Key Points
Myoclonic jerks and inattentiveness may be rare neurologic complications of ATO toxicity.
Clinicians must be aware of this rare toxicity given that the ATO and ATRA combination is now standard-of-care treatment of low-risk APL.
Introduction
All-trans retinoic acid (ATRA) and arsenic trioxide (ATO) are now standard of care in treating low-risk acute promyelocytic leukemia (APL). In this case report, we describe an unusual neurologic finding of a woman treated with ATRA/ATO who experienced myoclonic jerks and inattentiveness.
Case description and methods
A 69-year-old right-handed woman with a past medical history of hypothyroidism, migraines, fibromyalgia, and hypertension presented to an outside hospital with 2 months of fatigue and easy bruising accompanied by watery diarrhea. Review of systems was notable for mild gingival bleeding and a 15-pound weight loss over the previous 5 months. Evaluation was notable for pancytopenia with 32.0% promyelocytes (Table 1). Bone marrow biopsy revealed a hypercellular bone marrow (80% to 90%) with sheets of large immature blasts characterized by ample cytoplasm and lobulated nuclei. Flow cytometric analysis showed 38% of the cells expressed CD13, CD33, CD64, CD117, and HLA-DR but were negative for CD34. Peripheral blood fluorescence in situ hybridization confirmed PML-RARA translocation t(15;17)(q22;q12). She was transferred to our institution for treatment of low-risk (WBCs, 1.3 × 109/L with absolute neutrophils of 0.26 × 109/L) APL with ATRA/ATO.
Medications prior to hospitalization included levothyroxine, losartan, amitriptyline, pregabalin, duloxetine, and omeprazole. There was no history of chronic heavy metal exposure, including arsenic.
She was started on ATRA 45 mg/m2 per day and ATO 0.15 mg/kg per day and on day 6, and developed acute onset myoclonic jerks. Initially, these movements were infrequent, lasting briefly and occurring every 15 to 20 minutes and accompanied by inattentiveness. The frequency of myoclonic jerks increased to every minute and were evident in bilateral extremities and trunk. A neurologic examination revealed orientation to person, place, and year but due to inattentiveness, she was unable to focus on serial sevens. Cranial nerves were fully intact, and muscle strength was 5 of 5 throughout. She was hyperreflexive throughout on the right compared with the left, and her toes were downgoing bilaterally. There was no tremor or dysmetria.
Review of the medication list did not implicate a culprit agent and, specifically, pregabalin and duloxetine are not associated with both truncal and extremity myoclonus in combination with inattentiveness. Notable laboratory results included a creatinine increase from 1.0 to 1.67 mg/dL, blood urea nitrogen of 40 mg/dL, and calcium of 7.7 mg/dL. Both ATRA/ATO dosings were held. Neurology was consulted and the symptoms were attributed to either a toxic-metabolic process vs seizure activity secondary to toxin or due to central nervous system involvement. Lumbar puncture was deferred due to thrombocytopenia and concern for bleeding. Levetiracetam 500 mg twice daily was empirically started. Steroids were started on day 6 for concern of differentiation syndrome due to hypoxia and a rapid elevation of WBCs to 9.7 × 109/L accompanied by a low-grade fever of 100.6°F (38.1°C).
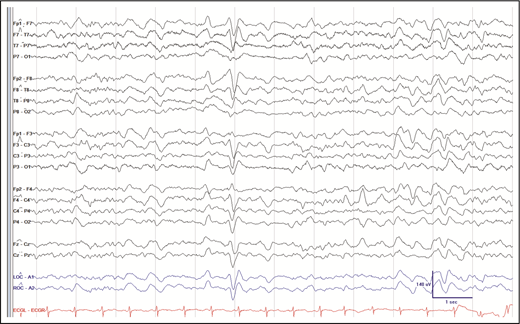
An electroencephalogram showed mild-to-moderate diffuse slowing with frequent triphasics, suggesting a toxic-metabolic etiology with no frank seizures or focal epileptiform activity (Figure 1). Brain magnetic resonance imaging with and without contrast did not show acute hemorrhage, mass, or infarction.
Snapshot of patient’s electroencephalogram from suspected ATO toxicity. There is mild-to-moderate slowing and frequent triphasics without frank seizures or focal epileptiform activity, suggesting toxic-metabolic etiology.
Snapshot of patient’s electroencephalogram from suspected ATO toxicity. There is mild-to-moderate slowing and frequent triphasics without frank seizures or focal epileptiform activity, suggesting toxic-metabolic etiology.
On day 9 (ATRA/ATO continued to be held), the myoclonic jerks and inattentiveness resolved. A decision was made to discontinue ATO and restart ATRA in combination with idarubicin to complete induction therapy. The patient had no further episodes of myoclonic jerks and her mental status remained normal. A bone marrow morphologic remission was documented on day 35 and she was discharged home for outpatient consolidation therapy with ATRA and anthracycline.
