Key Points
Allogeneic stem cell transplant is a well-tolerated and useful therapeutic option for relapsed/refractory pediatric NHL.
NHL histological subtype and disease status at time of transplant influence outcomes.
Abstract
Allogeneic hematopoietic stem cell transplant (HSCT) for relapsed pediatric non-Hodgkin lymphoma (NHL) is often reserved for patients with certain NHL subtypes or high-risk disease whereas the remainder receive autologous HSCT. Given the aggressive nature of pediatric NHL, we performed allogeneic HSCTs for all patients regardless of disease risk. We report overall survival (OS) and prognostic variables in 36 pediatric patients who underwent allogeneic HSCT between 1998 and 2016. OS at 3 years was 67%. The 3-year OS varied based on NHL subtype: 100% for anaplastic large cell lymphoma (n = 14), 63% for diffuse large B-cell lymphoma (n = 8), 17% for lymphoblastic lymphoma (LL; n = 9) and 80% for other subtypes combined (n = 5). Disease status influenced outcome with 3-year OS of 100% for patients in complete remission (n = 15), 59% with partial remission (PR; n = 17), and 0% with progressive/stable disease (n = 3) (P = .004). Of the 17 patients in PR, all 6 with LL died of relapsed disease, whereas the other 11 attained remission after HSCT and remained disease-free. The cumulative incidence of relapse after HSCT for LL was 78% compared with 15% for all other NHL subtypes combined (P < .0001). Cumulative incidence of nonrelapse mortality (NRM) was low in our cohort at 6%. Hence, allogeneic HSCT is a well-tolerated and useful therapeutic option with low rates of NRM and relapse for all NHL subtypes except LL with active disease at HSCT.
Introduction
Given the low incidence of childhood non-Hodgkin lymphoma (NHL) and excellent outcomes with current upfront therapies,1,-3 there are limited data on the use of hematopoietic stem cell transplant (HSCT) for treating relapsed or refractory disease. In particular, there are no clear guidelines for choosing between autologous or allogeneic HSCT for these patients and the decision is often dictated by local institutional practices. In most pediatric centers, autologous HSCTs are performed for the majority of patients, and allogeneic HSCT is reserved for those with certain NHL histological subtypes such as lymphoblastic lymphoma (LL) or with higher-risk or refractory disease. These decisions are largely based on older studies in adults, where higher nonrelapse mortality (NRM) after allogeneic HSCT compared with autologous HSCT offset any advantage of a lower relapse rate from putative graft-versus-lymphoma (GVL) activity.4,,,-8
It has been difficult to evaluate the suitability of this approach to selecting autologous vs allogeneic HSCT for pediatric NHL as small patient numbers have meant that most published pediatric reports describe combined data for autologous and allogeneic HSCTs.9,10 An older study from St. Jude Children’s Research Hospital reported a combined disease-free survival (DFS) of 50% in 24 patients receiving autologous or allogeneic HSCT.11 A more recent publication from Memorial Sloan Kettering Cancer Center (MSKCC) reports DFS of 53% in 36 patients again for both autologous and allogeneic HSCT combined.10 Gross et al evaluated the benefit of autologous vs allogeneic HSCTs by reviewing the Center for International Blood and Marrow Transplant Registry (CIBMTR) data from 182 patients whose transplants were performed between 1990 and 2005 at multiple centers.12 Depending on NHL disease subtype, DFS ranged from 4% to 52%. Benefit of allogeneic over autologous could only be established for the LL NHL subtype, where outcomes were superior after allogeneic HSCT, a benefit predominantly resulting from a lower relapse rate as compared with autologous HSCT. The indication for autologous vs allogeneic is not provided in these registry data and it is possible that patients with higher risk and those with chemotherapy refractory disease were chosen to undergo allogeneic HSCT.
Given the aggressive nature of pediatric as compared with adult NHL, for the last 2 decades our institution has used allogeneic HSCTs for all patients with relapsed or refractory disease, regardless of histological subtype or disease risk. This practice meant there was no bias in patient selection for those with higher-risk disease. We have now performed a retrospective chart review to evaluate outcomes because this single-center study means there is greater homogeneity in transplant practice without the center effects of registry studies. Moreover, our long-term follow-up data allow us to capture late events for our patients.
