Abstract
We have examined the role of caspase-mediated cleavage of the Ste20-like kinases, mammalian sterile 20-like 1 and 2 (Mst1/Mst2), in the mechanism of human eosinophil and neutrophil apoptosis. Initial measurements of kinase activity, using myelin basic protein (MBP) as a substrate in “in-gel” renaturation assays, showed that constitutive eosinophil and neutrophil apoptosis were associated temporally with the activation of a 36-kd MBP kinase (p36 MBPK) and a 34-kd MBP kinase (p34 MBPK), respectively. A constitutively active 63-kd MBP kinase (p63 MBPK) was also detected in freshly prepared eosinophils but not neutrophils, whose activity was transiently augmented during spontaneous apoptosis. Immunoblotting studies demonstrated the expression of Mst1 and Mst2 in eosinophils but not neutrophils whereas immunoprecipitation studies identified the p63 MBPK activity as being Mst1 and Mst2 and showed that the p36 MBPK activity represented the N-terminal catalytic fragment of Mst1. A role for the p36 MBPK in eosinophil cell death was supported by studies showing increased activation upon exposure to the proapoptotic Fas/CD95-activating antibody, CH-11, and attenuation in the presence of the survival-promoting cytokine, interleukin-5. Furthermore, spontaneous and Fas-induced activation of p36 MBPK was inhibited by catalase and the general caspase inhibitor, z-Asp-CH2-DCB, at concentrations that suppressed eosinophil apoptosis. These studies therefore implicate a role for caspase- and H2O2-mediated cleavage of the Mst1 and the subsequent release of the 36-kd catalytic fragment in the mechanism of eosinophil apoptosis. In contrast, neutrophil apoptosis occurs independently of Mst1 and Mst2 but instead is correlated with the activation of an as-yet-unidentified 34-kd MBPK.
Introduction
The accumulation of eosinophils and neutrophils within the airways has been implicated in the etiology of a range of acute and chronic inflammatory responses.1-3 The number of eosinophils and neutrophils in the airways reflects a balance between their rate of accumulation and removal. Whereas emigration of leukocytes from the circulation is mediated by a host of chemotactic factors released from various cells, the removal of tissue-associated cells can occur by the process of apoptosis that is regulated by a variety of factors, including cell-cell/cell-matrix interactions, as well as direct stimulation by cytokines. Apoptosis is a highly controlled nonphlogistic process of cell death, during which apoptotic cells shrink to form apoptotic bodies. These may then be recognized and removed from the tissue by phagocytes such as macrophages and epithelial cells, without discharge of their cytotoxic contents. This process is distinct from necrosis, during which the cell lyses and the uncontrolled release of cellular contents occurs, which may subsequently produce an inflammatory response. For this reason it has been speculated that the therapeutic induction of apoptosis may be desirable in the treatment of inflammatory diseases.2 3
Although little is known of the intracellular mechanisms that regulate cell death, recent investigations have suggested that the initiator events during apoptosis may be broadly divided into intrinsic and extrinsic pathways.4 Intrinsic apoptosis is centrally dependent upon the release into the cytosol of mitochondrial cytochrome c, as a consequence of an imbalance of expression of proapoptotic and antiapoptotic Bcl2 family members. Once in the cytosol, mitochondrial-derived cytochrome c may form a complex (known as the apoptosome) with apoptotic protease activating factor 1 (APAF1) and procaspase-9, thus catalyzing the autoprocessing and generation of active caspase-9. In contrast, extrinsic apoptosis is dependent upon the agonism of death receptors such as Fas/CD95. Fas is a type-1 transmembrane receptor belonging to the tumor necrosis receptor superfamily of proteins,5 which induces apoptosis in numerous cell types on ligation with its natural ligand, FasL, or with agonistic anti-Fas monoclonal antibodies. The intracellular domain of Fas contains a sequence required for induction of apoptosis, known as the death domain,6 which is conserved among several proteins including Fas-associated death domain (FADD)/MORT1.7 Thus the downstream signals propagated by Fas recruit FADD/MORT1 via interactions between the death domains of these 2 proteins.8 Subsequently, procaspase-8 is also recruited to Fas through an interaction between the death effector domains of FADD and procaspase-8,9 10 so initializing the caspase cascade that is crucial to the apoptotic signaling pathway. It has been suggested that the activation of initiator caspases by the extrinsic and intrinsic pathways converge with the activation of caspase-3 and caspase-7 which are the executioners of apoptosis. It is thought that these caspases may mediate their effects by cleavage of several proteins. Cleavage of some substrates renders them inactive, such as poly (ADP-ribose) polymerase (PARP), which has a role in DNA repair. Conversely, caspase-mediated cleavage of other targets, including caspases themselves, as well as protein kinases MEKK1 and PAK2, results in their activation.