Results and discussion
Neurotoxicity with arsenic treatment of APL
Neurologic toxicity associated with ATRA/ATO for APL is well described. Au et al described that arsenic levels were detectable in the cerebrospinal fluid in the case of oral1 and IV arsenic when administered for the treatment of APL.2 However, no case studies have described myoclonus as an adverse effect of ATO therapy.
The most frequent ATO-associated toxicities reported are vision changes, sensory peripheral neuropathy, and longer-term neurocognitive changes.3 Valentine et al described the observation of profound muscle fasciculations with ataxia and subsequent death in a herd of cows after ingesting grain pellets. Analysis of the cadaveric bovine kidneys found high levels of ATO as well as elevated levels of metaldehyde in the liver providing a possible relationship between ATO and the clinical findings.4 In another case study of acute arsenic poisoning in a suicide attempt, the man was found to have myoclonus, agitation, delirium, and intense tremor.5
Pathophysiology
Our patient’s neurologic symptoms persisted for ∼72 hours. ATO has a half-life of 10 to 14 hours,6 reaching a steady state at 50 to 70 hours, which corresponded with the time frame for the symptom resolution. Continued therapy with ATRA combined with an anthracycline did not result in reemergence of this neurologic sequala, further implicating ATO as the culprit medication.
ATO affects the brain according to various proposed mechanisms.7 Rodent models show that arsenic exposure affected DNA methylation in the cortex and hippocampus,8 and behavioral testing revealed progressive and dose-dependent deficits of memory implicating alterations in DNA methylation status involved in neuronal plasticity.8 Arsenic metabolism requires the addition of methyl groups derived from S-adenosylmethionine for excretion; it is possible that ATO depletes S-adenosylmethionine levels leading to alterations in DNA methylation status, which is required for myelination of neuronal cells.7 Exposure to arsenic may induce epigenetic modifications resulting in aberrant gene expression in neuronal cells.
Arsenic also affects hippocampal signaling, function, and morphology, affecting memory and learning.7 Arsenic demonstrated reduced global acetylation of lysine 9 of histone 3 in the cortex and hippocampus, correlated to altered learning in adulthood for rodent pups.7 Alterations in hippocampal function, morphology, and signaling lead to altered cognitive behavior in acquisition,9 recall,10 performance,10 coordination,9 ataxia,11 and locomotion.12 Our patient experienced short-term memory loss as well as cognitive deficits, ataxia, recall, and coordination issues, although these implicated epigenetic mechanisms would have been anticipated to occur later in the clinical course. ATO has also been shown to reduce acetylcholinesterase activity and choline acetyltransferase functioning affecting motor coordination and locomotion with hypolocomotion vs hyperlocomotion,7 and these 2 mechanisms may be implicated in our patient.
Increased susceptibility to ATO neurotoxicity may have been due to concomitant administration of amitriptyline, pregabalin, and duloxetine, all of which can depress mental status. Furthermore, amitriptyline is metabolized by the cytochrome P (CYP) pathway (CYP2C19 and CYP2D6)13 and duloxetine acts both as a substrate and inhibitor of CYP2D6.14 In neurons, several neurotransmitters stimulate the release of arachidonic acid (AA), which is important in the conversion of inflammatory metabolites as well as modulation of neural cell function.15 AA is subjected to metabolism through the CYP pathway, and arsenic toxicity has been shown to decrease brain AA levels,15 which may have further been enhanced by convergence on the CYP pathway by amitriptyline and/or duloxetine. Pregabalin and duloxetine may also be implicated as they were both held on day 8 correlating with resolution of symptoms on day 9. Duloxetine is less likely given it was restarted later in the hospital course without additional adverse events and pregabalin was not restarted given the neuropathic pain was well controlled. Lastly, cefepime can also be implicated but the intensity of jerks combined with inattentiveness made it a less likely culprit.
In conclusion, ATO treatment of APL may result in a rare adverse effect of myoclonic jerks/muscle fasciculations accompanied by inattentiveness. The reversible neurologic toxicity attributed to ATO reported here has yet to be described in the literature. Emergence of unusual neurologic symptoms in the setting of ATO and ATRA therapy should rapidly prompt a thorough neurologic evaluation, and, if appropriate, immediate discontinuation of ATO and implementation of alternative APL therapies.
Authorship
Contribution: J.Y.R., D.T., and J.M. formulated, designed, and wrote the paper, including taking care of the patient mentioned in the case report; and A.M.C. and M.S.T. provided significant edits, comments, and expertise to the case report.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John Mascarenhas, Division of Hematology/Oncology, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, 1 Gustave L. Levy Pl, Box 1079, New York, NY 10029; e-mail: john.mascarenhas@mssm.edu.