were susceptible to the cytotoxic effects of 30 nM bortezomib (Figure 1B-C). We confirmed this finding using trypan-blue exclusion of the dead cells (data not shown). In response to other Th1-related inducers, R848 or poly(I:C), LEN and POM did not affect the survival or CD86 expression of mDCs (supplemental Figure 2). We next investigated the effects of LEN and POM on the expression of OX40L, which is upregulated in response to TSLP and is responsible for the induction of inflammatory Th2 responses45-48 and Th2 memory cell expansion.35,49 mDCs treated with 1 μM LEN and POM showed no change in OX40L expression in response to TSLP (Figure 1A,D). mDCs exposed to 30 nM bortezomib have greatly reduced expression of these markers in response to LPS or TSLP. These results indicate that the clinical plasma concentration range of both LEN and POM did not influence the viability and maturation of mDCs.
Survival and maturation of mDCs in the presence of IMiDs. Human blood myeloid dendritic cells (mDCs) were incubated for 24 hours with lipopolysaccharide (LPS) (A-B) or thymic stromal lymphopoietin (TSLP) (A,C-D) and the different concentrations of lenalidomide (LEN), pomalidomide (POM), or bortezomib (BOR) (A,D: LEN or POM were used at 1 μM). After culture, viable cells were evaluated by Annexin-V staining. Before and after the culture, the expressions of CD86, CD80, CD83, and OX40L on mDCs was analyzed by flow cytometry. (A) Each histogram shows the expression of the indicated markers on mDCs freshly isolated or stimulated with LPS/TSLP alone or LPS/TSLP + 1 μM LEN for 24 (CD86, CD80, and CD83) or 48 hours (OX40L). Similar results were observed in at least 4 independent experiments and the results of a representative experiment are shown. (B-D) Data indicate the mean fluorescence intensity (MFI), calculated by subtracting the MFI of the isotype control-treated cells from the MFI of the cells treated with the indicated monoclonal antibodies. Each experiment was performed using DCs from a single donor, and data are shown as the mean ± SEM of 6 independent donors (cell viability and CD86) and 4 independent donors (CD80, CD83, and OX40L). Statistical significance was determined using a paired Student t test (**P < .01). Comparisons between data obtained for vehicle controls (indicated as LPS alone or TSLP alone) and for each concentration of the indicated drugs.
Survival and maturation of mDCs in the presence of IMiDs. Human blood myeloid dendritic cells (mDCs) were incubated for 24 hours with lipopolysaccharide (LPS) (A-B) or thymic stromal lymphopoietin (TSLP) (A,C-D) and the different concentrations of lenalidomide (LEN), pomalidomide (POM), or bortezomib (BOR) (A,D: LEN or POM were used at 1 μM). After culture, viable cells were evaluated by Annexin-V staining. Before and after the culture, the expressions of CD86, CD80, CD83, and OX40L on mDCs was analyzed by flow cytometry. (A) Each histogram shows the expression of the indicated markers on mDCs freshly isolated or stimulated with LPS/TSLP alone or LPS/TSLP + 1 μM LEN for 24 (CD86, CD80, and CD83) or 48 hours (OX40L). Similar results were observed in at least 4 independent experiments and the results of a representative experiment are shown. (B-D) Data indicate the mean fluorescence intensity (MFI), calculated by subtracting the MFI of the isotype control-treated cells from the MFI of the cells treated with the indicated monoclonal antibodies. Each experiment was performed using DCs from a single donor, and data are shown as the mean ± SEM of 6 independent donors (cell viability and CD86) and 4 independent donors (CD80, CD83, and OX40L). Statistical significance was determined using a paired Student t test (**P < .01). Comparisons between data obtained for vehicle controls (indicated as LPS alone or TSLP alone) and for each concentration of the indicated drugs.