Apoptosis appears to be the default state, during in vitro culture of both eosinophils and neutrophils in the absence of the hemopoietic cytokines (intrinsic death), that can be increased following agonism of the Fas (CD95) receptor (extrinsic death).11Interestingly, there appear to be significant differences in the mechanisms that mediate eosinophil and neutrophil apoptosis, which has raised the possibility of therapeutic intervention to induce selective cell death. It has previously been shown that although glucocorticoids attenuate neutrophil apoptosis, they induce cell death in eosinophils.12 Similarly, we have recently demonstrated that SB203580, an inhibitor of p38 mitogen-activated protein (MAP) kinase, promotes intrinsic eosinophil apoptosis,13 whereas other studies showed that these inhibitors either attenuate14 or have no effect on neutrophil apoptosis.15
Recently, investigations in hemopoietic-derived cell lines have suggested a possible role for members of the Ste20-related family of serine/threonine kinases in stress responses and apoptosis.16 Based on their structure, mammalian Ste20-homologues can be divided into either the germinal center kinase (GCK) or the p21-activated kinase (PAK) subfamilies.17 Of these, members of the GCK family can further be divided into 2 broad groups based on differences in their structure and function.17 Group I enzymes comprise GCK itself, together with GCK-related kinase, GCK-like kinase, hematopoietic progenitor kinase-1, Nck-interacting kinase, and Drosophilia misshapen. Group II GCKs include Ste20-like oxidant stress-activated kinase-1, mammalian sterile 20-like-1 (Mst1, also known as kinase responsive to stress-2 [Krs2]), Mst2 (also called Krs1), Mst3, lymphocyte-orientated kinase, and severin kinase. The functional roles of GCKs are currently obscure although it appears that group I members function as important regulators of stress-activated MAP kinase cascades whereas group II enzymes may be involved in the regulation of cellular responses to environmental stress and longevity. Thus, early studies showed that caspase-catalyzed cleavage of the group II enzyme Mst1 and the subsequent release of an active N-terminal 36-kd catalytic subunit is associated with Fas-induced apoptosis of the human B-lymphoma cell line, BJAB,18 and human thyoma-derived human peripheral blood–acute lymphoblastic leukemia cells.19 Proteolytic activation of Mst was also implicated in MT-21– and cytotrienin A–induced apoptosis of human leukemia HL-60 cells.20,21 Recently, extensive overexpression studies in a variety of cell lines have demonstrated that caspase-mediated Mst1 cleavage and the subsequent release of the 36-kd catalytic fragment are capable of inducing cell death.18,20 22-25
Although most of these investigations of Mst1 have been reported in cell lines, only one has described this kinase in a primary cell—the osteoclast.26 Therefore, we have extended investigations of the role of Mst1 and Mst2 in apoptosis to primary human peripheral blood eosinophils and neutrophils. Using “in gel” renaturation assays, we have detected Ste20-related serine/threonine kinases and have shown that constitutive and Fas-induced eosinophil but not neutrophil apoptosis is associated with H2O2-dependent and caspase-catalyzed cleavage of Mst1 and the subsequent release of a 36-kd catalytic N-terminal fragment.
Materials and methods
Drugs and analytical reagents
The following drugs and analytical reagents were used: human recombinant interleukin-5 (IL-5) and granulocyte macrophage–colony-stimulating factor (GM-CSF) (R&D Systems, Abingdon, United Kingdom); anti-Fas monclonal antibodies (clone CH-11; Coulter-Immunotech, Bedfordshire, United Kingdom); γ[32P]-adenosine triphosphate (γ[32P]ATP) (> 5000 Ci/mmol [185 TBq/mmol]) (NEN, Zaventium, Belgium); Elohaes (Fresenius Kabi, Warrington, United Kingdom); Ficoll-Hypaque PLUS, Percoll, Hybond ECL, Hyperfilm ECL, ECL Western blotting reagents, and protein molecular weight markers (Amersham International/Pharmacia, Buckinghamshire, United Kingdom); anti-CD16 microbeads and magnetic separation system (Miltenyi Biotec, Surrey, United Kingdom); protein G PLUS agarose beads, and goat antibodies to Mst1 and Mst2 (Santa Cruz Biotechnology/Autogen Bioclear, Wiltshire, United Kingdom); z-Asp-CH2-DCB (Peptide Institute/Scientific Marketing Associates, Herts, United Kingdom); horseradish peroxidase–conjugated antigoat and antimouse (Dako, Cambridgeshire, United Kingdom); myelin basic protein, catalase, propidium iodide (PI), fetal calf serum (FCS), RPMI 1640, aprotinin, pepstatin A, leupeptin, and all other chemicals (AnalaR grade) (Sigma, Poole, Dorset, United Kingdom).
Isolation and culture of human eosinophils
Eosinophils were isolated from the blood of steroid-naı̈ve asthmatic and eosinphilic donors as previously described.13 After sedimentation of red blood cells on Elohaes, supernatants were layered onto a Ficoll gradient. Following centrifugation, contaminating red blood cells were removed from the granulocyte-rich pellet by hypotonic cell lysis and neutrophils negatively selected by labeling with immunomagnetic anti-CD16 microbeads and elution through a magnetic field. Eosinophils eluted from the column were washed and cultured at 1.2 × 106 cells/mL in RPMI 1640 medium supplemented with 10% FCS and antibiotics (RPMI/FCS) and the indicated treatment on FCS-coated 12-well plates for various time points up to 24 hours. Plates were coated with FCS to prevent neutrophil and eosinophil binding. In immunoprecipitation studies, eosinophils were cultured at 2.5 × 106 cells/mL in RPMI/FCS on FCS-coated 6-well plates for 0 or 24 hours.
Isolation and culture of human neutrophils
Neutrophils were isolated from the peripheral blood of healthy donors. Briefly, red blood cells were allowed to sediment on Elohaes and the supernatant was layered onto a Percoll gradient. After centrifugation the neutrophil rich fraction, at the 70% to 80% interface, was collected, washed, resuspended at 5 × 106cells/mL in Iscoves modified Dulbecco medium (IMDM), supplemented with 10% FCS and antibiotics, and cultured upon FCS-coated 6-well plates for up to 24 hours.
Determination of apoptosis
Eosinophil and neutrophil apoptosis were determined by PI-staining of DNA fragmentation and flow cytometry (FACScan; Becton Dickinson, San Jose, CA). Cells showing decreased relative DNA content were considered apoptotic. With eosinophils, the cells were gently resuspended in 300 μL hypotonic PI (25 μg/mL in 0.1% sodium citrate and 0.1% Triton X-100) and stored in the dark at 4°C before flow cytometric analysis. In contrast, neutrophils were washed in phosphate buffered saline (PBS) solution, fixed by 70% ethanol, and incubated on ice for 30 minutes. The cell pellet was then resuspended in 200 μL PI (25 μg/mL in PBS), incubated for 1 hour at 4°C, and analyzed by flow cytometric analysis.