We report outcomes in 36 pediatric allogeneic recipients and describe risk factors contributing to survival. Our results show allogeneic HSCT to be effective for children with relapsed and refractory disease with low cumulative incidence rates of NRM and relapse. Risk factor analysis showed histological subtype and disease status at time of transplant influenced outcome, with worst outcomes seen in patients with LL with active disease.
Methods
Study design and inclusion criteria
This study was a retrospective chart review, which was approved by the Baylor College of Medicine (BCM) Institutional Review Board (IRB). Between 1 November 1998 and 31 December 2016, all patients with NHL who underwent allogeneic HSCT were included in this analysis. Patients who received autologous HSCT during this 18-year period were excluded from analysis.
The following data were collected: age, sex, lymphoma subtype, donor type, conditioning regimen used, time to relapse after initial diagnosis, disease status at HSCT, engraftment status, acute and chronic graft-versus-host disease (GVHD), NRM, relapse, and survival status. Engraftment was defined as an absolute neutrophil count >0.5 × 109/L for 3 consecutive days in those surviving at least 28 days after HSCT. GVHD was assessed using standard published criteria and disease status at transplant.13 Complete remission (CR), partial remission (PR), stable disease (SD), or progressive disease (PD) was determined using the Lugano classification.14
Statistical analysis
Descriptive statistics were used to summarize disease and transplant characteristics. Kaplan-Meier curves were constructed to estimate overall survival (OS) and disease-free survival (DFS), and comparisons between groups were carried out using the log-rank test. The cumulative incidence of engraftment, relapse, and NRM were analyzed and plotted by the competing risk method as described by Gray.15
Results
Patient and disease characteristics
Thirty-six patients made up the study cohort. None of these patients received prior autologous HSCT. The median age at HSCT was 14.5 years (range, 4-24 years) and 23 of 36 patients (63.9%) were male.
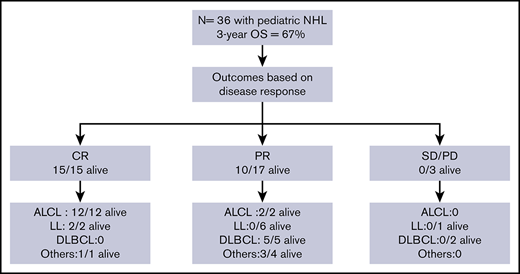
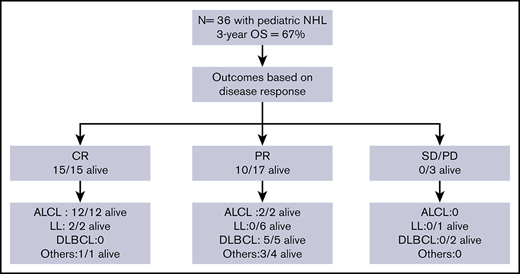
Anaplastic large cell lymphoma (ALCL) was the most common subtype of NHL (14 of 36), followed by LL (9 of 36; T-cell LL, 8 of 9; B-cell LL, 1 of 9), diffuse large B-cell lymphoma (DLBCL; 8 of 36), and 5 other histological subtypes (1 each: Burkitt lymphoma; small cell, non-Burkitt high-grade lymphoma; T-cell–rich, B-cell lymphoma; stem cell myeloproliferative/T-cell lymphoma; and hepatosplenic γδ T-cell lymphoma). At the start of conditioning therapy, 15 patients were in CR (CR1 = 3, CR2 = 12), 17 were in PR, and 3 had SD. The 3 CR1 patients received allogeneic HSCT for primary refractory disease. For 1 patient, staging was performed at an outside hospital and data were not available on chart review to verify disease status prior to transplant. Patient characteristics are summarized in Table 1.
