Key Points
Because CB units with the highest TNC dose may not have an adequate CD34+ dose, both should be considered in selecting CB units.
The national US inventory of adequately sized single CB units for larger patients (>70 kg) is small.
Abstract
CD34+ cell dose is critical for cord blood (CB) engraftment. However, the CD34+ content of the CB inventory in the United States is unknown. We examined the CD34+ cell content of 126 341 red blood cell–depleted US units banked from January 2007 to September 2017 with a total nucleated cell (TNC) count of ≥90 × 107 and a cryovolume of 24-55 mL. Median pre-cryopreservation TNC content was 127 × 107 (interquartile range [IQR], 108-156 × 107); CD34+ cell content was 44 × 105 (IQR, 29 to 67 × 105). The median CD34+:TNC ratio was 0.34%. TNC and CD34+ cell content correlation was weak (r = 0.24). Of 7125 units with TNCs of ≥210 × 107, only 47% had CD34+ content of ≥100 × 105. However, some units had high CD34+ content for a given TNC count. Only 4% of CB units were acceptable as single-unit grafts (TNCs, ≥2.5 × 107/kg; CD34+ cells, ≥1.5 × 105/kg) for 70-kg patients; 22% of units were adequate for 70-kg patients using lower dose criteria (TNCs, ≥1.5 × 107/kg; CD34+ cells, ≥1.0 × 105/kg) suitable for a double-unit graft. These findings highlight that units with the highest TNC dose may not have the highest CD34+ dose, units with unexpectedly high CD34+ content (a ratio of >1.0%) should be verified, and the US CB inventory of adequately sized single units for larger patients is small. They also support the ongoing use of double-unit grafts, a focus on banking high-dose units, and development of expansion technologies.
Introduction
Infused CD34+ cell dose is a critical determinant of the speed and success of engraftment after both single-1,2 and double-unit3 cord blood (CB) transplants. Although lack of standardization of CD34+ assays may result in measurements of CD34+ content being less reliable than those for total nucleated cells (TNCs), studies of postthaw CD34+ cell recovery have shown that viable CD34+ content can usually be predicted at the time of unit selection in more recently collected quality units that have undergone optimized processing and cryopreservation.3-5 Moreover, precryopreserved CD34+ cell dose has been associated with engraftment success and has a greater influence on hematopoietic recovery than TNC dose.5,6 Consequently, incorporation of the CD34+ cell dose, in addition to the TNC dose, into CB unit selection is now recommended.7,8 However, the CD34+ content of the current unrelated donor National Marrow Donor Program (NMDP) Be the Match CB inventory has not been analyzed.
Methods
We examined the CD34+ cell content of CB units originating from US CB banks listed in the NMDP Be the Match registry. Inclusion for analysis was limited to units banked between January 2007 and September 2017 that were red blood cell (RBC) depleted, had a TNC content of >90 × 107, a cryovolume of 24-55 mL (1 or 2 bags), and were either available or reserved; their CD34+ cell content postprocessing was reported. The cryovolume restriction of <55 mL was included because these units will have undergone optimized processing or cryopreservation, which results in RBC depletion. Most such units will have cryovolumes of approximately 25 mL (or 25 mL × 2 = ∼50 mL). TNC and CD34+ data were obtained from the existing NMDP Database. Excluded from this analysis were 2046 units with cryovolume <24 mL or not reported, 4054 units with frozen volume ≥56 mL, and 910 units that did not have CD34+ content reported.
Published NMDP and American Society of Blood and Marrow Transplantation CB Special Interest Group definitions of adequate cell doses were used to examine the number of units in the inventory that were considered adequate for single-unit (TNCs, ≥2.5 × 107/kg; CD34+ cells, ≥1.5 × 105/kg) and double-unit (TNCs, ≥1.5 × 107/kg/unit; CD34+ cells, ≥1.0 × 105/kg/unit) CB grafts.7 Descriptive statistics were used to describe the cell dose characteristics of the CB inventory, including calculations of CD34+:TNC ratio and cell doses by patient weight.
Results
In all, 126 341 units collected by 20 public banks fulfilled the criteria for analysis. Of the 126 341 analyzed units, the median pre-cryopreservation TNC content was 127 × 107 (interquartile range [IQR], 108 to 156 × 107); 89 126 units (71%) had a TNC content of <150 × 107, 20 618 units (16%) had a TNC content of 150 to 179 × 107, 9472 units (7%) had a TNC content of 180 to 209 × 107, and 7125 units (6%) had a TNC content of ≥210 × 107 (Table 1).
The median CD34+ cell content was 44 × 105 (IQR, 29 to 67 × 105). In all, 72 665 units (58%) had a CD34+ cell content of <50 × 105, 41 844 units (33%) had a CD34+ cell content of 50 to 99 × 105, 10 820 units (9%) had a CD34+ cell content of 100 to 200 × 105, and only 1012 units (<1%) had a CD34+ cell content of >200 × 105 (Table 1).
Because both TNC and CD34+ cell doses should be considered in unit selection,7,8 we examined the relationship between unit TNCs vs CD34+ content (Table 1). The correlation between the TNC and CD34+ cell content of the CB units was weak (r = 0.24). Thus, in a patient’s search there could potentially be many units with a high TNC count but with intermediate or low CD34+ cell content. For example, of the CB units with TNC content ≥210 × 107 (n = 7125 units; shown in bold in Table 1), less than half (n = 3332; 47%) had CD34+ cell content ≥100 × 105. Conversely, of units with low TNC content of <210 × 107 (n = 119 216), a small fraction (n = 8500; 7%) had high CD34+ content, defined as ≥100 × 105.
To evaluate the fraction of the TNC content that can be expected to be CD34+ cells, we analyzed the CD34+ cell:TNC content ratio of the units in the inventory (Figure 1). The median ratio was 0.34% (IQR, 0.23%-0.48%). The majority of units (n = 76 719; 61%) had a ratio between 0.2% and <0.5%. Only 20 263 (16%) had a ratio of <0.2%, whereas only 27 026 (21%) had a ratio of 0.5% to <1.0%. A ratio of ≥1.0% was very uncommon (n = 2333 units; 2%). Reported ratios did not vary significantly between banks.
Distribution of the CD34:TNC ratio of CB units in the domestic CB unit inventory. A total of 23 units with ratios of 3.0 to 30.9 are excluded from this figure.
Distribution of the CD34:TNC ratio of CB units in the domestic CB unit inventory. A total of 23 units with ratios of 3.0 to 30.9 are excluded from this figure.
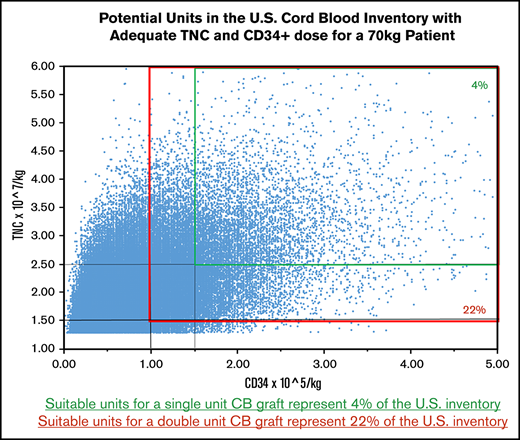
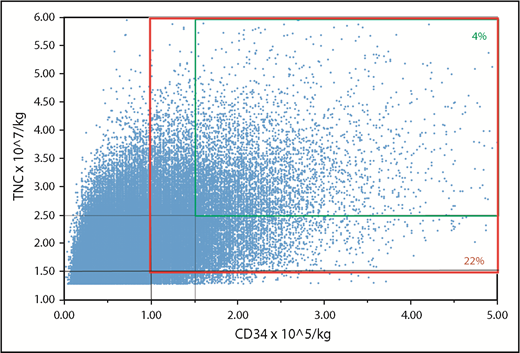
We next examined the number of units that fulfill minimum cell dose criteria for a single-unit graft (TNCs, ≥2.5 × 107/kg; CD34+ cells, ≥1.5 × 105/kg per current guidelines7,9 ) by patient weight. Although approximately half the inventory (n = 62 282; 49%) units met minimum criteria for a 30-kg patient and 37 788 (30%) of the units met minimum criteria for a 40-kg patient, the number of acceptable units for adult-size patients was small. For example, only 5418 units (4% of the inventory) had TNC and CD34+ cell doses adequate for a patient weighing 70 kg (Figure 2, smaller panel). The number of acceptable units all US CB units for larger patients progressively diminished: 2826 (2%) for 80-kg, 1495 (1%) for 90-kg, and 776 (<1%) for 100-kg patients.
Potential units that are adequately dosed for a 70-kg patient. Suitable units for a single-unit graft (TNC dose, ≥2.5 × 107/kg; CD34+ cell dose, ≥1.5 × 105/kg) represent 4% of the inventory (smaller panel). Units that would be suitable as 1 of 2 for a double-unit graft (TNC dose, ≥1.5 × 107/kg; CD34+ cell dose, ≥1.0 × 105/kg per unit) represent 22% of the inventory (larger panel).
Potential units that are adequately dosed for a 70-kg patient. Suitable units for a single-unit graft (TNC dose, ≥2.5 × 107/kg; CD34+ cell dose, ≥1.5 × 105/kg) represent 4% of the inventory (smaller panel). Units that would be suitable as 1 of 2 for a double-unit graft (TNC dose, ≥1.5 × 107/kg; CD34+ cell dose, ≥1.0 × 105/kg per unit) represent 22% of the inventory (larger panel).
Reducing the cell dose criteria to the minimum acceptable for each unit of a double-unit graft (TNCs, ≥1.5 × 107/kg; CD34+ cells, ≥1.0 × 105/kg) increased adequately sized units to 93 717 (74% of the inventory) for a 30-kg patient and 72 125 (57%) units for a 40-kg patient. For a 70-kg patient, the number increased to 27 274 (22%) (Figure 2, larger panel). The number of acceptable units for larger patients was 18 494 (15%) for 80-kg, 12 400 (10%) for 90-kg, and 8363 (7%) for 100-kg patients.
Discussion
This is the first analysis to evaluate the CD34+ content of the US CB inventory. It should be noted that our analysis did not consider units collected before 2007. However, we do not consider this a limitation because units more recently collected are associated with optimized banking practices and are much more likely to be used. Our findings do require detailed validation, however, and could be limited by changes in postprocessing prefreeze CD34+ cell enumeration assays over time. They also do not account for variations in unit quality as reflected by the postthaw CD34+ cell viability.3 Nonetheless, they provide critical information that has important implications for CB transplantation, CB banking, and CB bank funding.
From a practical standpoint, these data clearly reinforce incorporating both TNC and CD34+ cell dose into CB unit selection, because units with an adequate TNC dose do not necessarily have an adequate CD34+ cell dose. Centers should recognize that once CD34+ dose is considered, the best units may not necessarily have the highest TNC dose. Importantly, incorporation of this guidance into unit selection could improve engraftment without the need for CB expansion and therefore could have major advantages in terms of transplantation costs and use of CB.
The potential clinical implications of the CD34+:TNC content ratio are unknown and require investigation. It is critically important that if transplant centers incorporate CD34+ dose into unit selection, the precryopreserved CD34+ dose must approximate what will be recovered after thawing. Current guidance states that centers should request unit reports and CB bank verification of units with a CD34+ dose much higher than expected based on both the TNC dose and the CD34+:TNC ratio (eg, >1%). The implications of an unexpectedly high CD34+ dose are complex. Such a unit may be very good because it is enriched with CD34+ progenitors. Conversely, the reported CD34+ cell content could be wrong. This would most likely result from an error in software data entry.
A valid cutoff of the expected CD34+ content for a given TNC dose has not been established and requires further detailed investigation. However, the observed range of the CD34+:TNC ratio in this study provides guidance on what may be an expected CD34+ content relative to the reported precryopreservation TNC count. In this analysis, a ratio of ≥1.0% was very uncommon (2% of the inventory). Therefore, a CD34+:TNC content ratio of 2% or 3%, for example, should be questioned. The CD34+ cell content and potency of a prefreeze specimen, an associated cryovial or attached segment10-12 could potentially be informative in this scenario, and each requires investigation. In addition, whether the CD34+:TNC ratio has any clinical association with engraftment, independent of the infused doses of TNCs and viable CD34+ cells, also requires investigation. Such analyses will require compiling data from multiple dedicated CB transplant centers from their cell therapy laboratories and their clinical databases. Analyses will need to include a patient population transplanted with relatively uniform myeloablation and immunosuppression, as well as examining any variations in thaw techniques and CD34+ cell enumeration assays between centers.
Our analysis highlights the fact that there is a large inventory of CB units with adequate TNC and CD34+ doses for patients of low to intermediate weight. This has major implications for pediatric transplantation (and smaller adults), because these units can be obtained quickly, and high survival rates have been achieved with pediatric CB transplant.9,13 The number of single units for patients weighing at least 70 kg, however, is relatively small, which supports the continued use of double-unit CB grafts for patients without adequately sized single CB units. Such grafts have been associated with very high engraftment rates in larger patients,3,14,15 albeit with delayed count recovery relative to transplantation of mobilized peripheral blood. Moreover, we found that lowering the cell dose threshold to the minimum considered acceptable for double-unit grafts notably increased the number of CB units that would be available for larger children and adults.
Our analysis also supports the need for concerted efforts and ongoing funding to enrich the CB inventory with units that have high cell doses. This approach has recently been the focus of many CB banks and offers the advantage of increased cost-effectiveness because CB units with higher cell content are more likely to be used. Finally, new technologies such as ex vivo CB expansion or the addition of cells from a third party could potentially further improve engraftment and are currently under investigation.16-22 These technologies could also enlarge the usable CB inventory, which has relevance when a better HLA-matched unit is required for transplantation.
To request original data, contact Juliet N. Barker (barkerj@mskcc.org).
Acknowledgments
The authors thank the participating banks that provided and verified the unit data.
This work was supported in part by US Health Resources and Services Administration contracts HHSH234200637021C and HHSH250201200018C.
Authorship
Contribution: All authors designed and performed the research, analyzed the data, and helped write the paper.
Conflict-of-interest disclosure: J.N.B. received clinical trial or educational funding from NYSTEM, Angiocrine Bioscience, and Gamida Cell. J. Kempenich and J.D. are employees of the National Marrow Donor Program (NMDP). J. Kurtzberg is medical director of CryoCell Cord Blood Bank. C.D. is chief scientific officer of Nohla Therapeutics. J. Kurtzberg, A.S., and E.J.S. are medical directors of public CB banks (New York Blood Center’s National Cord Blood Program, Carolinas CB Bank Duke University, and MD Anderson Cancer Center CB Bank, respectively). J.N.B., J. Kurtzberg, F.M., E.J.S., and A.S. are medical advisors to the NMDP. The remaining authors declare no competing financial interests.
Correspondence: Juliet N. Barker, Memorial Sloan Kettering Cancer Center, 1275 York Ave, Box 259, New York, NY, 10065; e-mail: barkerj@mskcc.org.