IMiDs inhibited the induction of Th1-related molecules in mDCs
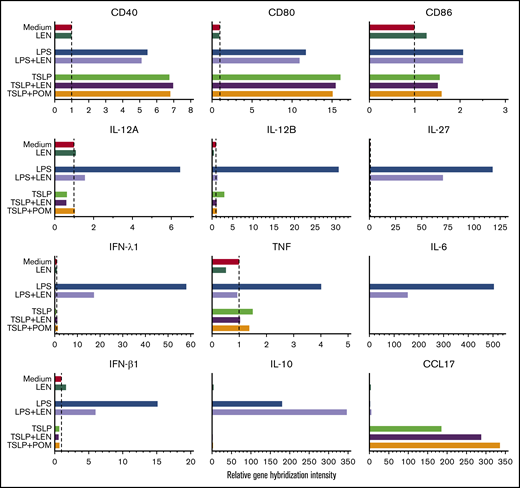
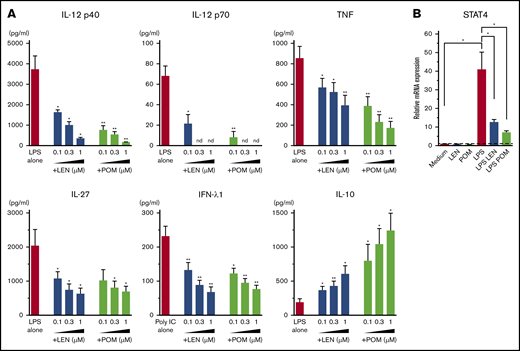
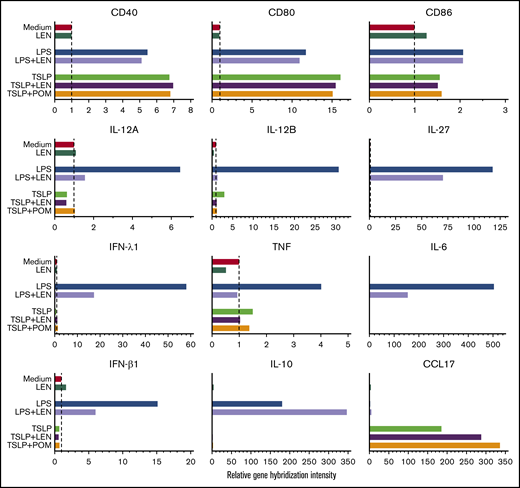
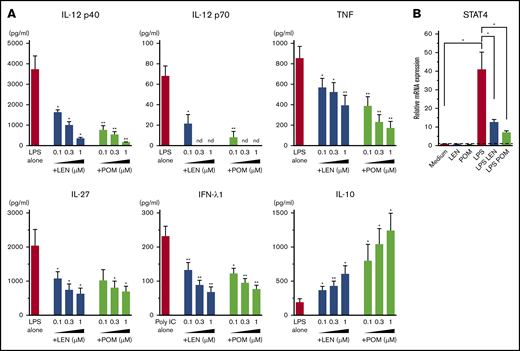
DCs have the ability to induce Th1 or Th2 response depending on the maturation signals they receive. To investigate the immunomodulatory actions of IMiDs on the function of human mDCs in Th1- or Th2-related activation pathways, we initially performed microarray gene expression analysis of mDCs activated by LPS or TSLP in the presence or absence of IMiDs. DC-related genes that were up- and downregulated when treated with LEN are listed in supplemental Figure 3, and representative genes are shown in Figure 2. Gene expression of CD40 and CD80 were upregulated by both LPS and TSLP but were not substantially changed after the addition of IMiDs (Figure 2). LPS stimulation strongly upregulated the gene expression of Th1-related cytokines IL-12 (IL12A and IL12B), IFN-λ1, IFN-β, and IL-27 and inflammatory cytokines TNF and IL-6, whereas the addition of LEN to LPS-stimulated mDCs repressed the expression of these genes (Figure 2). Expression of anti-inflammatory cytokine IL-10 gene was also induced by LPS, and the addition of LEN further upregulated IL-10 expression. To confirm the gene expression data in Th1-activation pathway, we analyzed cytokine production by ELISA from mDCs stimulated with LPS. For detection of IFN-λ1, we used poly(I:C) instead of LPS, as poly(I:C) is a strong inducer of IFN-λ.50 We confirmed that either LEN or POM inhibited the LPS-induced production of IL-12p40, IL-12p70, TNF, and IL-27 and poly(I:C)-induced IFN-λ1 in a dose-dependent manner (Figure 3A). When stimulated with poly(I:C) or R848, both IMiDs inhibited IL-12p70 production by mDCs (supplemental Figure 2). Notably, both IMiDs dose-dependently enhanced LPS-induced IL-10, indicating a downregulation of the Th1 response. Thus, IMiDs showed inhibitory activity toward the Th1-activation pathway in mDCs. In addition, we revealed that both 0.3 μM LEN and POM downregulated LPS-induced STAT4 messenger RNA (mRNA) in mDCs (Figure 3B), which is important for DC-mediated Th1 responses and IL-12 production.51,52
Gene expression of DC-related molecules in mDCs in response to IMiDs. Microarray gene expression profiles of DC-related molecules in mDCs activated by medium, LPS, or TSLP with or without LEN or POM at 0.3 μM after 8 hours of culture. Results of the gene expression profiles in a single run are shown as the relative gene hybridization intensity level.