Extraction of cytosolic proteins for Western blotting and “in gel” kinase assays
Eosinophils (1.2 × 106 cells/mL) and neutrophils (5 × 106 cells/mL) were harvested, washed with cold Hanks buffered saline, and resuspended in 100 μL and 200 μL immunoprecipitation buffer, respectively (final concentration: 10 mM Tris base, pH 7.4, 1% Triton X-100, 0.5% Na-deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid [EDTA], 0.5 mM phenylmethylsulfonyl fluoride, 2 mM Na-orthovanadate, 10 μg/mL leupeptin, 25 μg/mL aprotinin, 10 μg/mL pepstatin A, 1.25 mM NaF, and 1 mM Na-pyrophosphate). After vortex mixing, cells were incubated on ice for 30 minutes before centrifuging at 12000 rpm for 10 minutes to remove cell debris. Laemmli buffer (× 6) was then added to eosinophil (20 μL) and neutrophil (40 μL) supernatants. Samples were then boiled and stored at −20°C until immunoblotting and “in gel” renaturation assay analysis.
Immunoprecipitation
Eosinophils (2.5 × 106 cells/mL) and neutrophils (5 × 106 cells/mL) were lysed in 100 μL and 200 μL immunoprecipitation buffer, respectively. Following cytosolic extraction, lysates were incubated with 1 μg per 106cells of the relevant antibody or control IgG overnight at 4°C before addition of 2.5 μL per 106 cells of protein G PLUS agarose beads and incubation at 4°C for 2 hours. Samples were then centrifuged, supernatants retained, and made up to 200 μL with immunoprecipitation buffer. Agarose pellets were washed 3 times with immunoprecipitation buffer and finally resuspended in 200 μL immunoprecipitation buffer. Laemmli buffer (40 μL) was then added to supernatant and immunoprecipitated samples before boiling and storing at −20°C for “in gel” kinase assays.
Immunoblotting
The expression and cleavage of Mst1 and Mst2 were assessed by Western immunoblot analysis. Protein samples (containing ∼ 2.0 × 105 eosinophils or ∼ 5 × 105neutrophils) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on 10% polyacrylamide gels. Following transfer to nitrocellulose (HybondECL), proteins were labeled using antibodies (1:500) raised to the catalytic fragments and subsequently detected using horseradish peroxide–linked anti–goat IgG (diluted 1:4000) and ECL Western blotting detection agents on ECL hyperfilm. Relevant bands intensities were quantified by laser-scanning densitometry.
Determination of protein kinase activity by “in gel” renaturation assay
Protein kinase activity was determined using an “in gel” renaturation assay employing myelin basic protein (MBP) as a substrate. Briefly, eosinophil and neutrophil samples were separated on 10% SDS polyacrylamide gels containing 0.2 mg/mL MBP. After electrophoresis, SDS was removed by 3 × 20 minutes (50 mL) washes with 20% isopropyl alcohol in 50 mM TrisHCl, pH 8.0, followed by 3 × 20 minutes (50 mL) washes in 50 mM TrisHCl, pH 8.0, 5 mM 2-mercaptoethanol. Proteins were denatured by 2 × 30 minutes (50 mL) incubations with 6 M guanidineHCl, 5 mM 2-mercaptoethanol, 50 mM TrisHCl, pH 8.0, and subsequently renatured by 5 washes (50 mL) over 12 to 18 hours with 5 mM 2-mercaptoethanol, 0.04% Tween 40, and 50 mM TrisHCl, pH 8.0. Gels were then re-equilibrated by washing 2 × 30 minutes (50 mL) with 40 mM HEPES, pH 8.0, 10 mM MgCl2, and 2 mM dithiothreitol (DTT). For the kinase reaction the gels were incubated for 3 hours with 10 mL of 40 mM HEPES, pH 8.0, 10 mM MgCl2, 0.055 mM ATP, 0.1 μM PKA inhibitor, and 25 μCi (925 KBq) [32P]ATP. The reaction was stopped and excess [32P]ATP removed by 5 washes in 5% trichloroacetic acid and 1% sodium pyrophosphate over 12 to 18 hours, the gels dried, and radioactivity detected by autoradiography.
Statistical analysis
Data points, and values in the text and figure legends, represent the mean ± SEM of 3 to 4 independent determinations taken from different cell preparations. When statistical evaluation was required, data were analyzed parametrically by 2-tailed ttest for paired data. The null hypothesis was rejected whenP < .05.