Transplant procedures
Transplant characteristics including donor types, stem cell sources, and conditioning regimens are summarized in Table 1. In our cohort, one-third of patients (13 of 36) received HSCTs from a MRD, 10 of 36 patients from MUDs, 9 of 36 from MMUDs, and 4 of 36 from haploidentical donors. More than two-thirds of patients (26 of 36) received bone marrow grafts whereas 10 of 36 patients received peripheral blood stem cell grafts.
The majority of patients received MAC regimens (27 of 36) and all but 4 patients received TBI. The most frequent myeloablative regimen used was TBI-based (TBI; 1200 cGy for MRD, 1400 cGy for unrelated and haploidentical donors) along with cyclophosphamide and cytosine arabinoside. For unrelated and haploidentical donors, serotherapy with anti-CD52 alemtuzumab (Campath) was added. Lungs were shielded after 600 cGy. For patients who were unable to tolerate full-dose TBI, a lower dose (600 cGy) was used along with fludarabine, and serotherapy with Campath was incorporated for unrelated donors. Three patients received busulfan-based myeloablative regimens due to history of prior radiation and the 1 patient with underlying ataxia telangiectasia received a fludarabine-and-melphalan–based conditioning regimen.
For all patients other than haploidentical recipients, GVHD prophylaxis consisted of low-dose methotrexate and tacrolimus or cyclosporine. Patients who underwent haploidentical transplants received ex vivo T-cell–depleted grafts and were not given additional posttransplant GVHD prophylaxis. CD34+ cells were selected from leukapheresis products using the Isolex (Nexell Therapeutics, Irvine, CA) or CliniMacs (Miltenyi Biotec, Auburn, CA) magnetic-activated cell-sorting system.
Engraftment
The cumulative incidence of engraftment was 97.2% (95% confidence interval [CI], 81.2% to 99.6%). One patient died at day +16 posttransplant, prior to engraftment, and therefore could not be evaluated for this event.
GVHD
Seven of the 36 patients had acute GVHD (4 grade I-II; 3 grade III-IV). Four of the 36 patients developed chronic GVHD (3 limited, 1 extensive). There was no correlation between occurrence of GVHD and the type of donor or conditioning regimen used.
Survival analysis
Over the 18-year period, 9 patients received transplants between 1998 and 2002, 8 patients between 2003 and 2007, 7 patients between 2008 and 2012, and 11 patients between 2013 and 2016. The median follow-up for the 36 patients in the cohort was 31 months and all but 3 patients had >1-year follow-up.
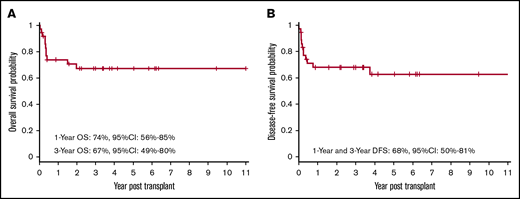
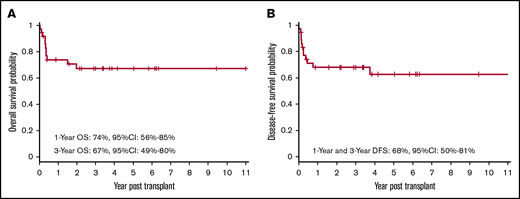
Twenty-five of the 36 patients who received transplants remain alive and disease-free. The OS at 1 year and 3 years was 74% (95% CI, 56% to 85%) and 67% (95% CI, 49% to 80%), respectively (Figure 1A). The DFS at 1 year and 3 years was 68% (95% CI, 50% to 81%) (Figure 1B).
OS and DFS after allogeneic HSCT. Kaplan-Meier curve showing probabilities of OS (A) at 1 year and 3 years are 74% and 67%, respectively, and probabilities of DFS (B) at 1 year and 3 year are 68%.
OS and DFS after allogeneic HSCT. Kaplan-Meier curve showing probabilities of OS (A) at 1 year and 3 years are 74% and 67%, respectively, and probabilities of DFS (B) at 1 year and 3 year are 68%.
