Key Points
GRFS is similar between older and younger patients using FCC conditioning but comorbidities impact on outcome of SAA HSCT.
Immunomodulatory B lymphocytes potentially contribute to control of alloreactivity and low GVHD after FCC HSCT.
Abstract
Survival after allogeneic hematopoietic cell transplantation (HSCT) for severe aplastic anemia (SAA) among older patients remains poor and associated with increased risk for graft-versus-host disease (GVHD). In this retrospective study of 65 consecutive patients with acquired SAA who were transplanted using fludarabine, low-dose cyclophosphamide, and alemtuzumab (FCC), outcomes of 27 patients aged at least 50 years were compared with those of 38 patients younger than 50 years. The median age of the older cohort was 61 years (range, 51-71 years); 21 (78%) patients were transplanted from unrelated donors (3 of 21 from HLA 9/10 mismatch donors) and 6 from matched sibling donors. One-year GVHD-free, relapse-free survival (GRFS) was comparable to that of patients younger than 50 years (84% vs 94%, respectively; P = .23). Both groups showed low rates of acute (5% vs 4%) and chronic (18% vs 14%) GVHD, with no cases of severe GVHD among matched donor transplants, and similar 1-year transplant-related mortality (14% vs 5.4%, older vs younger; P = .23). HSCT comorbidity index (HTC-CI) scores were similar between the groups, but overall survival with an HCT-CI of at least 3 was lower compared with a score less than 3 (76% vs 98%; P = .005). Median donor T-cell chimerism among older patients was 64% and 60% at 1 and 3 years, respectively, and was similar to that of younger patients. Increased B regulatory cells potentially contributed to low alloreactivity and mutual donor-recipient tolerance in older patients. Effect of comorbidities rather than age alone may be a more important determinant of suitability for FCC HSCT in older patients.
Introduction
Age is one of the strongest predictive factors for survival in patients with severe acquired aplastic anemia (SAA) undergoing allogeneic hematopoietic cell transplantation (HSCT) or immunosuppressive therapy (IST) with antithymocyte globulin (ATG) and ciclosporin (CSA). After IST, older age adversely affects survival, progressively worsening over the age of 40 years, and particularly among patients older than 60 years.1-3 For patients aged 50 to 60 years and older than 60 years, 5-year overall survival (OS) is 57% and from 50% to 52%, respectively; response to ATG and CSA among patients older than 40 years and with very severe AA is only 15%.4,5
In contrast, significant improvement in outcomes after HSCT using matched sibling donor (MSD) and matched unrelated donor (MUD) over time has led to a progressive increase in the upper age limit for consideration of HSCT.6-8 Improvements include better supportive care; improved HLA tissue typing; changes in conditioning regimens including the use of low-dose cyclophosphamide (CY) with fludarabine to replace high-dose CY for MUD HSCT and, in older patients,9-12 the use of in vivo T-cell depletion with ATG or alemtuzumab8,13,14 ; and avoidance of peripheral blood stem cells (PBSC) when using ATG-based conditioning regimens.15,16 European and international registry data report worse outcomes for patients older than 40 years transplanted from MSD/MUD compared with younger patients.17,18 In contrast, a large, single-center study reported excellent OS (>90%) among patients older than 40 years, using a uniform conditioning of fludarabine, low-dose CY, and ATG and matched sibling donors,19 and another dose de-escalating study showed improvement in tolerance with acceptable graft failures by further reducing the doses of CY. The optimal dose of CY appeared to be 50 mg/kg when given with fludarabine, ATG, and 2 Gy total body irradiation (TBI).20
With an increasingly aging population globally, a more realistic definition of older patients when assessing transplant outcomes may be older than 50 years instead of the historical definition of older than 40 years.21 For hematologic malignancies, increasing numbers of patients older than 70 years are undergoing allogeneic HSCT, with more emphasis on comorbidities than age alone for patient selection.22,23 A higher risk for acute and chronic graft-versus-host disease (GVHD) among older patients with SAA has restricted consideration of HSCT in this age group, especially for a disease in which there is no benefit of a graft-versus-disease effect. A recent retrospective study of 499 patients with SAA older than 50 years showed that mortality was increased while using a MUD compared with MSD and when performance status was lower than 90%; however, age per se did not affect survival. Grade II-IV acute GVHD was higher among patients older than 65 years. However, among those patients receiving alemtuzumab as part of conditioning, rate of chronic GVHD was low, at 17%.24 A retrospective comparison from the European Blood and Marrow Transplant Registry reported a significant reduction in acute and chronic GVHD with alemtuzumab compared with ATG-based conditioning regimens.25
Alemtuzumab-based conditioning with fludarabine and low-dose CY (FCC) in SAA HSCT is an irradiation-free regimen for unrelated donor (UD) HSCT, and is associated with stable mixed T-cell chimerism and very low rates of acute and chronic GVHD and sustained myeloid engraftment persisting beyond withdrawal of immunosuppression as a result of a state of mutual donor and recipient T-cell tolerance.13 This study shows that outcomes of patients older than 50 years compared with younger patients are similar, using the composite end-point of GVHD-free, relapse-free survival (GRFS).26,27 We have further investigated the immunologic basis for control of alloreactivity, and a potential contributory role for B regulatory cells was demonstrated.