Results
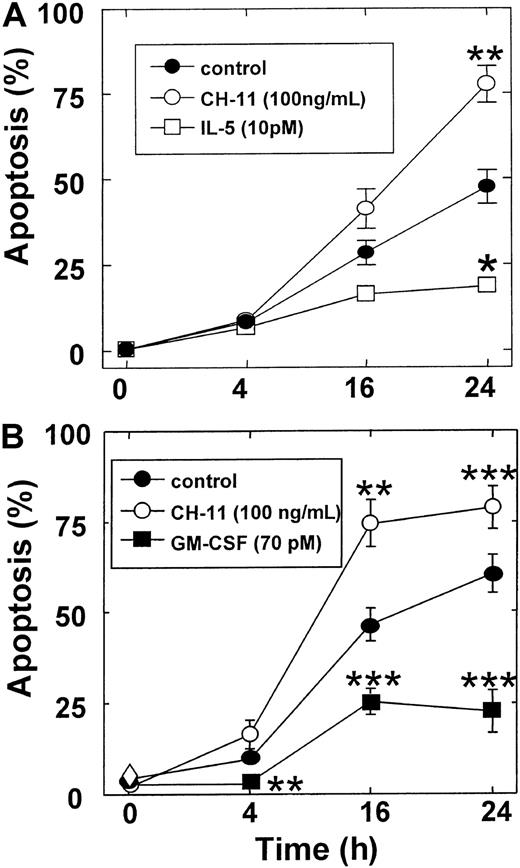
Following in vitro culture in the absence of hematopoietic cytokines, eosinophils and neutrophils underwent constitutive apoptosis, as measured by PI-staining of DNA fragmentation, resulting in 47% and 61% cell death at 24 hours, respectively (Figure1). At 24 hours, this process was significantly attenuated by the survival-promoting cytokines, IL-5 (10 picomolar [pM]; eosinophils: 19% death,P < .05), and GM-CSF (1 ng/mL; neutrophils: 23% death,P < .05) and enhanced by the proapoptotic ligand CH-11 (100 ng/mL), which activates cell-surface Fas receptors (eosinophils: 78% death, P < .01; neutrophils: 79% death,P < .005) (Figure 1).
Time-course of eosinophil and neutrophil apoptosis.
Eosinophils (A) and neutrophils (B) were cultured for the indicated times in medium alone (●), or in the presence of the Fas-activating monoclonal antibody (clone CH-11; 100 ng/mL; ○), IL-5 (10 pM; ■), or GM-CSF (1 ng/mL; ▪) and apoptosis assessed by flow cytometric analysis of PI-stained DNA fragmentation. Data points show the percentage of apoptotic cells and are the mean ± SEM of 3 (A) or 6 (B) independent experiments. P versus control: * < .05, ** < .01, and *** < .005.
Time-course of eosinophil and neutrophil apoptosis.
Eosinophils (A) and neutrophils (B) were cultured for the indicated times in medium alone (●), or in the presence of the Fas-activating monoclonal antibody (clone CH-11; 100 ng/mL; ○), IL-5 (10 pM; ■), or GM-CSF (1 ng/mL; ▪) and apoptosis assessed by flow cytometric analysis of PI-stained DNA fragmentation. Data points show the percentage of apoptotic cells and are the mean ± SEM of 3 (A) or 6 (B) independent experiments. P versus control: * < .05, ** < .01, and *** < .005.
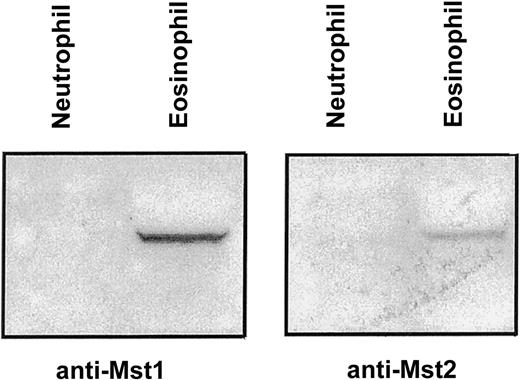
To investigate the possible role of Mst1 and Mst2 in eosinophil and neutrophil apoptosis, preliminary experiments were undertaken using antibodies directed toward the catalytic N-terminal domain. Western immunoblotting detected the presence at approximately 63 kd of Mst1 and Mst2 in eosinophils but not neutrophils (Figure2), even when the total protein loading of the latter was increased by 4 times (data not shown).
Immunoblot detection of Mst1 and Mst2 in human eosinophils and neutrophils.
Eosinophil and neutrophil lysates were subjected to 10% SDS-PAGE and the presence of Mst1 and Mst2 was detected by immunoblotting with antibodies to the N-terminal catalytic fragment. A representative autoradiograph obtained from 3 independent experiments is shown.
Immunoblot detection of Mst1 and Mst2 in human eosinophils and neutrophils.
Eosinophil and neutrophil lysates were subjected to 10% SDS-PAGE and the presence of Mst1 and Mst2 was detected by immunoblotting with antibodies to the N-terminal catalytic fragment. A representative autoradiograph obtained from 3 independent experiments is shown.
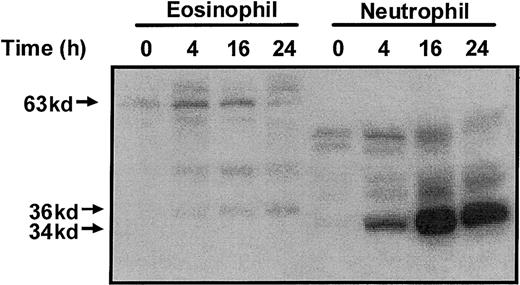
Activation of the Ste20-like kinases was determined using an MBP “in gel” renaturation assay. In freshly isolated eosinophils, we detected the presence of a 63-kd MBP kinase (p63 MBPK) whose activity was elevated at 4 hours and gradually returned to basal activity by 24 hours (Figure 3). In addition, in eosinophils aged for 4 hours, we detected a 36-kd MBP kinase (p36 MBPK), whose activity increased in a time-dependent manner up to 24 hours (Figure 3). Parallel studies with neutrophils failed to detect the p63 MBPK but identified the activation of a 34-kd MBP kinase (p34 MBPK) which correlated with constitutive apoptosis (Figure 3).
Profile of myelin basic protein kinase activation during constitutive eosinophil and neutrophil apoptosis.
Constitutive eosinophil and neutrophil apoptosis was induced by incubation in the absence of survival-promoting cytokines. At the indicated times, the cells were lysed by the addition of ice-cold immunoprecipitaton buffer, and soluble fractions were prepared and subjected to electrophoresis on 10% SDS-PAGE. Protein kinase activities were assayed using an “in gel” renaturation kinase assay employing MBP as the substrate. A representative autoradiograph obtained from 3 independent experiments is shown.