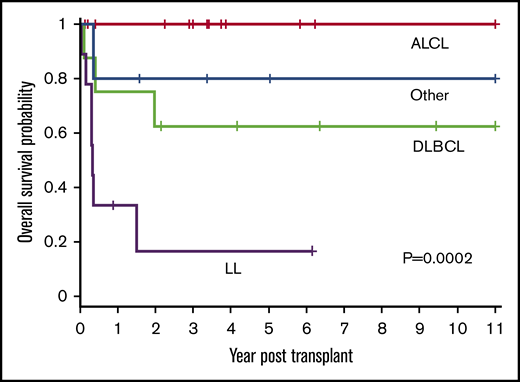
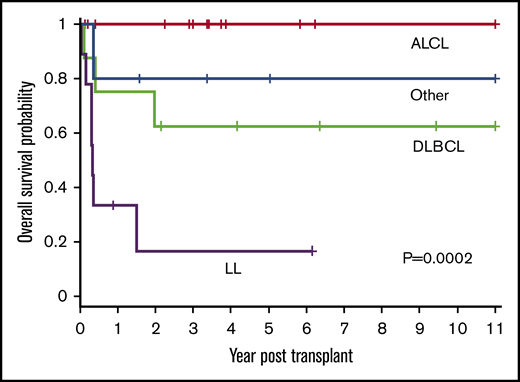
Patient outcomes were affected by the underlying lymphoma subtype (P = .0002) (Figure 2). The 1-year OS for ALCL, DLBCL, LL, and other was 100%, 75% (95% CI, 31% to 93%), 33% (95% CI, 8% to 62%), and 80% (95% CI, 20% to 97%), respectively. All 14 patients with ALCL are alive with a 3-year OS of 100%. Patients with DLBCL had a 3-year OS of 63% (95% CI, 23% to 86%) with 5 of 8 alive. In contrast, only 2 of 9 patients with LL are alive with a 3-year OS of 17% (95% CI, 1% to 49%). The remainder were a heterogeneous group of rare pediatric NHL subtypes (n = 5) and analyzed together had an 80% OS at 3 years (95% CI, 20% to 97%) (4 of 5 alive).
OS based on histological NHL subtype. The 1-year OS for ALCL, DLBCL, LL, and other was 100%, 75% (95% CI, 31% to 93%), 33% (95% CI, 8% to 62%), and 80% (95% CI, 20% to 97%), respectively. The 3-year OS for ALCL, DLBCL, LL, and other was 100%, 63%, (95% CI, 23% to 86%), 17% (95% CI, 1% to 49%), and 80% (95% CI, 20% to 97%), respectively (P = .0002).
OS based on histological NHL subtype. The 1-year OS for ALCL, DLBCL, LL, and other was 100%, 75% (95% CI, 31% to 93%), 33% (95% CI, 8% to 62%), and 80% (95% CI, 20% to 97%), respectively. The 3-year OS for ALCL, DLBCL, LL, and other was 100%, 63%, (95% CI, 23% to 86%), 17% (95% CI, 1% to 49%), and 80% (95% CI, 20% to 97%), respectively (P = .0002).
Patients with LL are known to have poor outcomes and, unlike for other histological subtypes, most institutes perform allogeneic HSCTs for this subtype. To ascertain the effect of allogeneic HSCT on all subtypes except LL, further analysis was performed excluding patients with LL. When grouped together, all other histological subtypes (n = 27) had significantly better OS compared with patients with LL (n = 9) (P < .0001). The OS at 1 year and 3 year for all histological subtypes, excluding LL, was 88% (95% CI, 68% to 96%) and 84% (95% CI, 62% to 94%), respectively, and DFS was 84% (95% CI, 63% to 94%) at 1 year and 3 year with low relapse rate (3 of 27 patients) for the entire subgroup, suggesting the benefit of allogeneic graft for this cohort of patients.
Relapse
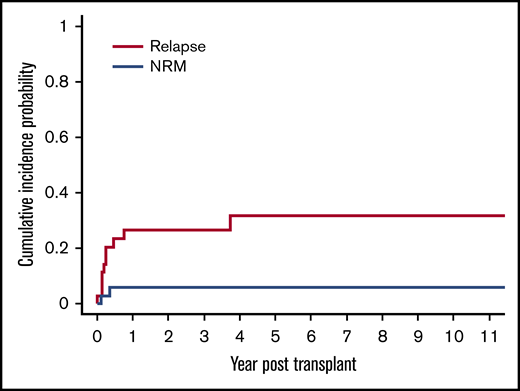
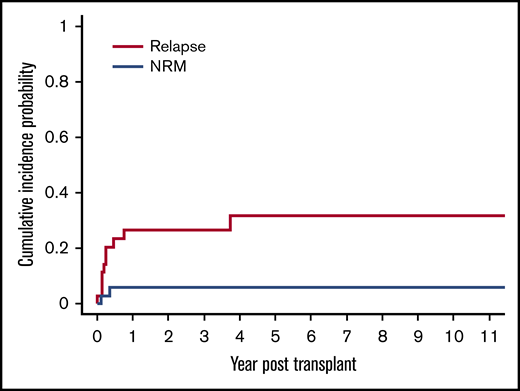
The cumulative incidence of relapse was 20% (95% CI, 9% to 35%) at day 100 and 26% (95% CI, 13% to 42%) at 1 year and 3 years, respectively (Figure 3). There were a total of 10 relapses in the 36 patients in our cohort. Seven of these 10 relapses occurred in patients with LL. The cumulative incidence of relapse for patients with LL was significantly higher at 78% (95% CI, 28% to 95%), compared with 15% (95% CI, 3% to 36%) for patients with other lymphoma subtypes (P < .0001).
Cumulative incidence of relapse and NRM after allogeneic HSCT. The cumulative incidence of relapse was 20% (95% CI, 9% to 35%) at day 100 and 26% (95% CI, 13% to 42%) at 1 year and 3 years, respectively. The cumulative incidence of NRM was 3% (95% CI, 0.2% to 13%) at day 100 and 6% (95% CI, 1% to 17%) at 1 year, respectively.
Cumulative incidence of relapse and NRM after allogeneic HSCT. The cumulative incidence of relapse was 20% (95% CI, 9% to 35%) at day 100 and 26% (95% CI, 13% to 42%) at 1 year and 3 years, respectively. The cumulative incidence of NRM was 3% (95% CI, 0.2% to 13%) at day 100 and 6% (95% CI, 1% to 17%) at 1 year, respectively.
Timing of relapse posttransplant also varied by histological subtype. Patients with LL relapsed early at a median of 54 days (range, 0-166 days) post-HSCT and patients with DLBCL at a median of 187 days (range, 94-280 days). Only 1 of 14 patients with ALCL relapsed and this patient had a very late relapse (44 months post-HSCT). This patient had received a RIC regimen and remained with ∼40% donor chimerism despite donor lymphocyte infusions. He had an unusual pattern of relapse with evidence of leptomeningeal enhancement and positive cerebrospinal fluid cytology but with no evidence of lymphomatous involvement at any other site. This patient is still alive receiving reinduction salvage therapy.
NRM
There was a low rate of NRM in our cohort with only 2 of the 11 total deaths attributable to this cause, resulting in a cumulative incidence of NRM of 3% (95% CI, 0.2% to 13%) at day 100 and 6% (95% CI, 1% to 17%) at 1 year, respectively (Figure 3). The cause of NRM was respiratory failure in 1 patient and multiorgan failure in another patient who had underlying ataxia telangiectasia.
Prognostic factors
We next evaluated the effect of patient and treatment variables on outcome.