Patients and methods
Among 65 patients with acquired SAA transplanted consecutively at King’s College Hospital, London, United Kingdom, with an FCC conditioning regimen, we compared 27 patients older than 50 years with 38 patients aged 50 or fewer years. Inherited AA was excluded by a negative diepoxybutane test, absence of clinical features suggestive of constitutional AA, and family history of AA. Patients were transplanted from a MSD or a 9-10/10 (allele or antigen level) HLA UD between 2007 and 2018. One-year GRFS was defined by absence of grade 3 to 4 acute GVHD, severe cGVHD, relapse (graft failure), or death from any cause at 1 year after HSCT.26,27 Baseline organ dysfunction was defined using the hematopoietic cell transplant comorbidity index (HCT-CI).28 The severity of aGVHD and cGVHD was defined according to the Center for International Blood and Marrow Transplant Research.29,30 Patients were evaluable for engraftment if they survived more than 21 days from transplant. Cytomegalovirus (CMV) disease was defined as possible, probable, or proven.31 Chimerism was assessed in unfractionated bone marrow (BM)/peripheral blood (PB) and fractionated CD3+ T cells and CD15+ granulocytes in PB, as previously described.32 PB lymphocyte phenotyping and detection of BM somatic mutations are described in the supplemental Data. This study was reviewed and approved by the Institutional Review Board of Kings College Hospital, and all patients were consented in accordance with the Declaration of Helsinki. This is a retrospective analysis of a prospectively maintained and updated database, and data were analyzed in January 2019.
Conditioning regimen and GVHD prophylaxis
All patients were transplanted using FCC conditioning regimen comprising fludarabine 30 mg/m2 each day for 4 days (days −7 to −4), CY 300 mg/m2 each day for 4 days (days −7 to −4), and in vivo T-cell depletion with alemtuzumab (0.2 mg/kg each day, days −7 to −3). Ciclosporin alone, without methotrexate, was used as postgraft immunosuppression commencing day −1 until 9 months posttransplant, at a dose of 2.5 mg/kg twice daily. Target plasma trough CSA levels were 250 to 300 µg/L, followed by a 3-month tapering, provided there was stable hematologic parameters and absence of declining donor chimerism. If renal impairment occurred, half-dose CSA was used in combination with mycophenolate mofetil (MMF). Next, 2 Gy TBI was added to FCC for 9/10 mismatched UD (MMUD) HSCT.
Statistical analysis
Categorical variables were summarized as frequency counts and compared using 2 × 2 tables, and continuous variables were summarized as medians and compared using Mann-Whitney U test. OS was calculated from the time of transplant to the last follow-up date. GRFS was calculated as the time from transplantation until the earliest occurrence of any event: relapse, death, or GVHD. GRFS and OS were estimated by the Kaplan-Meier method and compared using the log-rank test. Cumulative incidences of treatment-related mortality (TRM), graft failure, and GVHD were compared using Fine and Gray model,26,33 and were considered as competing risks for each other. Statistical analysis was conducted using SPSS 24.0 and R 3.4.3 software. All statistical tests were 2-sided, and a P value of .05 was used to indicate statistical significance.
Results
Demographic profile
Patient characteristics are summarized in Table 1. The median age of the older group was 61 years (range, 51-71 years) compared with 36 years (range, 22-50 years) for the younger group. In the older and younger groups, most patients received HSCT from an unrelated donor (78% vs 79%, respectively). The 2 groups were well matched in terms of time from diagnosis to HSCT, prior IST given, donor type, HLA allo-immunization, HCT-CI score, and donor age. A similar donor age between the 2 groups likely reflected the high proportion of patients who received an unrelated donor transplant. The majority of patients (100% older vs 81% younger patients; P = .03) received PBSC. The median CD34+ stem cell dose was 6.7 × 106/kg (range, 1.97-123 × 106/kg), and median duration of follow-up was 41 months (range, 4 months-11 years). One older patient had Robertsonian translocation (der 13, 14), and of 40 patients tested for somatic mutations, 1 had an ASXL1 mutation (Gly646TrpfsTer12; variant allele frequency, 10%).
Engraftment and chimerism
Median time to neutrophil and platelet engraftment and rate of graft failure were similar for older and younger patients (Table 2). Three older patients with invasive fungal disease at time of transplant were nonevaluable for engraftment, as they died between days +14 and 21. In the older group, there was 1 graft failure (3.7%) at 6 months post-HSCT in a 71-year old patient associated with subtherapeutic CSA blood levels. In the younger group, there were 2 graft failures (5.3%): 1 early graft failure occurred in a patient who received a low BM stem cell dose, and 1 late graft failure was associated with low CSA levels because of renal impairment. All 3 patients with graft failure were successfully re-transplanted using fludarabine with ATG conditioning and PBSC and with no subsequent GVHD. Median time to stopping CSA was 40.2 months (range, 11-123 months), and 4 patients currently continue receiving CSA, and 2 continue receiving MMF. There was a high incidence of stable mixed T-cell chimerism, which was similar among older and younger patients (Table 2; supplemental Figure 1A-B).
Viral infections and comorbidities
Rates of CMV and Ebstein-Barr virus reactivation were similar between older and younger patients (33% vs 24% [P = .4] and 37% vs 39% [P = .79], respectively; Table 2). There was 1 case of probable CMV disease31 in both age groups, and 6 patients (4 aged ≥ 50 years) had recurrent (>1) CMV reactivation. One patient with recurrent CMV reactivation was successfully treated with third-party CMV-specific cytotoxic T lymphocytes after persistent cytopenias resulting from prior CMV treatment with ganciclovir/valganciclovir. There were 2 cases of Ebstein-Barr virus posttransplant lymphoproliferative disorder in the younger cohort. Although HCT-CI scores were similar between the 2 age groups, older patients with renal dysfunction required dose reduction of CSA with addition of MMF posttransplant. None of the patients in the older cohort with cardiac or hepatic dysfunction pretransplant developed worsening of cardiac problems posttransplant (supplemental Table 1).
GRFS, OS, and TRM
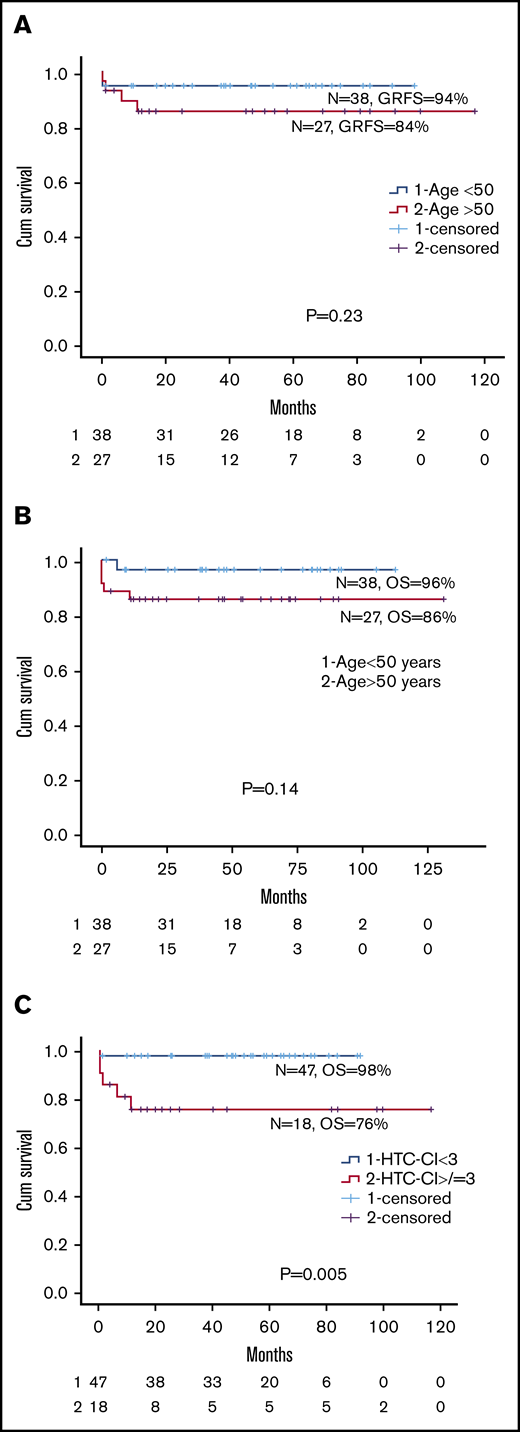
Comparing the older group with the younger group, there was no significant differences in I-year GRFS (84% [range, 62.9%-94.5%] vs 94% [range, 78.6%-98.6%]. P = .23), 5-year OS (86% [range, 66.2%-95.7%] vs 96% [range, 83.3%-99.4%]; P = .14), or 1-year TRM (14% vs 5.4%; P = .23; Figure 1 A-B; supplemental Figure 2; Table 2). Among the older group, there was no difference in 5-year OS between patients aged 51 to 60 or 61 to 71 years (89% vs 82%, respectively; P = .82). Because there was no difference in HTC-CI scores between the 2 groups, we examined the effect of HTC-CI on OS for the whole cohort. An HTC-CI of at least 3 was associated with a significantly worse 5-year OS compared with HTC-CI lower than 3 (76% [range, 55%-92%] vs 98% [range, 88%-100%], respectively; P = .005; Figure 1C). Causes of death in the 2 groups are summarized in Table 2.
Overall survival of patients according to GRFS, age, and comorbidity index. (A) GRFS for age older than 50 vs younger than 50 years. (B) OS for age older than 50 vs younger than 50 years. (C) HCT-CI for less than 3 vs more than 3 for entire cohort
Overall survival of patients according to GRFS, age, and comorbidity index. (A) GRFS for age older than 50 vs younger than 50 years. (B) OS for age older than 50 vs younger than 50 years. (C) HCT-CI for less than 3 vs more than 3 for entire cohort
Graft-versus-host disease
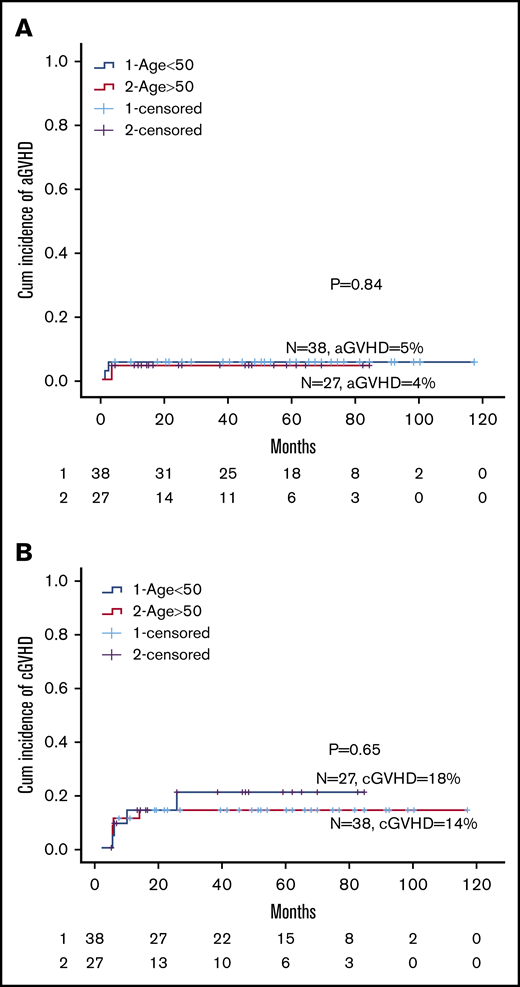
Results for GVHD are summarized in Table 3. There was no acute or chronic GVHD among MSD transplants. There were 3 cases of acute GVHD: 2 severe skin GVHD in patients transplanted with MMUD, and 1 (2.7%) grade 2 skin GVHD in a MUD transplant. Cumulative incidence of acute GVHD was similar for older and younger patients (4% vs 5%; P = .84; Figure 2A). Among MUD transplants, 7 (19%) had mild chronic skin GVHD, and severe skin chronic GVHD was seen in the 2 patients transplanted from 9/10 UDs. There was no difference in cumulative incidence between older and younger patients (18% vs 14%; P = .65; Figure 2B).
Cumulative incidence of GVHD. (A) Cumulative incidence of acute GVHD for age 50 years or older vs younger than 50 years. (B) Cumulative incidence of chronic GVHD for age 50 years or older vs younger than 50 year.
Cumulative incidence of GVHD. (A) Cumulative incidence of acute GVHD for age 50 years or older vs younger than 50 years. (B) Cumulative incidence of chronic GVHD for age 50 years or older vs younger than 50 year.
Cytopenias post HCT
Posttransplant cytopenias were observed in 11 patients and are summarized in Table 4. These comprised 6 autoimmune cytopenias: 4 with warm-type autoimmune hemolytic anemia, 1 probable autoimmune neutropenia and 1 immune thrombocytopenia purpura. The median time of onset was 25 months (range, 12-207 months) for the 4 patients who developed autoimmune hemolytic anemia and 17.5 and 25 months, respectively, for the patients with immune thrombocytopenia purpura and autoimmune neutropenia, respectively. Outcomes are summarized in Table 4. There were 3 cases of drug-induced cytopenia, and 1 patient with ganciclovir-induced cytopenias resulting from recurrent CMV disease developed hemophagocytic syndrome, BM hypocellularity, and transfusion dependence. Late mild thrombocytopenia with reduced BM megakaryocytes occurred in a patient at 51 months posttransplant, with a fall in fractionated CD15 PB chimerism and normalization of platelet count on CSA reintroduction.
Second malignancies
Hepatocellular carcinoma developed 41 months posttransplant in a 39-year-old man who had received prolonged oxymetholone and required resection of hepatic adenoma pretransplant. He was treated with partial hepatectomy, and later sorafenib for lung metastases. A 22-year-old woman developed squamous cell cervical carcinoma associated with human papilloma virus 101 months posttransplant, which was successfully treated with radiotherapy. This patient was refractory to 2 courses of ATG pretransplant, and developed primary graft failure posttransplant as the result of a low BM stem cell dose. She was successfully retransplanted and remains in hematological remission with no GVHD. A 19-year-old man developed classical Hodgkin disease 82 months posttransplant (Table 2).
Reconstitution of lymphocytes with immunomodulatory potential
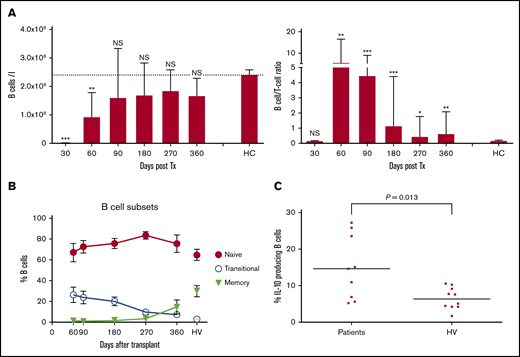
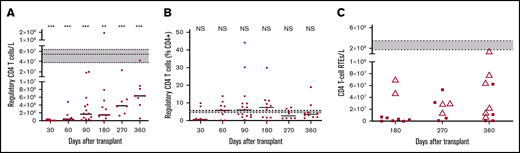
After HSCT, there is rapid recovery of B cells in contrast to a profound T-cell immunoparesis, as shown in our previously published work (Figure 3A).32 A more detailed analysis of recovery of B-cell subsets showed a substantial proportion of naive and transitional B cells early after HSCT (Figure 3B), and a significantly higher proportion of interleukin 10 (IL-10)–producing B cells compared with healthy volunteers after in vitro stimulation with CD40L (14.6% compared with 6.3% for B cells from healthy volunteers; P = .013; Figure 3C). IL-10 was produced by both transitional and naive B-cell subsets (data not shown). Numbers of T cells with a potential regulatory phenotype (CD4+, CD25high, CD27+, CD127low, FoxP3+) were significantly deficient (Figure 4A), but their proportions within the CD4 T-cell population were normal (median range, 4.2%-7.4% compared with 5.3% in healthy volunteers; Figure 4B). Among the naïve CD4 T cells, those expressing CD31 (indicative of recent thymic emigration) were substantially lower in patients older than 50 years (filled circles) compared with those younger than 50 years (white triangles; Figure 4C).
Recovery of B cells and B-cell/T-cell ratio, B-cell subsets, and potential to produce IL-10. (A) B-cell recovery (left) and B-cell/T-cell ratio recovery (right). (B) Recovery of B-cell subsets. Percentage of transitional, naive, and memory B cells. Mean and standard error of the mean are shown, and values for 11 adult healthy volunteers (HV) are indicated. (C) Percentage of IL-10-producing B cells present early (day 90) after HSCT and in healthy volunteers (HV) was determined by stimulation of PBMCs with transfected L cells expressing CD40L for 24 hours with leukocyte activation cocktail added for the last 6 hours, followed by fixation, permeabilization, and assessment of IL-10 production by B cells, using flow cytometry. Short horizontal lines indicate the median values. IL-10 production in response to stimulation with L cells expressing CD40L was at least threefold higher than background response to nontransfected L cells. Comparison between patients and healthy volunteers were performed using a 2-tailed Mann-Whitney U test. Significant differences are indicated with ***P < .0005; **P < .005; *P < .05. NS, not significant.
Recovery of B cells and B-cell/T-cell ratio, B-cell subsets, and potential to produce IL-10. (A) B-cell recovery (left) and B-cell/T-cell ratio recovery (right). (B) Recovery of B-cell subsets. Percentage of transitional, naive, and memory B cells. Mean and standard error of the mean are shown, and values for 11 adult healthy volunteers (HV) are indicated. (C) Percentage of IL-10-producing B cells present early (day 90) after HSCT and in healthy volunteers (HV) was determined by stimulation of PBMCs with transfected L cells expressing CD40L for 24 hours with leukocyte activation cocktail added for the last 6 hours, followed by fixation, permeabilization, and assessment of IL-10 production by B cells, using flow cytometry. Short horizontal lines indicate the median values. IL-10 production in response to stimulation with L cells expressing CD40L was at least threefold higher than background response to nontransfected L cells. Comparison between patients and healthy volunteers were performed using a 2-tailed Mann-Whitney U test. Significant differences are indicated with ***P < .0005; **P < .005; *P < .05. NS, not significant.
Recovery of T cells with regulatory and recent thymic emigrant phenotypes. Numbers (A) and percentages (B) of CD4 T cells with a regulatory phenotype. Short horizontal lines indicate the median values at each time. Horizontal dotted lines enclosing gray boxes represent the median and interquartile range of 11 adult healthy volunteers. Comparisons between patients at each time and healthy volunteers were performed using a 2-tailed Mann-Whitney U test. (C) Numbers of naive CD4 T cells expressing CD31, which indicates recent thymic emigrants (RTEs). Patients older than 50 years are indicated by filled circles and those younger than 50 years are indicated by white triangles. Horizontal dotted lines enclosing gray boxes represent the median and interquartile range of 11 adult healthy volunteers. Significant differences are indicated with ***P < .0005; **P < .005.
Recovery of T cells with regulatory and recent thymic emigrant phenotypes. Numbers (A) and percentages (B) of CD4 T cells with a regulatory phenotype. Short horizontal lines indicate the median values at each time. Horizontal dotted lines enclosing gray boxes represent the median and interquartile range of 11 adult healthy volunteers. Comparisons between patients at each time and healthy volunteers were performed using a 2-tailed Mann-Whitney U test. (C) Numbers of naive CD4 T cells expressing CD31, which indicates recent thymic emigrants (RTEs). Patients older than 50 years are indicated by filled circles and those younger than 50 years are indicated by white triangles. Horizontal dotted lines enclosing gray boxes represent the median and interquartile range of 11 adult healthy volunteers. Significant differences are indicated with ***P < .0005; **P < .005.
Discussion
Using a uniform conditioning with FCC, we report similar outcomes for patients aged at least 50 years compared with younger adult patients, using the composite endpoint of GRFS.27 As OS of patients with SAA transplanted using MSD and MUD now exceeds 80%, evaluation of outcomes that take into account not only failure-free survival but also GVHD becomes the optimal assessment tool.26,27 Despite a higher risk for GVHD in older transplanted patients in the reported literature,24 we show that FCC conditioning is as effective as in younger patients at reducing the risk for GVHD, and significantly lower than other groups using predominantly ATG-based settings.18,24 The low sample size and retrospective nature of study remains a drawback to this study. The study lacks the power to detect small differences in survival between the 2 groups. The number of patients required to detect a 10% difference in OS and GRFS would be 150 per group (300 total), with 80% power at 5% significance. However, this being a rare disease, and given the difficulty in designing a prospective study as well as scarcity of literature in this age group, this study highlights new issues relating to HSCT in older patients with SAA.
HCT-CI scores were similar between older and younger patients, but for all ages taken together, an HTC-CI score of at least 3 was associated with a significantly worse OS compared with a score lower than 3. This is in keeping with a recent Center for International Blood and Marrow Transplant Research study when applying HCT-CI to a cohort of patients transplanted for nonmalignant marrow disorders.34 Thus, comorbidities instead of age per se are more important when considering HSCT for SAA in older patients.
FCC transplantation resulted in a low TRM, even for older patients, which was not significantly different from younger patients (14% vs 5.4%). Invasive fungal disease at time of HSCT accounted for most of the 1-year TRM in older patients, accounting for 4 of 5 deaths. This low TRM is in contrast to worse survival after IST with ATG among older patients.1-4
A potential concern has been raised, when using alemtuzumab, of an increase in risk for viral infections.6,35 There has been no direct comparison between alemtuzumab and ATG-based conditioning regimens in SAA HSCT. A large study from Korea reported data on CMV reactivation after FCATG HSCT for SAA. Among 177 patients, 59% of patients older than 40 years and 44% of younger patients needed preemptive CMV treatment. This compares with 33% and 37% in the current study. This is despite the need to continue CSA after HSCT for 12 months to prevent late graft failure. A smaller single-center study reported that CMV reactivation occurred in 76% of patients transplanted using alemtuzumab-based conditioning.35 Nevertheless, the myelosuppressive effect of CMV treatment can be problematic, and necessitated the use of CMV-specific cytotoxic T lymphocytes in 1 patient with recurrent CMV reactivation.
We have previously highlighted the risk for secondary autoimmune cytopenias after FCC conditioning, and this is a recognized complication of using alemtuzumab in other settings,35 such as treatment of multiple sclerosis. Although the proportions of T cells with potential regulatory phenotype within the CD4 T-cell population were normal compared with in healthy volunteers (Figure 4B), in view of overall profound T-cell deficiency, the absolute numbers were significantly low (Figure 4A) and prolonged, which may contribute to dysregulated B-cell function, possibly explaining the high incidence of autoimmune cytopenia.
In this study, we confirm that it is safe to use PBSC instead of BM for older patients. The rationale for using PBSC was to minimize the risk for a low stem cell dose that is more likely with a BM harvest. A low stem cell dose is a risk factor for graft failure in SAA. There were 2 cases of late graft failure associated with low CSA blood levels, and 1 early graft failure in a patient who received a suboptimal dose of infused BM cells, which compares favorably with other studies using ATG and BM harvest.18,19 The use of PBSC is contraindicated using ATG-based conditioning because of an increased risk for acute and chronic GVHD.15,16 Using FCC, despite the use of PBSC, the incidence of GVHD among older patients was similarly low compared with younger patients. Furthermore, there were no cases of severe acute or chronic GVHD among MUD transplants, and all cases were confined to the skin.
Among older patients, an increase in renal impairment was noted, likely because of CSA toxicity. Because relatively high CSA blood levels and prolonged CSA administration are required posttransplant for SAA, a higher number of older patients needed a reduction in CSA dose with simultaneous administration of MMF to help prevent late graft failure.
We report a similar incidence of stable mixed T-cell chimerism among older and younger patients. We have previously shown that mixed chimerism is associated with sustained myeloid chimerism and hematologic engraftment that is accounted for by persistence of recipient CD8+ T effectors that survive the conditioning.32 This provides the rationale for the requirement for extended CSA for at least 12 months posttransplant. Despite stable mixed T-cell chimerism that continued at least 3 years posttransplant and 2 years after CSA withdrawal, patients retained full donor myeloid chimerism and a low rate of chronic GVHD, indicating a state of mutual donor-recipient tolerance.
In this study, we investigated further the basis for low alloreactivity. The potential basis for tolerance might be multifactorial, possibly related to B regulatory cells, defined as IL-10-producing B cells,36,37 and naive T cells that are thymic emigrants, defined by CD31 expression.38,39 Potential factors that may be controlling alloreactivity include, first, the production of naive T cells of recent thymic emigration, which was higher in patients younger than 50 years, consistent with age-related thymic atrophy in older patients, but indicating that in older age, their contribution to preventing alloreactivity may not be substantial. Furthermore, proportions of T cells with potential regulatory phenotype within the CD4 T-cell population were normal, although T regulatory cell numbers were low during the first year after HSCT, reflecting the profound CD4 T lymphopenia. Second, in contrast, we demonstrated the presence of B lymphocytes with immunomodulatory potential. More significantly, the B-cell population present early after transplantation contained a significantly higher proportion of cells that produce the immunosuppressive cytokine IL-10, after in vitro stimulation with CD40L (14.6% compared with 6.3% for B cells from healthy volunteers; P = .013; Figure 3C). IL-10 was produced by both transitional and naive B-cell subsets (data not shown). Last, a factor that may potentially contribute to low alloreactivity of the T effectors that survive lymphodepletion with alemtuzumab is the relatively mild conditioning with fludarabine and low-dose CY (without TBI, except for 3 patients transplanted from 9/10 MMUDs, possibly resulting in limited tissue damage and reduced cytokine storm). However, in view of low sample size, the role of B regulatory cells in controlling alloreactivity is speculative but for future immunological scientific studies.
Among older patients with SAA, excluding a possible diagnosis of hypoplastic myelodysplastic syndrome can sometimes be challenging, but in our study, none had evidence of hypoplastic myelodysplastic syndrome pretransplant, as assessed by morphology and metaphase cytogenetics.40,41 There was only 1 case of ASXL1 at low variant allele frequency of 10%.
Outcomes of alternative therapies for patients older than 60 years, such as the combination of ATG and CSA or CSA alone, are dismal.1-4 The retrospective contemporary population-based study from Sweden highlighted the significantly worse survival among patients with AA aged at least 60 years treated with IST compared with younger patients, with less than 40% alive after 5 years and an excess mortality of 45%.4,5 Furthermore, after IST and eltrombopag, patients are at later risk for myelodysplastic syndrome/AML and solid tumors, especially lymphomas, in addition to risk for relapse of AA in up to 38% of patients.42-46
We show for the first time, using a composite end-point of GRFS, that outcomes among older patients with SAA, transplanted mainly from MUDs, were comparable with those for younger patients undergoing FCC transplantation, with low morbidity and lower-than-expected TRM generally observed after IST for SAA in this older age group. The incidence of GVHD was extremely low in older patients, despite using predominantly peripheral stem cell harvest from UDs. This was mainly attributed to persistent mixed T-cell chimerism because of tolerance developed between host and donor, mainly attributed to B regulatory cells. This regimen challenges the myth related to the safety of transplants in the elderly subgroup with SAA, and shows that careful patient selection based on comorbidities is likely more important than age per se.
Authorship
Contribution: V.S.S. collected data, analyzed data, prepared the manuscript, and designed the study; V.P. designed the study, reviewed data, and reviewed the manuscript; S.A.G., A.G.K., H.d.L., P.M., A.P., C.F.M.R., and V.M. treated patients and reviewed the manuscript; F.G. and L.D.B. carried out experiments; S.I. collected and analyzed data; G.J.M. designed the study, reviewed the data, and prepared and mentored the manuscript; and J.C.M. designed the study, reviewed the data, and prepared and mentored the manuscript
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Judith C. Marsh, Department of Haematological Medicine, King's College Hospital, Denmark Hill, London SE5 9RS, United Kingdom; e-mail: judith.marsh@nhs.net.
References
Author notes
The full-text version of this article contains a data supplement.