Profile of myelin basic protein kinase activation during constitutive eosinophil and neutrophil apoptosis.
Constitutive eosinophil and neutrophil apoptosis was induced by incubation in the absence of survival-promoting cytokines. At the indicated times, the cells were lysed by the addition of ice-cold immunoprecipitaton buffer, and soluble fractions were prepared and subjected to electrophoresis on 10% SDS-PAGE. Protein kinase activities were assayed using an “in gel” renaturation kinase assay employing MBP as the substrate. A representative autoradiograph obtained from 3 independent experiments is shown.
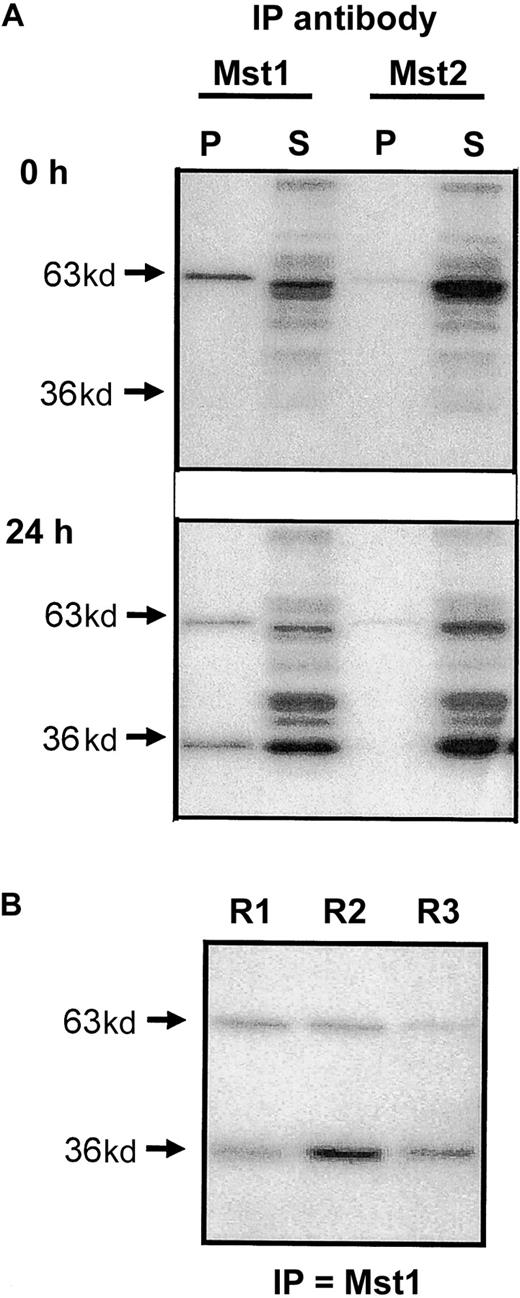
In an attempt to identify the MBP kinases activated during cell death, we undertook immunoprecipitation studies using lysates of freshly purified cells and those undergoing constitutive apoptosis (ie, after 24-hour culture). As would be expected following our inability to detect these proteins by Western blotting, antibodies to Mst1 and Mst2 failed to immunoprecipitate the p34 MBPK activity from neutrophil lysates (data not shown). In contrast, anti-Mst1, and to a lesser extent anti-Mst2, antibodies immunoprecipitated the p63 MBPK activity from both freshly isolated and apoptotic eosinophils, as detected by MBP “in gel” kinase assay. Moreover, anti-Mst1, but not anti-Mst2, also precipitated the p36 MBPK in lysates from apoptotic eosinophils at 24 hours (Figure 4A), suggesting that this represented the cleaved catalytic fragment of Mst1. A comparison of the activity of p63 MBPK and p36 MBPK recovered in the pellet and supernatant of eosinophil lysates at 24 hours following immunoprecipitation showed that the anti-Mst1 antibody only partially precipitated these activities. However, this appeared to be due to low avidity of the antibody for the enzyme since subsequent rounds of precipitation continued to pull down significant p36 and p63 MBP activity (Figure 4B).
Immunoprecipitation of Mst1 and Mst2 from apoptotic eosinophils.
(A) Eosinophil lysates were obtained from freshly purified eosinophils and from cells cultured for 24 hours in cytokine-free medium. Following immunoprecipitation with antibodies to the N-terminal catalytic fragments of Mst1 and Mst2, the pellet (P) and supernatant (S) fractions were separated on 10% gels by SDS-PAGE and the kinase activities measured by “in gel” renaturation assays using MBP as a substrate. (B) Supernatant obtained following initial immunoprecipitation (R1) was immunoprecipitated for a second (R2) and third (R3) time using the antibody to Mst1 and the activity in the pellets determined by MBP “in gel” renaturation assay. Representative autoradiographs are presented and are typical of 3 independent experiments.
Immunoprecipitation of Mst1 and Mst2 from apoptotic eosinophils.
(A) Eosinophil lysates were obtained from freshly purified eosinophils and from cells cultured for 24 hours in cytokine-free medium. Following immunoprecipitation with antibodies to the N-terminal catalytic fragments of Mst1 and Mst2, the pellet (P) and supernatant (S) fractions were separated on 10% gels by SDS-PAGE and the kinase activities measured by “in gel” renaturation assays using MBP as a substrate. (B) Supernatant obtained following initial immunoprecipitation (R1) was immunoprecipitated for a second (R2) and third (R3) time using the antibody to Mst1 and the activity in the pellets determined by MBP “in gel” renaturation assay. Representative autoradiographs are presented and are typical of 3 independent experiments.