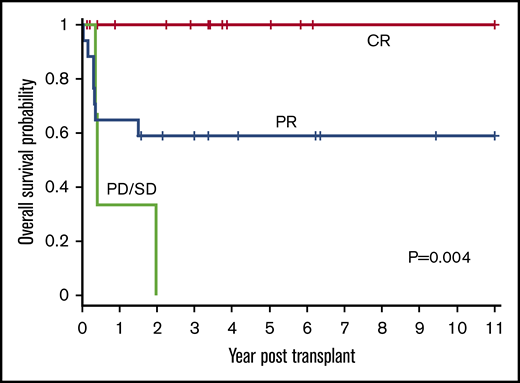
Disease status
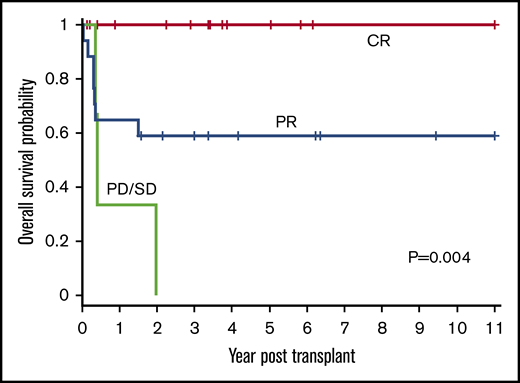
Disease status (CR, PR, or PD/SD) at time of HSCT influenced outcomes (P = .004) (Figure 4). The 1-year OS for CR, PR, and PD/SD was 100%, 65% (95% CI, 38% to 82%), and 33% (95% CI, 9% to 77%), respectively. The 3-year OS for CR, PR, and PD/SD was 100%, 59% (95% CI, 33% to 78%), and 0%, respectively.
OS based on disease status at time of HSCT. The 1-year OS for CR, PR, and PD/SD was 100%, 65% (95% CI, 38% to 82%), and 33% (95% CI, 0.9% to 77%), respectively. The 3-year OS for CR, PR, and PD/SD was 100%, 59% (95% CI, 33% to 78%), and 0%, respectively (P = .004).
OS based on disease status at time of HSCT. The 1-year OS for CR, PR, and PD/SD was 100%, 65% (95% CI, 38% to 82%), and 33% (95% CI, 0.9% to 77%), respectively. The 3-year OS for CR, PR, and PD/SD was 100%, 59% (95% CI, 33% to 78%), and 0%, respectively (P = .004).
All 15 patients who received transplants in CR were alive and all but 1 remained in continued remission after HSCT regardless of NHL subtype. Only 1 of these 15 patients suffered a late and unusual pattern of relapse (as described in “Relapse”). All 11 patients who received transplants in PR, for all NHL subtypes except those with LL, attained remission after HSCT and remained disease-free. In the LL group, all 6 patients with PR died of relapse and only the 2 treated in CR were relapse-free survivors. All 3 patients who received transplants with SD or PD irrespective of disease histology succumbed to early posttransplant relapse.
Of note, the majority of patients with ALCL (12 of 14) were in CR at time of HSCT compared with other subtypes. Ten of these patients had received novel therapeutic agents to treat their relapse (7 received crizotinib, 2 received brentuximab, and 1 a novel Alk inhibitor LDK378)
Other influences on outcome
We also evaluated the effects of other variables such as conditioning regimens and donor type on OS. However, the small numbers in each group limit the strength of these observations. The majority of patients (75%) received MAC regimens and the remaining 9 patients received RIC regimens. There was no difference in OS between patients receiving MAC vs RIC (P = .779). The 3-year OS was 67% (95% CI, 44% to 82%) for MAC and 67% (95% CI, 28% to 88%) for RIC. There was no significant difference in OS among donor stem cell source (MRD, 13; MUD/MMUD, 19; haploidentical, 4) used for HSCT (P = .400). Unlike other studies, we found no significant difference in OS between patients who relapsed early (≤12 months) compared with those relapsed late (>12 months) after initial diagnosis (P = .107) prior to being referred to HSCT. The incidence of GVHD was too low to ascertain an influence on outcome.
Discussion
We report outcomes for 36 patients after allogeneic HSCT performed over 18 years at a single institution. We found allogeneic HSCTs to be effective with OS/DFS of 67%/68%, respectively, for the entire cohort and 84%/84% excluding patients with LL. We also noted low rates of NRM following allogeneic HSCT and low rates of relapse for all patients other than patients with LL with active disease. Histological subtype and disease status at time of HSCT influenced outcome.
NHLs are heterogeneous diseases with differences in biology and response to therapy.16 The large cell lymphomas ALCL and DLBCL have better outcomes than the small cell lymphomas such as LL, which tend to be aggressive. Outcomes after HSCT, correspondingly varied in our cohort based on underlying histological subtype (P= .0002).
For patients with ALCL, CIBMTR registry data show DFS of 46% (n = 12) after allogeneic HSCT as compared with 35% (n = 24) after autologous HSCT with relapse rates of 20% and 40% in the allogeneic and autologous HSCT groups, respectively, suggesting trend to superior outcomes after allogeneic HSCT; however, numbers were too small to show significance.12 Outcomes after autologous HSCT vary from event-free survival (EFS) of 33% to 58% in the reported literature likely due to different reinduction strategies after relapse.17 Data for outcomes after allogeneic HSCT are limited to few reports but these reports show good outcome with low rates of relapse. The Berlin-Frankfurt-Münster (BFM) group report data from a multicenter European cohort showing DFS of 75% in 20 patients who received allogeneic HSCT.18 A smaller study from Japan demonstrated that all 6 patients who received allogeneic HSCT were alive and disease-free as compared with 3 of 8 who underwent an autologous HSCT.19 Our data in a larger cohort show excellent outcomes with all 14 patients alive and 13 of 14 being disease-free. The relatively good outcomes in our cohort compared with previous reports may be a consequence of low relapse rates from GVL allograft activity and favorable pretransplant remission status (12 of 14 in CR), along with low rates of NRM and use of targeted therapies pre-HSCT in the majority of patients (10 of 14), minimizing chemotherapy exposure and pretransplant comorbidities. These potentially contributing factors are not mutually exclusive. Overall, these outcomes support the use of allogeneic HSCT for patients with ALCL.
By contrast, allografting of LL offered little evident benefit over previous outcomes. LL is a highly aggressive subtype of childhood NHL, and, as increasingly intense frontline therapies are being offered, the smaller number of patients who relapse have more aggressive and resistant disease. A recent BFM study reports just 34 of 324 patients (10%) relapsed after intensive front-line treatment, compared with a 33% relapse rate in adult LL.20 Unfortunately, however, fewer than one-half of the relapsed pediatric patients achieved second remission, leading to survival of only 14% for those with relapsed disease. Outcomes after HSCT for patients with LL are uniformly reported as poor with all series showing DFS of <50%.17,20,21 CIBMTR registry data show 5-year EFS of 40% (n = 39) after allogeneic HSCT as compared with 4% (n = 14) after autologous HSCT.12 The corresponding 5-year probability of relapse was 86% after autologous HSCT as compared with 23% after allogeneic HSCT, suggesting significant GVL activity for these patients, and/or that autologous HSCT can convey malignant cells contributing to recurrence. For LL, remission status prior to SCT has been noted as the most important prognostic factor for outcome, and our own results confirm this experience.12 The only 2 patients of 9 with LL in this series who survived without early relapse after HSCT were the 2 patients in CR at the time of this treatment. Although allogeneic HSCT is associated with superior outcome compared with autologous HSCT for LL, disease status at HSCT influences outcome and it may be necessary for patients with LL to be in remission prior to conditioning for allogeneic HSCTs to be successful.
For the other rarer lymphoma subtypes, there are few pediatric data and it is still unclear whether autologous or allogeneic transplant is superior.10,12,17 For patients with DLBCL, the CIBMTR study found no difference in EFS after autologous and allogeneic HSCT (EFS, 52% and 50%).12 The outcomes in our cohort for patients with DLBCL are comparable to registry data with OS of 63%. The 2 patients who relapsed had refractory disease at HSCT. OS for all of the other rare NHL subtypes (n = 5) in our cohort combined together was 80%. None of these patients relapsed.
Disease status at time of HSCT is an important predictor of OS across studies9,,-12 and was a highly significant predictor of OS in this cohort, where all patients underwent allogeneic HSCT (P = .004). Additionally, we found that for all histological subtypes other than LL, even those with PR, could attain long-term DFS.
The rate of NRM (6%) in our series is lower than in other reports, which describe NRM of 19% to 41%.8,10,12 Possible explanations for the lower NRM in our series include the following. (1) Timing and choice of allogeneic HSCT. All patients with relapsed NHL underwent allogeneic HSCT unlike in other reports where allogeneic HSCT was reserved for higher-risk patients with chemorefractory disease. Also, none of our patients received prior autologous HSCTs. (2) Treatment era. Most studies, including the CIBMTR study, report outcomes after HSCTs performed up to 2005. Our report includes transplants performed in a more recent era, through 2016, and lower transplant-related mortality rates are likely secondary to improvements in transplant supportive care and the more targeted drugs used for reinduction. Indeed, although the CIBMTR registry data showed an overall rate of NRM after allogeneic HSCT of over 25%, the risk was lower in allogeneic HSCT performed in the latter part of the study period. (3) Age. Our cohort is restricted to pediatric patients, who are known to tolerate allogeneic HSCTs better than older patients. (4) Homogeneity in transplant practices. Unlike registry studies, our study is not biased by heterogeneous practices across centers that can skew outcomes. (5) Finally, use of novel therapies pre-HSCT minimized chemotherapy exposures. Ten of 14 patients with ALCL received novel therapies such as Alk inhibitors minimizing toxicities and comorbidities prior to transplant that likely contributed to the low NRM for the cohort.
Our study suffers from the limitations of a retrospective design over an 18-year study period, modest patient numbers with heterogeneous diseases, and lack of direct comparison with autologous HSCT. Nevertheless, the data provided here do support a potential benefit of allogeneic HSCT for children with relapsed and refractory lymphomas. The predominantly TBI-based conditioning regimens were well tolerated and associated with low rates of relapse for all but LL patients and low rates of NRM for the entire cohort. Future comparison with the outcome of autologous transplants will be of value.
A major consideration for management of children with relapsed and refractory NHL in the modern era is availability of targeted and/or immune therapy alone or in combination with chemotherapy. There are several new agents, including Alk inhibitors, antibodies against CD30, immune checkpoint inhibitors, and cell therapies including cytotoxic T cells and engineered chimeric antigen receptor T-cell options to reinduce remission in relapsed NHL.22,,-25 If these can be incorporated into treatment regimens pre-HSCT aimed at reducing disease burden with minimal toxicities, then allogeneic HSCTs may be both feasible and successful for a higher proportion of patients in need.
Acknowledgment
The authors thank the staff of the BMT Unit and Outpatient Clinic at Texas Children's Hopital for patient care, and the staff of the cell-processing facility for the preparation of stem cell products. The authors also thank the parents, who entrusted them with the care of their children.
Authorship
Contribution: S.N., R.A.K., K.L., C.A.M., G.S., B.O., K.Y., S.G., and K.K. performed the research; S.N. and R.A.K. designed the research study; S.N., R.A.K., M.W., and R.O. analyzed the data; and S.N., M.K.B., C.E.A., H.E.H., and R.A.K. wrote the paper.
Conflict-of-interest disclosure: H.E.H. is a founder with equity in Viracyte and Marker Therapeutics; has received research support from Tessa Therapeutics and Cell Medica; and has served on advisory boards for Cytosen, Gilead Biosciences, and Novartis. M.K.B. is a founder with equity in Viracyte, Marker Therapeutics, and Tessa Therapeutics; has received research support from Cell Medica; and has served on advisory boards for Bluebird Bio, Torque Therapeutics, Unum Therapeutics, and Turnstone. S.G. has patents and patent applications in the field of gene and cell therapy for cancer; is on the data safety monitoring board of Immatics; receives research support from Tessa Therapeutics; and has consulted for EMD Serono, Sanofi, Servier, and ViraCyte within the last 2 years. The remaining authors declare no competing financial interests.
Correspondence: Swati Naik, Center for Cell and Gene Therapy, Baylor College of Medicine, 1102 Bates St, Suite 1640.14, Houston, TX 77030; e-mail: sxnaik@txch.org.