Gene expression of DC-related molecules in mDCs in response to IMiDs. Microarray gene expression profiles of DC-related molecules in mDCs activated by medium, LPS, or TSLP with or without LEN or POM at 0.3 μM after 8 hours of culture. Results of the gene expression profiles in a single run are shown as the relative gene hybridization intensity level.
Effects of IMiDs on LPS-induced responses in mDCs. Blood mDCs were treated with media alone, LPS, LPS + LEN, or LPS + POM (in some experiments, poly(I:C) was used instead of LPS). (A) After 24 hours of culture, the cytokines in the supernatants were analyzed by an ELISA. (B) After 4 hours of mDC culture (with LPS in the presence of LEN or POM at 0.3 μM), STAT4 mRNA in mDCs was analyzed by real-time PCR. Each experiment was performed using DCs from a single donor, and data are shown as the mean ± SEM of 4 independent donors (A) and 5 independent donors (B). Statistical significance was determined using a paired Student t test (*P < .05, **P < .01). Comparisons between data obtained for vehicle controls (indicated as LPS alone or poly(I:C) alone) and for each concentration of LEN or POM. nd, not detected.
Effects of IMiDs on LPS-induced responses in mDCs. Blood mDCs were treated with media alone, LPS, LPS + LEN, or LPS + POM (in some experiments, poly(I:C) was used instead of LPS). (A) After 24 hours of culture, the cytokines in the supernatants were analyzed by an ELISA. (B) After 4 hours of mDC culture (with LPS in the presence of LEN or POM at 0.3 μM), STAT4 mRNA in mDCs was analyzed by real-time PCR. Each experiment was performed using DCs from a single donor, and data are shown as the mean ± SEM of 4 independent donors (A) and 5 independent donors (B). Statistical significance was determined using a paired Student t test (*P < .05, **P < .01). Comparisons between data obtained for vehicle controls (indicated as LPS alone or poly(I:C) alone) and for each concentration of LEN or POM. nd, not detected.
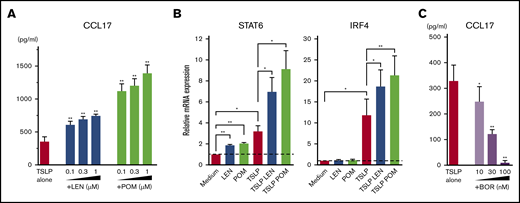
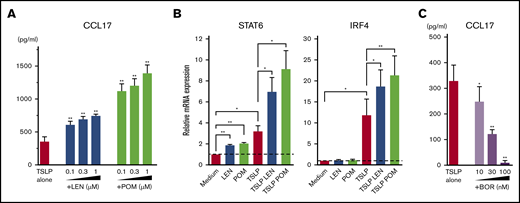
IMiDs upregulated CCL17 production and STAT6 and IRF4 expression in mDCs
Microarray gene expression profiles in mDCs activated by TSLP showed high gene expression of CCL17 (Figure 2). LEN and POM further upregulated the TSLP-driven expression of CCL17. Next, we performed an ELISA for CCL17 (Figure 4A) and real-time PCR for Th2-related transcription factors IRF453 and STAT654 mRNA in mDCs (Figure 4B). We confirmed that both LEN and POM statistically enhanced the TSLP-induced CCL17 secretion by mDCs in a dose-dependent manner. Both IMiDs enhanced IRF4 and STAT6 mRNA, which is important for DC-mediated CCL17 production.54-56 Meanwhile, bortezomib dose-dependently inhibited TSLP-induced CCL17 secretion by mDCs (Figure 4C).
Effects of IMiDs on TSLP-induced responses in mDCs. Blood mDCs were treated with media alone, TSLP, TSLP + LEN, TSLP + POM, or TSLP + BOR. (A,C) After 24 hours of culture, CCL17 concentration in the supernatants was analyzed by an ELISA. (B) After 4 hours of mDC culture (with TSLP in the presence of LEN or POM at 0.3 μM), STAT6 and IRF4 mRNA in mDCs were analyzed by real-time PCR. Each experiment was performed using DCs from a single donor, and data are shown as the mean ± SEM of 6 independent donors. Statistical significance was determined using a paired Student t test (*P < .05, **P < .01). Comparisons between data obtained for vehicle controls (indicated as TSLP alone) and for each concentration of the indicated drugs.
Effects of IMiDs on TSLP-induced responses in mDCs. Blood mDCs were treated with media alone, TSLP, TSLP + LEN, TSLP + POM, or TSLP + BOR. (A,C) After 24 hours of culture, CCL17 concentration in the supernatants was analyzed by an ELISA. (B) After 4 hours of mDC culture (with TSLP in the presence of LEN or POM at 0.3 μM), STAT6 and IRF4 mRNA in mDCs were analyzed by real-time PCR. Each experiment was performed using DCs from a single donor, and data are shown as the mean ± SEM of 6 independent donors. Statistical significance was determined using a paired Student t test (*P < .05, **P < .01). Comparisons between data obtained for vehicle controls (indicated as TSLP alone) and for each concentration of the indicated drugs.
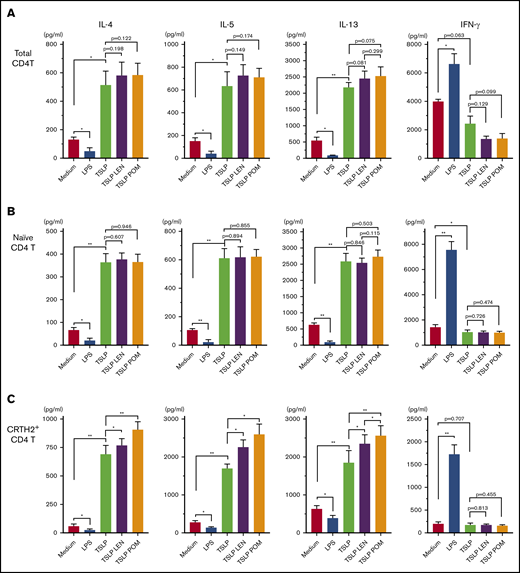
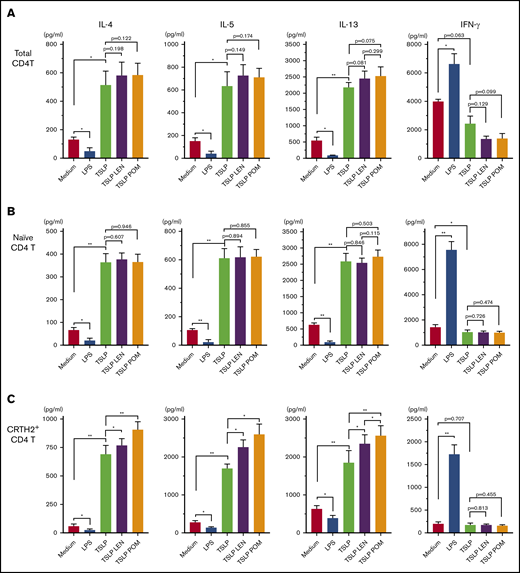
Treatment of DCs with IMiDs enhanced the polarization of Th2 memory cells induced by TSLP
CCL17 is an important chemokine for the progression of allergic inflammation by recruiting memory Th2 cells,57,58 and circulating CRTH2+CD4+ T cells represent Th2 central memory cells35 ; thus, we next examined the modulatory effects of IMiDs on the DC-mediated Th2 memory response. Total CD4+ T cells, naïve CD4+ T cells, and CRTH2+CD4+ Th2 cells in the blood were cocultured for 7 days with mDCs under different stimuli (Figure 5). TSLP-stimulated mDCs enhanced the production of Th2 cytokines IL-4, IL-5, and IL-13 from all CD4+ T-cell populations tested, but not IFN-γ, indicating an enhanced Th2 cell phenotype, as previously described.35,47 LPS-stimulated mDCs inhibited the Th2 phenotype of all CD4+ T cell populations and promoted the production of IFN-γ. Notably, the addition of either LEN or POM to mDCs cultured with TSLP significantly enhanced the production of IL-4, IL-5, and IL-13 from CRTH2+CD4+ Th2 cells only (Figure 5C). There was a trend indicative of a more Th2-polarized phenotype induced by POM compared with LEN but a significant difference was seen only in the IL-13 production. Modest Th2 skewing was observed in total CD4+ T cells (Figure 5A) but not in naïve T cells (Figure 5B). These results suggest IMiDs have the potential to further polarize the DC-related Th2 response by activating the existing Th2 memory cells but not by inducing the differentiation of Th2 cells from naive T cells.
Effect of IMiDs on the function of DCs to total, naïve, and memory Th2 memory cells. Total (A), naïve (B), and CRTH2+CD4+ (C) T cells were cultured for 7 days with allogeneic mDCs pretreated with media alone, LPS, TSLP, TSLP + 0.3 μM LEN, or TSLP + 0.3 μM POM for 24 hours. After culture, cytokine production by primed CD4+ T cells was measured in supernatants after restimulation for 24 hours with anti-CD3 and soluble anti-CD28 antibodies at a concentration of 106 cells/mL. A single experiment was performed using DCs from 1 donor and T cells from another allogenic donor, and data are the means ± SEM of 4 (A-B) and 5 (C) independent experiments. Statistical significance was determined using a paired Student t test (*P < .05, **P < .01).
Effect of IMiDs on the function of DCs to total, naïve, and memory Th2 memory cells. Total (A), naïve (B), and CRTH2+CD4+ (C) T cells were cultured for 7 days with allogeneic mDCs pretreated with media alone, LPS, TSLP, TSLP + 0.3 μM LEN, or TSLP + 0.3 μM POM for 24 hours. After culture, cytokine production by primed CD4+ T cells was measured in supernatants after restimulation for 24 hours with anti-CD3 and soluble anti-CD28 antibodies at a concentration of 106 cells/mL. A single experiment was performed using DCs from 1 donor and T cells from another allogenic donor, and data are the means ± SEM of 4 (A-B) and 5 (C) independent experiments. Statistical significance was determined using a paired Student t test (*P < .05, **P < .01).
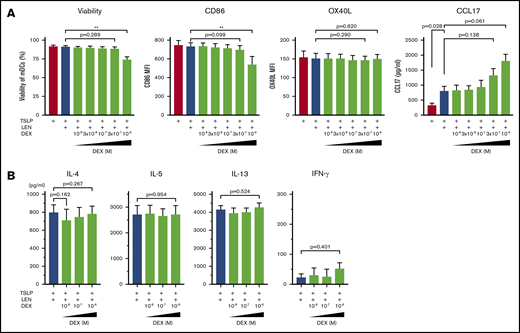
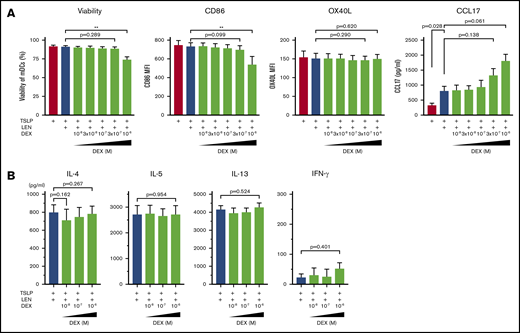
Dexamethasone does not negatively affect LEN-induced DC responses
DEX exerts immunomodulatory effects on many immune cells including DCs.59 Indeed, DCs derived from human monocytes in the presence of 10-7 M DEX express low CD80 and CD86 and have a low IL-12-producing capacity.60 Because treatment with DEX once a week is administered to patients in combination with LEN in clinical practice, we next examined the effect of LEN-DEX on mDCs. Different concentration (10-8-10-6 M) of DEX was added to mDC cultures in the presence of TSLP + 0.3 μM LEN for 24 hours. DEX at 10-6 M, but not ≤3×10-7 M, moderately inhibited annexin-V and CD86 expressions (Figure 6A). OX40L and CCL17 expression from mDCs (Figure 6A) and Th2-polarizing activity of LEN-treated mDCs (Figure 6B) were not significantly suppressed by ≤10-6 M DEX. Rather, DEX tended to enhance CCL17 secretion. Thus, DEX not used at a high concentration might be less toxic and may not negatively affect LEN-induced DC functions in the presence of TSLP.
Impact of dexamethasone on mDCs in the presence of IMiDs. (A) mDCs were incubated with LEN and/or TSLP and the indicated concentrations of dexamethasone (DEX). Annexin-V and CD86 expressions after 24-hour culture and OX40L expression after 48 hours on mDCs were analyzed by flow cytometry. Data indicate the mean fluorescence intensity (MFI), calculated by subtracting the MFI of the isotype control-treated cells from the MFI of the cells treated with the indicated monoclonal antibody. After 24 hours of culture, CCL17 concentration in the supernatants was analyzed by an ELISA. Each experiment was performed using DCs from a single donor, and data are shown as the mean ± SEM of 4 independent donors. (B) CRTH2+CD4+ T cells were cultured for 7 days with allogeneic mDCs pretreated for 24 hours with TSLP + 0.3 μM LEN in the presence or absence of the indicated concentrations of DEX. After culture, cytokine production by primed CD4+ T cells was measured in supernatants after restimulation for 24 hours with anti-CD3 and soluble anti-CD28 antibodies at a concentration of 106 cells/mL. A single experiment was performed using DCs from 1 donor and T cells from another allogenic donor, and data are the means ± SEM of 3 independent experiments. Statistical significance was determined using a paired Student t test (**P < .01).
Impact of dexamethasone on mDCs in the presence of IMiDs. (A) mDCs were incubated with LEN and/or TSLP and the indicated concentrations of dexamethasone (DEX). Annexin-V and CD86 expressions after 24-hour culture and OX40L expression after 48 hours on mDCs were analyzed by flow cytometry. Data indicate the mean fluorescence intensity (MFI), calculated by subtracting the MFI of the isotype control-treated cells from the MFI of the cells treated with the indicated monoclonal antibody. After 24 hours of culture, CCL17 concentration in the supernatants was analyzed by an ELISA. Each experiment was performed using DCs from a single donor, and data are shown as the mean ± SEM of 4 independent donors. (B) CRTH2+CD4+ T cells were cultured for 7 days with allogeneic mDCs pretreated for 24 hours with TSLP + 0.3 μM LEN in the presence or absence of the indicated concentrations of DEX. After culture, cytokine production by primed CD4+ T cells was measured in supernatants after restimulation for 24 hours with anti-CD3 and soluble anti-CD28 antibodies at a concentration of 106 cells/mL. A single experiment was performed using DCs from 1 donor and T cells from another allogenic donor, and data are the means ± SEM of 3 independent experiments. Statistical significance was determined using a paired Student t test (**P < .01).
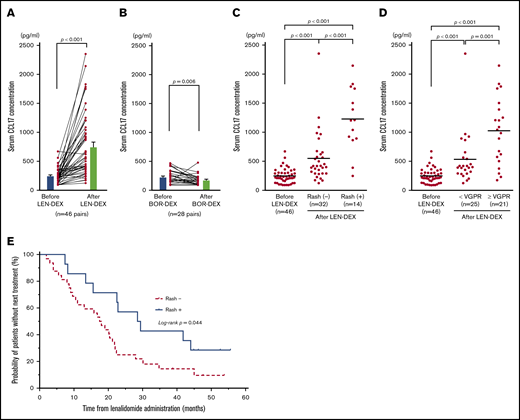
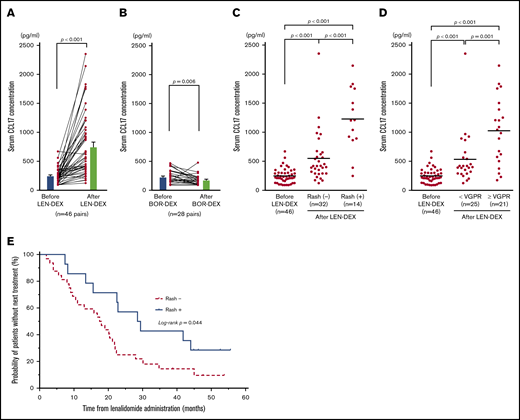
Serum CCL17 was elevated and correlated with skin rashes in patients after administration of lenalidomide
Serum CCL17 levels are a parameter identifying disease severity specific to atopic dermatitis.58,61 To examine whether IMiDs induce allergy-related response in MM patients, we investigated the serum levels of CCL17 in MM patients before and after LEN-DEX treatment (at onset of skin rash or after 2 cycles of treatments). Serum was collected from 46 MM patients receiving LEN-DEX and 28 MM patients receiving bortezomib with DEX without discontinuation because of adverse events (supplemental Table 1). We found that serum CCL17 levels were significantly higher after LEN administration compared with before LEN treatment (mean ± standard error of the mean [SEM]; 757.2 ± 82.7 pg/mL vs 248.2 ± 18.8 pg/mL; P < .001) (Figure 7A). Furthermore, serum CCL17 levels in patients after 2 cycles of bortezomib treatment (175.8 ± 16.6 pg/mL) were significantly lower than those at baseline (230.5 ± 21.5 pg/mL; P = .006) (Figure 7B). The incidences of skin rashes were observed in 14 of 46 patients (30.4%) after LEN-DEX administration (supplemental Table 2), and, only 1 patient developed skin rash after bortezomib treatment. Serum CCL17 levels in patients even without rashes (32 of 46 patients, 69.6%) after LEN-DEX treatment (n = 32; 551.8 ± 76.3 pg/mL) were significantly higher than baseline (n = 46; 248.2 ± 18.8 pg/mL) (P < .001) (Figure 7C). Notably, serum CCL17 levels in patients at the onset of LEN-associated rash (n = 14; 1227 ± 147 pg/mL) were significantly higher compared with patients without rashes after treatment (P < .001). In the 14 patients who developed rashes and the 32 patients who did not develop rashes after LEN-DEX treatment, serum CCL17 levels were significantly higher compared with samples taken before treatment (supplemental Figure 4A-B). It is possible that the rashes caused by LEN could be associated with CCL17-mediated allergic responses, such as atopic dermatitis. Of the 46 patients treated with LEN-DEX, 21 patients (45.7%) achieved very good partial response (VGPR) or better and 25 patients (54.3%) had partial response or less during 6 cycles of LEN-DEX treatment. Serum CCL17 levels in patients who acquired at least VGPR (1025 ± 125 pg/mL) were significantly higher (P = .001) compared with those acquiring less than a VGPR (532.7 ± 89.3 pg/mL) after LEN-DEX treatment (Figure 7D). In either the 21 patients who acquired VGPR or better or the 25 patients acquiring less than VGPR, serum CCL17 levels after LEN-DEX treatment were significantly higher compared with baseline levels (supplemental Figure 4C-D).
Serum CCL17 levels in MM patients before and after treatment. Mean serum CCL17 in 46 MM patients before and after LEN-DEX treatment (A) and in 28 MM patients before and after bortezomib-DEX treatment (B) at the onset of skin rash or after 2 cycles of treatments in cases of without rash formation. Serum CCL17 levels in 46 patients before LEN-DEX treatment, 32 patients who did not have rashes after LEN-DEX treatment, and 14 patients who developed rashes after LEN-DEX treatment (C); and serum CCL17 levels in 46 patients before LEN-DEX treatment, 25 patients who did not acquire a very good partial response (VGPR) after LEN-DEX treatment, and 21 patients who acquired VGPR or better after LEN-DEX treatment (D). (A-D) Each dot indicates 1 patient. (E) Kaplan-Meier curves of time to the next treatment in 14 patients who developed rashes vs 32 patients who did not have rashes after LEN-DEX treatment. (A-B) Bars indicate mean ± SEM and statistical significance was calculated by Wilcoxon matched-pairs signed rank test. (C-D) Bars indicate mean values and statistical significances were calculated by Mann-Whitney U tests. (E) Data of time to the next treatment were compared between the groups by using log-rank tests.
Serum CCL17 levels in MM patients before and after treatment. Mean serum CCL17 in 46 MM patients before and after LEN-DEX treatment (A) and in 28 MM patients before and after bortezomib-DEX treatment (B) at the onset of skin rash or after 2 cycles of treatments in cases of without rash formation. Serum CCL17 levels in 46 patients before LEN-DEX treatment, 32 patients who did not have rashes after LEN-DEX treatment, and 14 patients who developed rashes after LEN-DEX treatment (C); and serum CCL17 levels in 46 patients before LEN-DEX treatment, 25 patients who did not acquire a very good partial response (VGPR) after LEN-DEX treatment, and 21 patients who acquired VGPR or better after LEN-DEX treatment (D). (A-D) Each dot indicates 1 patient. (E) Kaplan-Meier curves of time to the next treatment in 14 patients who developed rashes vs 32 patients who did not have rashes after LEN-DEX treatment. (A-B) Bars indicate mean ± SEM and statistical significance was calculated by Wilcoxon matched-pairs signed rank test. (C-D) Bars indicate mean values and statistical significances were calculated by Mann-Whitney U tests. (E) Data of time to the next treatment were compared between the groups by using log-rank tests.
There were 10 patients and 11 patients who achieved VGPR or better within the patients with skin rashes (n = 14) and without skin rashes (n = 32), respectively (supplemental Table 3). For this categorical data, the P value using Fisher’s exact test was .027, indicating that the onset of skin rashes with LEN-DEX treatment is correlated with a response of VGPR or better. Furthermore, the median time to the next treatment as a surrogate measure for progression-free survival was significantly longer in patients who developed rashes than those without rashes (29.0 vs 17.8 months; P = .044) (Figure 7E). Thus, the therapeutic effect of LEN was superior in patients with onset of rash to those without rash development.
Discussion
DC-mediated T-cell responses comprise cell-mediated immunity (Th1) and humoral responses (Th2).62 The present study suggests IMiDs have the potential to shift the DC-mediated response from Th1 to Th2 humoral immunity.
Clinically, serum CCL17 levels are associated with the disease activity of atopic dermatitis, and mDCs such as LCs are the major producers of CCL17 in allergic Th2 conditions.30,63 TSLP-related Th2 responses initiated via LC activation act as a pathogenic trigger in atopic dermatitis.64,65 Human blood mDCs used in the present study are the direct precursors of LCs66 ; thus, our experimental setting using TSLP and blood mDCs is an appropriate in vitro model of atopic dermatitis. mDCs are the primary mediators of allergic responses67 and IRF4 expression in DCs is required for Th2 responses in both humans and mice.53,68-70 Moreover, STAT6 activation by TSLP has been implicated in CCL17 production by human mDCs54 with STAT6 shown to bind to the promoter regions of the CCL17 and IRF4 genes,55 indicating an intimate line between STAT6 and IRF4 in DC-mediated Th2 responses. Given these findings, our results showing that IMiDs promote TSLP-induced IRF4 and STAT6 activation suggest a possible signal-transduction mechanism for the immunopotentiating activity of IMiDs for a Th2 humoral immune response. Furthermore, the present study also showed that both IMiDs suppressed the Th1-inducing capacity of DCs by downregulating STAT4, in line with the previous observations that LEN suppresses IL-12 and IL-23 production and enhances IL-10 production by mDCs in response to TLR-ligands.22,23
Our findings also provide insight into the pathogenesis of IMiD-induced rashes. The incidence of all-grade LEN-associated rashes was 30.4% in our study. This is similar to a meta-analysis of 10 trials showing rashes in 27.2% of cases71 and a report of 144 MM patients receiving LEN therapy in a 4-institutional cohort study with 35% of cases developing rashes.72 These observations suggest IMiDs polarize the immune response toward the allergic axis by promoting mDC activation and upregulating CCL17 expression. The development of atopy-like rashes following LEN treatment is likely a result of Th2-mediated immunostimulation. In support of our study, there are many reports showing cases of eosinophilia during LEN treatment,73,74 and the incidence of eosinophilia after LEN administration is higher in patients with rashes.72 Our results showed IMiD-treated DCs enhanced the Th2 polarization of memory Th2 cells but not naïve T cells; therefore, administration of IMiDs might induce atopic-like rash by exacerbating existing Th2 memory cells rather than inducing the differentiation of Th2 cells from naive T cells.
In contrast to IMiDs, bortezomib has been shown to suppress immune responses,75-77 and our data showed that serum CCL17 levels were significantly downregulated after bortezomib administration. Furthermore, a recent report has shown that LEN-induced rash incidence was decreased in patients with prior bortezomib therapy72 ; therefore, it is possible that the immunosuppressive effects of bortezomib inhibit the Th2 responses induced by subsequent administration of LEN.
Our data showing clinical outcomes of IMiD treatment of MM patients have limitations as this is a retrospective database study with a small number of patients; however, it suggests that the LEN-associated rashes could be a potential predictor of clinical outcome. MM involves humoral immune dysfunction78-80 because MM cells are malignant plasma cells. Furthermore, immune dysfunction is associated with poor clinical outcomes in MM and clinical outcomes can be improved by the recovery of immune status.81,82 LEN has been shown to improve humoral immunity with non-neoplastic globulin recovery in MM patients, and the patients with humoral responses improved by LEN experience better outcomes, both progression-free survival and overall survival.83,84 However, the mechanisms of cellular basis associated with a LEN-enhanced humoral response are unclear. Our data showing that IMiDs activate DCs at upstream cascade of immunoglobulin production provide a plausible explanation for the observed benefits of LEN in improving humoral immunity in MM. This function of LEN is relevant to the treatment of MM patients with significant humoral immune dysfunction. Our results also showed that unless the concentration of DEX is high, it has fewer harmful effects on LEN-treated DC function; therefore, the LEN-DEX regimen might be effective at the immunopotentiating activity. In support of our findings, the addition of DEX to the DC vaccination plus IMiDs does not have a negative antimyeloma effect compared with the combination without DEX in a model of murine MM.20
This is the first report of a potential mechanism whereby IMiD treatment leads to the onset of itchy skin with rash-like atopy. Understanding LEN-related rashes provides valuable insight into the response to LEN treatment and may enable improved treatment regimens for MM. Furthermore, CCL17 may be useful to clinically predict the therapeutic outcome of IMiDs. Therefore, IMiD-related rash might be considered as an immunologically relevant adverse effect linking to therapeutic effects of IMiDs through the DC-mediated humoral immune activation.
Collectively, our results suggest that IMiDs function as enhancers of humoral immunity via mDCs, thereby contributing to tumoricidal effects against MM.
Send data sharing requests via e-mail to the corresponding author, Tomoki Ito (itot@hirakata.kmu.ac.jp).
Acknowledgments
The authors thank S. J. Win from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
This study was supported in part by grant 17K09963 from Grant-in-Aid for Scientific Research (C), Japan Society for the Promotion of Science (T.I.).
Authorship
Contribution: T.I. designed the research and wrote the manuscript; V.P., M.I., K.K., N.I.-K., and A.T. performed the research; A.S. and S.N. contributed patient samples and clinical data; T.I. and V.P. analyzed the data; and all authors read and agreed to the final version of the manuscript.
Conflict-of-interest disclosure: T.I. has received honoraria for speaking at education conferences sponsored by Celgene, Novartis, Takeda, and Bristol-Myers Squibb. The remaining authors declare no competing interests.
Correspondence: Tomoki Ito, First Department of Internal Medicine, Kansai Medical University, 2-5-1, Shinmachi, Hirakata-city, Osaka 573-1010, Japan; e-mail: itot@hirakata.kmu.ac.jp.
References
Author notes
The full-text version of this article contains a data supplement.