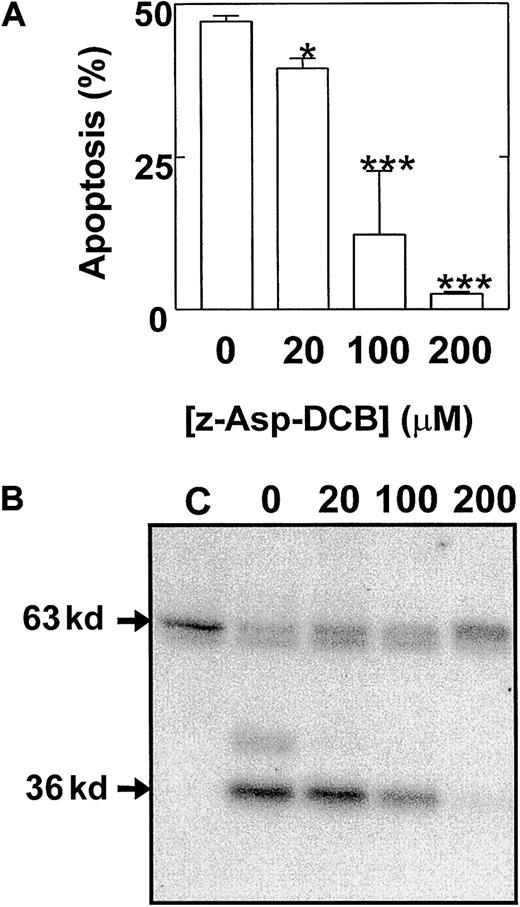
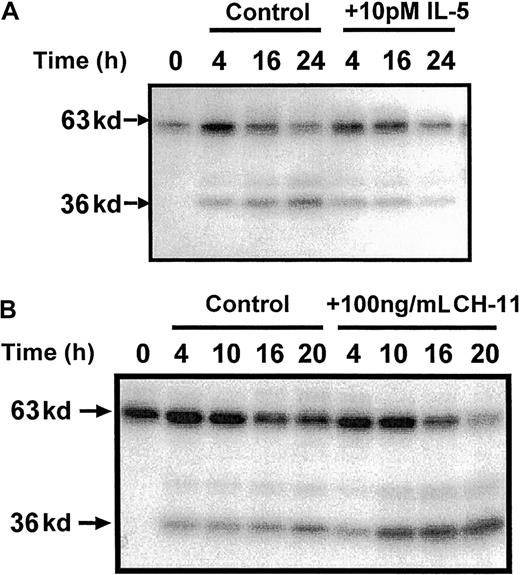
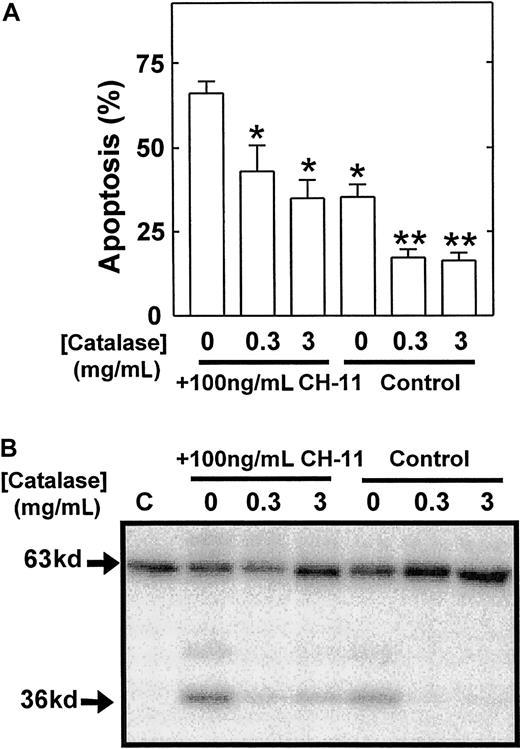
These studies therefore identified a possible role for the cleavage and release of a catalytic 36 kd of Mst1 in the mechanism of eosinophil apoptosis. To provide further evidence of a role of p36 MBPK and to investigate its mechanism of activation we examined the action of a number of proapoptotic and antiapoptotic stimuli. The role of caspases in the mechanism of Mst1 cleavage and spontaneous eosinophil apoptosis was investigated using the broad specificity caspase inhibitor, z-Asp-CH2-DCB. As shown in Figure5, incubation with z-Asp-CH2-DCB caused dose-dependent suppression of both constitutive apoptosis and activation of p36 MBPK at 24 hours, with 200 μM producing 5.4 ± 0.8% and 12 ± 8% of control, respectively. In addition, the finding that the activation of p36 MBPK was augmented to 338 ± 102% of control at 24 hours by the proapoptotic Fas/CD95-activating antibody, CH-11 (100 ng/mL; Figure6B), while it was attenuated to 15 ± 4% of control by the antiapoptotic IL-5 (10 pM; Figure 6A) further implicated p36 MBPK in eosinophil apoptosis. Similarly, GM-CSF (1 ng/mL; not shown) attenuated eosinophil apoptosis as well as abolished p36 MBPK activity by 24 hours. Furthermore, the antioxidant catalase (0.3 mg/mL and 3 mg/mL), which prolonged eosinophil survival (Figure 7A), attenuated p36 MBPK activity during both constitutive and CH-11–induced cell death (30 ± 11% and 1 ± 1% of controls at 0.3 mg/mL, respectively; Figure 7B), implicating H2O2 as having a role in p36 MBPK activation. Interestingly, p63 MBPK activation did not correlate with eosinophil cell death since exposure to CH-11 (Figure 6B) and IL-5 (Figure 6A) resulted in a similar activation profile as that observed during constitutive apoptosis (Figure 3) whereas catalase (3 mg/mL) augmented the activity of p63 MBP at 24 hours (Figure 7B).
Eosinophil apoptosis and activation of p36 MBPK is mediated by caspase cleavage.
Cells were pretreated for 2 hours in the absence and presence of 20 μM to 200 μM of the broad specificity caspase inhibitor, z-Asp-CH2-DCB, and then cultured for a further 24 hours. The percentage apoptotic cells (mean ± SEM of 3 independent determinations) was then enumerated by PI-labeling of DNA fragmentation (A). Kinase activity was measured by “in gel” renaturation assays using MBP as a substrate. A representative autoradiograph obtained from 3 independent experiments is shown in B. C indicates control cells at 0 hours. P versus control: * < .05 and *** < .005.
Eosinophil apoptosis and activation of p36 MBPK is mediated by caspase cleavage.
Cells were pretreated for 2 hours in the absence and presence of 20 μM to 200 μM of the broad specificity caspase inhibitor, z-Asp-CH2-DCB, and then cultured for a further 24 hours. The percentage apoptotic cells (mean ± SEM of 3 independent determinations) was then enumerated by PI-labeling of DNA fragmentation (A). Kinase activity was measured by “in gel” renaturation assays using MBP as a substrate. A representative autoradiograph obtained from 3 independent experiments is shown in B. C indicates control cells at 0 hours. P versus control: * < .05 and *** < .005.
p36 MBPK activation is inhibited by the survival-promoting cytokine, IL-5, and enhanced by the Fas/CD95 agonist, CH-11.
Eosinophils were incubated in the absence and presence of either IL-5 (10 pM; A), or the Fas-activating antibody, CH-11 (100 ng/mL; B) for the indicated times. Cells were then lysed by the addition of ice-cold immunoprecipitaton buffer, and soluble fractions were prepared, separated by 10% SDS-PAGE, and the kinases detected by “in gel” renaturation assays using MBP as a substrate. Representative autoradiographs obtained from 3 independent experiments are shown.
p36 MBPK activation is inhibited by the survival-promoting cytokine, IL-5, and enhanced by the Fas/CD95 agonist, CH-11.
Eosinophils were incubated in the absence and presence of either IL-5 (10 pM; A), or the Fas-activating antibody, CH-11 (100 ng/mL; B) for the indicated times. Cells were then lysed by the addition of ice-cold immunoprecipitaton buffer, and soluble fractions were prepared, separated by 10% SDS-PAGE, and the kinases detected by “in gel” renaturation assays using MBP as a substrate. Representative autoradiographs obtained from 3 independent experiments are shown.
Eosinophil apoptosis and the activation of p36 MBPK is attenuated by catalase.
Eosinophils were aged for 24 hours in the absence or presence of the Fas-activating antibody, CH-11 (100 ng/mL), together with the indicated concentrations of catalase. The percentage of apoptotic cells (mean ± SEM of 3 independent determinations) was then enumerated by PI-labeling of DNA fragmentation (A). Kinase activity was measured by “in gel” renaturation assays using MBP as a substrate. A representative autoradiograph obtained from 3 independent experiments is shown in B. C indicates control cells at 0 hours. P versus no catalase: * < .05 and ** < .01.
Eosinophil apoptosis and the activation of p36 MBPK is attenuated by catalase.
Eosinophils were aged for 24 hours in the absence or presence of the Fas-activating antibody, CH-11 (100 ng/mL), together with the indicated concentrations of catalase. The percentage of apoptotic cells (mean ± SEM of 3 independent determinations) was then enumerated by PI-labeling of DNA fragmentation (A). Kinase activity was measured by “in gel” renaturation assays using MBP as a substrate. A representative autoradiograph obtained from 3 independent experiments is shown in B. C indicates control cells at 0 hours. P versus no catalase: * < .05 and ** < .01.
Discussion
Caspase-mediated cleavage of full-length 63-kd Mst1 and Mst2 and the subsequent release of a catalytically active 36-kd kinase during apoptosis has been demonstrated in several investigations using hematopoietic cell lines, implicating these kinases in this mechanism of cell death.18,22,23 Thus, studies in the human promyelocytic leukemia HL-60 cells showed that induction of apoptosis, using a range of anticancer drugs, is associated with caspase-mediated cleavage and release of the catalytic domain of Mst1, which was detected as a 36-kd activity by “in gel” kinase assay employing MBP as a substrate.20,21 27 In this report, we have extended these observations by examining the role of Ste20-like kinases in the intracellular pathways that determine apoptosis in the primary, nondividing hemopoietic cells, eosinophils and neutrophils. Surprisingly, although immunoblot studies identified the presence of Mst1 and Mst2 in eosinophils, these enzymes were not expressed in neutrophil lysates. This observation was supported by measurement of kinase activity by an MBP “in gel” renaturation assay, which showed the presence of constitutive activity at 63 kd in eosinophils, but was absent in neutrophils. It might be envisioned that the failure to identify Mst1 results from its protease cleavage following neutrophil lysis. However, we believe that this is improbable given the large excess of protease inhibitors included in these experiments and the absence of this phenomenon in the protease-rich eosinophils.
Subsequent studies suggested a role for Mst1 but not Mst2 cleavage during constitutive eosinophil cell death, since p36 MBPK could only be immunoprecipitated with antibodies to the N-terminal catalytic domain of Mst1. In contrast, neutrophil constitutive apoptosis was correlated with activation of an as-yet-unidentified 34-kd MBPK. Since ERK-1/2 and p38 activation can be detected by MBP “in gel” renaturation assay, it might be imagined that the p36 MBPK activity represents these MAP kinases. However, it was while investigating the role of ERK-1/2 and p38 MAPK in eosinophil apoptosis that we first identified the activation of an unidentified p36 MBPK. During these studies, 2 pieces of evidence indicated that the p36 MBPK did not represent ERK-1/2 and p38. First, MBP “in gel” renaturation assays showed that the p36 MBPK activity was detected at a lower molecular weight than activated ERK-1/2 and p38 MAPK controls. Second, Western blot studies using polyclonal antibodies to the inactive and activated (phosphoantibodies) ERK-1/2 and p38 did not label the p36 MAPK activity.13
Due to the lack of selective pharmacologic inhibitors, we attempted to identify the functional role of p36 MBPK by demonstrating that enzyme activation correlates with eosinophil cell death following exposure to a range of proapoptotic and antiapoptotic mediators. Thus, the time-course of constitutive-induced p36 MBPK activation paralleled spontaneous apoptosis and both of these events could be augmented upon agonism of the proapoptotic Fas/CD95 receptor. In contrast, incubation with the survival-promoting cytokines, GM-CSF (data not shown) and IL-5, attenuated activation of p36 MBPK. Similarly, catalase, an enzyme that inhibits apoptosis by catalyzing the conversion of endogenous H2O2 to H2O and O2, also inhibited p36 MBPK activity. Indeed, the latter result implicates H2O2 in the mechanism of eosinophil apoptosis and is in agreement with a recent report by Wedi et al,28 which showed that both constitutive and Fas-mediated apoptosis is associated with intracellular H2O2 production and that antioxidants such as glutathione (GSH) and N-acetyl-cysteine (NAC) could inhibit eosinophil cell death. In addition, our finding that H2O2 acts either upstream or in parallel to p36 MBPK is in accordance with 2 recent studies of MT-21– and H2O2-induced apoptosis in leukemic HL-60 cells.20 21
Since the appearance of the p36 MBPK and apoptosis could be attenuated in the presence of z-Asp-CH2-DCB, this suggested that this kinase represented a caspase-cleaved catalytic product and that this mechanism was important to eosinophil cell death. Indeed, this result is in agreement with early studies that identified the presence of a caspase-cleavage sequence, DEMD326S, between the catalytic and regulatory domain of Mst1, which is cleaved during apoptosis to release the 36-kd catalytic subunit.18,29 Recently, 2 reports have identified the presence of an additional caspase site, TMTD349G, whose cleavage releases a 40-kd to 41-kd MBPK activity.22,23 Of note, we also observed the activation of an MBPK of approximately 41 kd during eosinophil apoptosis. Although there is strong evidence suggesting a role for caspase 3 in mediating Mst1 cleavage, a recent in vitro study suggests that the 2 sites may be selectively cleaved since the DEMD326S site is acted upon by caspases 3, 6, 7, and 9 whereas TMTD349G is cleaved only by caspases 6 and 7.22
Examination of the profile of p63 MBPK activation showed that it was constitutively active and appeared to be regulated independently of apoptosis and p36 MBPK release. Analysis of the regulatory domain of Mst1 has demonstrated the presence of a kinase inhibitory domain within the central region and a C-terminal self-association sequence.30,31 It has therefore been proposed that Mst1 exists in vivo as an autoinhibitory homodimer that is activated following posttranslational modification such as phosphorylation and/or proteolytic cleavage. Strong evidence now exists demonstrating that phosphorylation is required for the activation of both the 63-kd Mst1 and its 36-kd cleavage products.22,23 Indeed, Graves et al have identified an autophosphorylation site at Ser 327 that attenuates caspase cleavage at the DEMD326S site.22
Following caspase cleavage, the Mst1 catalytic subunit has been shown to migrate from the cytosol to the nucleus where it mediates its proapoptotic responses.23,24 Recently, Ura et al have demonstrated that full-length Mst1 is excluded from the nucleus by 2 nuclear export signals (NESs) located at 361-370 and 441-451 within the C-terminal regulatory domain.24 They show that removal of these NCSs following caspase cleavage results in the translocation of the catalytic Mst1 fragment into the nucleus where it promotes chromatin condensation.24 Unfortunately, because we have been unable to detect Mst1 by immunohistochemistry either as a result of the low antibody affinity and/or low protein expression levels, it has not been possible to examine whether Mst nuclear translocation occurs during eosinophil apoptosis.
In conclusion, we have implicated a possible role for caspase-mediated cleavage Mst1 and subsequent release of the N-terminal catalytic fragment in the mechanism of constitutive and Fas-mediated eosinophil apoptosis that appears to be at least partially dependent upon H2O2 production. In contrast, neutrophils do not express either Mst1 or Mst2, although cell death is temporally associated with the activation of an as-yet-unidentified 34-kd MBPK. These differences in the mechanism of apoptosis may be useful for the selective induction of eosinophil and neutrophil apoptosis. Several recent studies have suggested that a number of antitumor drugs such as cytotrienin A, camptothecin, taxol, and 5-fluorouracil may mediate their actions through Mst1 cleavage and the subsequent induction of apoptosis.21 27 Therefore, our demonstration of a similar role for Mst1 in eosinophil apoptosis implies that this could provide a possible selective therapeutic regimen in the resolution of eosinophilic inflammation.
Supported by grants from AstraZeneca Pharmaceuticals, Wellcome Trust (grant no. 056814), GlaxoWellcome United Kingdom, Medical Research Fund of Tampere University Hospital, Finland and the Jalmari and Rauha Ahokas Foundation Academy, Finland.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mark A. Lindsay, EST-Biology, AstraZeneca Pharmaceuticals, Alderley Park, Cheshire, United Kingdom, SK10 4TG; e-mail: mark.lindsay@astrazeneca.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal