Abstract
Erythrocytes have a defined lifespan in vivo, and the signals that maintain their survival in circulation or trigger their death are unknown. Here, we investigated the control of erythrocyte survival and death in an in vitro culture system where erythrocytes survived for 10 days in serum-free medium in the presence or absence of bovine serum. Death of the cells in culture was correlated with increased exposure of phosphatidylserine and increased levels of intracellular calcium. Cell death could be suppressed by supplementing the medium with human plasma or serum, resulting in a doubling of the lifespan to 20 days. Freshly isolated erythrocytes and cultured erythrocytes were both found to express Bcl-XL and, to a lesser extent, Bak in membrane protein extracts. Treatment of the cells with a Bak-derived BH3 peptide fused to the internalization sequence of the antennapedia protein, which has previously been shown to enter cells by diffusion and antagonize Bcl-XL, resulted in substantial cell death in erythrocyte cultures. BH3-induced death was accompanied by an immediate increase in accumulation of intracellular calcium and could be suppressed by plasma, but not by the caspase inhibitor zVAD. A BH3 peptide mutated at amino acid 78 of full-length Bak required for heterodimerization with Bcl-XL had no effect on cell viability or calcium levels. We conclude that the BH3 peptide accelerates erythrocyte death through antagonization of Bcl-XL. The data suggest that erythrocyte survival is promoted by survival factors in plasma and by membrane-associated Bcl-XL.
Introduction
Cell death and survival in hematopoietic cells is regulated by signals from cell-surface receptors that either promote apoptosis, such as Fas and tumor necrosis factor (TNF), or repress cell death, such as receptors for cytokines and survival factors such as erythropoietin (EPO), interleukin 3, and insulinlike growth factor I (IGF-I).1-3 Ligation of the receptors for Fas/TNF can lead directly to activation of caspases, which can act as effectors of cell death by catalyzing structural changes associated with apoptosis, including cytoskeletal reorganization and DNA fragmentation. Survival factors mediate signals through several kinases, such as phosphoinositol 3 kinase and AKT, ultimately leading to transcriptional events or to regulation of Bcl-2 family proteins.4 The Bcl-2 family acts as central regulators of cell death in many cells5-7 and is divided into 2 main groups consisting of suppressors of apoptosis, including Bcl-2, Bcl-XL, Bcl-w, and Mcl-1, and potent apoptosis promoters, including Bax, Bak, Bad, and Bid. Bcl-2 and Bcl-XL survival activity can be antagonized by heterodimerization with proteins containing a BH3 domain, which is the essential domain for the binding and proapoptotic activity of Bak, Bax, Bik, and Bid.8Bcl-2 and Bcl-XL are expressed in outer mitochondrial membranes where antagonization of their function is associated with rupture of the membrane, release of the proapoptotic factors cytochromec, and activation of caspases.9
Human erythrocytes are terminally differentiated cells that circulate in the blood for an average of 120 days, at which time they are removed by macrophages of the reticuloendothelial system.10Changes in morphology occur as erythrocytes age and newly externalized phosphatidylserine (PS) appears on the surface of the senescent erythrocytes.11 Recognition of PS by phagocytic cells is a mechanism used for removal of different kinds of apoptotic cells, including circulating leukocytes,12 as well as the removal of senescent red blood cells. Erythrocytes do not possess mitochondria, now considered to be the key cellular sites where cell death is controlled, nor do they possess nuclei, which are one of the key targets for caspases during the execution of apoptosis. Therefore, it is not known how erythrocyte cell death is regulated. Mature erythrocytes are derived from precursor cells in the bone marrow whose requirement for survival signals is well documented. EPO, IGF-I, and expression of Bcl-XL are all essential for the normal differentiation of erythroid progenitor cells into mature red blood cells.13-15 However, although EPO induces the expression of Bcl-XL late in erythroid differentiation and is thought to mediate at least part of its survival activity through Bcl- XL,16 responses to EPO decrease as the cells mature. Therefore, it is not known if EPO or any other survival factors are involved in keeping fully mature erythrocytes alive during their 120 days in circulation. It is also not known what triggers or mediates erythrocyte death leading to externalization of PS. However, it is well established that there is an association of increased levels of intracellular calcium with the events surrounding PS translocation in mature erythrocytes.17-19 The original hypothesis by Raff et al20 that all nucleated cells require survival signals to be maintained has been well supported over time. In terminally differentiated hematopoietic cells of the granulocytic lineage, distinct cell death and cell survival pathways are mediated by cytokines, Fas, the Bcl-2 family, and p38 mitogen-activated protein kinases.21,22 Platelets have been shown to express Bcl-2 family members and caspases, but they die in a caspase-independent manner and are cleared from circulation via recognition of cell-surface PS.23 24 The lifespan of erythrocytes in vivo is apparently programmed, and it is therefore likely that erythrocyte survival, lifespan, and death may be as tightly regulated as in other cells. However, the proteins and signals involved in regulating the survival or death of mature erythrocytes are not known.
In this study, we have investigated regulation of erythrocyte cell survival and death. We first established a cell-culture system in serum-free medium (SFM), where the lifespan of cultured erythrocytes could be significantly extended by addition of plasma. Both Bcl-XL and, to a lesser extent, its proapoptotic partner Bak were found to be expressed in erythrocyte membrane extracts. Treatment of erythrocytes with a synthetic Bak-derived BH3 peptide fused to the internalization domain of the antennapedia homeodomain protein25 resulted in a rapid induction of cell death. This peptide could specifically interact with Bcl-XL in erythrocyte membrane extracts. A Bak-BH3 peptide mutated at amino acid at position 78 in full-length Bak required for heterodimerization with Bcl-XL did not affect cell viability and could not bind to membrane-associated Bcl-XL. The Bak-BH3 peptide rapidly and transiently increased erythrocyte intracellular calcium levels, and its killing activity could be blocked by plasma. These data indicate that Bcl-XL is expressed in erythrocyte membranes, that antagonization of Bcl-XL accelerates their death, and that this death can be suppressed by plasma. This suggests that erythrocyte survival and lifespan in vivo may be regulated by membrane-associated Bcl-XL and Bak and by survival factors in plasma that functionally interact with these proteins to control ion homeostasis.
Materials and methods
Materials
Annexin-V fluorescein isothiocyanate (FITC), the calcium probe Fluo-3 AM, and the peroxide probe H2DCFH DA were obtained from Molecular Probes (Leiden, The Netherlands). Phalloidin FITC and the RNA probe thiazole orange were purchased from Sigma-Aldrich (Wexford, Ireland). The anti–Bcl-XL and anti-Bad antibodies were purchased from Transduction Laboratories (Lexington, KY). Anti–Bcl-2 antibody was obtained from Dako (Glostrup, Denmark). Anti-Bak and anti–SHPTP-1 antibodies were purchased from Upstate Biotechnology (Lake Placid, NY), and the anti–cytochrome coxidase antibody was obtained from Molecular Probes. Anti-CD45 antibody was obtained from Neomarkers (Lab Vision, Fremont, CA), and the platelet-specific antibody anti-CD61 was obtained from Serotec (Oxford, United Kingdom). The Bak-BH3 peptides were synthesized as antennapedia-fused proteins as described previously.25 Cell-culture medium was purchased from Biowhittaker (Verviers, Belgium).
Cell lines
The cell lines used were HL-60 myeloid cells, Jurkat T-cells, and FL5.12 B-lymphoblastoma cells. The HL-60 and Jurkat cells were cultured in RPMI supplemented with 10% fetal bovine serum (FBS). The FL5.12 B-lymphoblastoma cells were cultured in Iscoves modified Dulbecco medium supplemented with 10% FBS and 10% WEHI-conditioned medium.
Erythrocyte cell culture
Whole blood was collected from healthy donors, and erythrocytes were immediately isolated by centrifugation through a Ficoll hypaque (Amersham, Little Chalfont, United Kingdom) density gradient followed by washing with serum-free RPMI 1640 medium. The plasma layer was also isolated from the gradient, and platelets were collected by centrifugation at 960g for 20 minutes. Serum was prepared by allowing whole blood to clot by incubation at 37°C for 1 hour followed by centrifugation at 514g for 10 minutes. The supernatant (serum) was removed and recentrifuged to remove any remaining blood cells. The freshly isolated erythrocytes were then cultured in RPMI 1640 supplemented with 1 mM glutamine either alone or in medium supplemented with 10% FBS or 10% platelet-poor plasma (PPP) or 10% freshly prepared human serum.
Depletion of reticulocytes by flow cytometry and cell sorting
Freshly isolated erythrocyte fractions (5 × 106cells) were washed in phosphate-buffered saline (PBS) and incubated with 100 ng/mL thiazole orange in PBS containing 2 mM CaCl2for 20 minutes at room temperature to label the RNA-containing subset of cells (reticulocytes).26 Flow cytometry was carried out with a Becton Dickinson FACScalibur flow cytometer (Becton Dickinson, Oxford, United Kingdom), and the RNA-containing subset of cells was identified as consisting of cells displaying a fluorescence signal in excess of that obtained with an unlabeled control. Erythrocytes that did not display thiazole orange staining were gated, separated from the thiazole orange population by flow sorting, and collected for further analysis.
Erythrocyte viability assay and induction of erythrocyte cell death by Bak-BH3 peptides
Erythrocytes were cultured in triplicate at 5 × 106 cells per milliliter either alone or in the presence of 10% FBS, 10% PPP, or 10% human serum. Cell viability in the cultures was determined by removing an aliquot of cells and by counting morphologically intact erythrocytes with a hemocytometer. Erythrocytes always excluded trypan blue dye, and viable erythrocytes were morphologically defined as intact discocytes or echinocytes. At the indicated time points, samples were taken from erythrocyte cell suspensions that were cultured in SFM or medium supplemented with 10% PPP and were loaded with 5 μg/mL annexin-V FITC. Cell-associated fluorescence was then analyzed by flow cytometry. For Bak-BH3 induction of erythrocyte cell death, cells were cultured in medium in the presence or absence of plasma, to which the peptides antennapedia protein (Ant), Ant-BH3, or Ant-BH3-A78 (all at a final concentration of 50 μM) were added. Aliquots of cells were removed to assess cell number and annexin-V binding at the indicated time points. Phalloidin FITC (1 μg/mL in RPMI medium) was incubated with cells for 15 minutes, after which cells were washed once, resuspended in PBS, and assessed for cell-associated fluorescence. As a positive control for phalloidin FITC staining, cells were fixed and permeabilized by treatment with 1% paraformaldehyde and methanol.
Whole cell and erythrocyte plasma membrane protein extracts
Freshly isolated erythrocytes (5 × 106 cells), reticulocyte-depleted erythrocytes, HL-60 and Jurkat cells (1 × 106), and packed platelets that were isolated from 5 mL of plasma were washed once in PBS and solubilized in 80 μL or 30 μL (reticulocyte-depleted erythrocytes) RIPA buffer (150 mM NaCl, 1% NP40, 0.5% deoxycholate, 0.15% sodium dodecyl sulfate [SDS], and 50 mM Tris, pH8) containing 1 mM phenylmethyl sulfonyl fluoride (PMSF), 1 μM pepstatin, and 1 μM aprotinin at 4°C for 15 minutes. Unsolubilized material was removed by centrifugation at 16 000g for 20 minutes at 4°C. The supernatants were removed and used immediately for Western blotting.
To prepare membranes, freshly isolated erythrocytes were suspended in PBS and centrifuged to form a pellet constituting 10% of normal hematocrit. To these packed erythrocytes, 5 vol isotonic buffer (5 mM Na2HPO4, pH 8, buffer containing 1 mM PMSF, 1 μM pepstatin, and 1 μM aprotinin) was added. The membranes were then concentrated by centrifugation at 20 000g for 20 minutes at 4°C. The supernatant was decanted off, and membranes were washed twice in the same buffer. Membrane proteins were then solubilized in RIPA buffer containing the same protease inhibitors at 4°C for 15 minutes.
SDS polyacrylamide gel electrophoresis and Western blotting
The protein concentrations of the erythrocyte membrane and whole cell lysates were determined by the Bradford assay (Biorad, Hemel-Hemstead, United Kingdom). Samples of lysate containing 50 μg or 30 μg (reticulocyte-depleted erythrocytes) protein were boiled with 5 × Laemmli sample buffer and fractionated by 4% to 20% gradient SDS polyacrylamide gel electrophoresis (SDS-PAGE). Resolved protein was electrophoretically transferred onto nitrocellulose membranes (Schleicher and Schull, Dassel, Germany). The nitrocellulose was blocked in Tris-buffered saline–Tween (TBS-T) (20 mM Tris, 150 mM NaCl, 0.05% Tween-20, pH 7.6) containing 5% milk for 1 hour (2 hours for anti–cytochrome c oxidase) at room temperature. All primary antibodies were then incubated overnight at 4°C at their recommended dilutions in TBS-T/5% milk. Secondary antibodies used were horseradish peroxidase–conjugated goat anti-rabbit and rabbit antimouse (Dako) diluted 1/1000 and incubated for 1.5 hours at room temperature. Following antibody incubations, the nitrocellulose was washed 3 times in TBS-T for 3 minutes (10 minutes for anti–cytochrome c oxidase). Bound antibody was detected using enhanced plus chemiluminescence (Amersham). For analysis of the expression of the Bcl-2 family members in erythrocyte membranes, samples of lysate representing 90 μg erythrocyte or 30 μg HL-60 lysate were used for Western blotting.
Detection of Bak-BH3 peptide/Bcl-XLinteraction
Erythrocyte plasma membrane extracts and FL5.12 cells were lysed in Hepes buffer (10 mM Hepes, 142.5 mM NaCl, 1 mM ethyleneglycotetraacetic acid, pH 7.2) containing 0.25% NP40 and protease inhibitors for 15 minutes at 4°C. This was followed by centrifugation at 16 000g for 15 minutes at 4°C to remove cellular debris. Streptavidin-coated agarose beads (Sigma, Dorset, United Kingdom) were washed 3 times in lysis buffer. Then, 20 μL beads were added to the erythrocyte and FL5.12 cell samples, and the samples were further incubated at 4°C for 2 hours. The streptavidin beads were then centrifuged at 960g for 3 minutes, and the supernatant was removed. A small sample (20 μL) of the supernatant was retained from each experimental condition to determine the amount of Bcl-XL that was not interacting with the beads. The beads were then washed 3 times in lysis buffer and boiled in 2 × Laemmli sample buffer for 5 minutes to dissociate protein from the beads. Following centrifugation at 16 000gfor 1 minute to pellet the beads, the supernatants were removed and resolved by 4% to 20% gradient SDS-PAGE. Western blotting to detect Bcl-XL was performed as described above.
Caspase activity assay
Erythrocytes (5 × 106/mL) were cultured in serum-free RPMI 1640 medium in the presence or absence of 80 μM zVAD-fmk (Enzyme Systems Products, Livermore, CA). Following 15 minutes of pretreatment with zVAD, the Ant or Ant-BH3 peptides (50 μM) were added to the cell suspension. Viable cell number was determined at time 0 and after 24 hours' incubation. As a positive control for zVAD-fmk activity, 1 × 106 Jurkat T cells were treated with anti-Fas immunoglobulin (Ig)–M to induce cell death, which was detected by both annexin-V binding and propidium iodide uptake.
Intracellular calcium measurement
Freshly isolated erythrocytes (5 × 106/mL) were cultured in serum-free RPMI 1640 medium in the presence or absence of 10% PPP. Aliquots of cells were removed at different time points and were loaded with the calcium probe Fluo-3 AM (250 nM) in the dark for 15 minutes. Cell-associated fluorescence was then analyzed by flow cytometry. Erythrocyte autofluorescence was also determined for both treated and untreated cells in the absence of the Fluo-3. Intracellular calcium was measured for cells that were cultured in the presence and absence of plasma on day 0 of culture and following 4 and 6 days culture. To investigate the effect of the BH3 peptides on erythrocyte intracellular calcium, cells (2 × 106/mL) were loaded with 250 nM Fluo-3 AM and incubated for 15 minutes in the dark. To determine cell-associated background fluorescence due to Fluo-3, the erythrocytes were analyzed in the absence of peptides by flow cytometry over an approximately 2-minute period. The Ant-BH3 or Ant-BH3-A78 peptides (50 μM) were then added to the cell suspension, and the cells were immediately analyzed for Fluo-3 fluorescence.
Results
Erythrocyte viability can be modulated in vitro
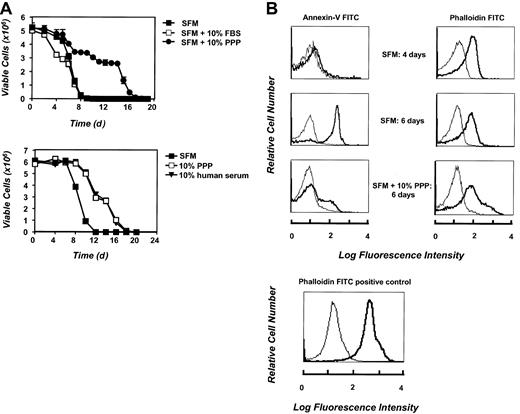
The impact of different cell-culture conditions on the lifespan of erythrocytes and the factors that induce death or promote the survival of these cells in vitro is unknown. Therefore, we sought to establish conditions whereby erythrocyte survival could be modulated. We first examined whether supplementation of culture medium with FBS or plasma was essential for or contributed to erythrocyte survival. Freshly isolated cells were cultured either in SFM alone or in medium supplemented with FBS, 10% human serum, or 10% PPP, and total cell viability was assessed over time. As shown in Figure1A, erythrocytes that were cultured in SFM displayed a marked reduction in the number of viable cells following 6 days of culture, and by 10 days of culture, very few cells were left alive. Cells cultured in medium supplemented with 10% FBS died at a similar rate. In contrast, erythrocytes that were maintained in the PPP or in human serum–supplemented medium displayed prolonged viability until day 15 of culture. The initial slow phase of death (up to day 4 in the absence of survival factors, or day 12 with the addition of plasma) may be a measure of the demise of the more aged erythrocytes in the cell population, while the steep decline in the cell viability at later times may be due to the absence of essential erythrocyte survival factors in vitro. Overall, these data demonstrate that the lifespan of erythrocytes cultured in SFM or FBS is only 10 days and that this lifespan can be doubled by the addition of PPP or human serum to the cell-culture medium. The data suggest that culture of erythrocytes either in SFM alone or in the presence of FBS leads to an acceleration of a death process that is constitutively active in these cells, and that plasma- or human serum–supplemented medium delays this death process. The data further suggest that plasma contains essential erythrocyte survival factors.
Effect of culture in SFM on erythrocytes.
Erythrocytes display reduced viability and increased cell-surface PS exposure upon culture in SFM. (A) Freshly isolated erythrocytes were cultured at 5 × 106 cells per milliliter in medium, either alone or supplemented with 10% FBS, 10% PPP, or 10% human serum. Cell number was determined for triplicate cultures at time 0 and every 24 hours or 48 hours thereafter. Data are presented as mean cell number ± SD. (B) PS translocation was determined by annexin-V FITC binding at 0, 4, and 6 days following initial culture of the cells in SFM. The thin line represents erythrocyte autofluorescence in the absence in annexin-V FITC. Bound annexin-V FITC fluorescence is represented in bold. Erythrocyte membrane integrity at 0, 4, and 6 days of culture in SFM was determined by incubating the cells with phalloidin FITC. As a positive control for phalloidin FITC uptake by erythrocytes, 5 × 106 cells per milliliter were fixed with 1% phosphonoformatic acid and permeabilized with methanol prior to addition of phalloidin FITC. The thin line represents erythrocyte autofluorescence in the absence of phalloidin FITC. Phalloidin FITC fluorescence is represented in bold.
Effect of culture in SFM on erythrocytes.
Erythrocytes display reduced viability and increased cell-surface PS exposure upon culture in SFM. (A) Freshly isolated erythrocytes were cultured at 5 × 106 cells per milliliter in medium, either alone or supplemented with 10% FBS, 10% PPP, or 10% human serum. Cell number was determined for triplicate cultures at time 0 and every 24 hours or 48 hours thereafter. Data are presented as mean cell number ± SD. (B) PS translocation was determined by annexin-V FITC binding at 0, 4, and 6 days following initial culture of the cells in SFM. The thin line represents erythrocyte autofluorescence in the absence in annexin-V FITC. Bound annexin-V FITC fluorescence is represented in bold. Erythrocyte membrane integrity at 0, 4, and 6 days of culture in SFM was determined by incubating the cells with phalloidin FITC. As a positive control for phalloidin FITC uptake by erythrocytes, 5 × 106 cells per milliliter were fixed with 1% phosphonoformatic acid and permeabilized with methanol prior to addition of phalloidin FITC. The thin line represents erythrocyte autofluorescence in the absence of phalloidin FITC. Phalloidin FITC fluorescence is represented in bold.
To further characterize the onset of cell death in cultured erythrocytes, we investigated the expression of PS on their cell surface. As a reduction in cell viability was observed in erythrocytes cultured in SFM, we therefore investigated whether the loss of viability was accompanied by a translocation of PS to the outer leaflet of the plasma membrane in these cells. Cells cultured in SFM were analyzed for the expression of PS by means of annexin-V FITC and flow cytometry. Results, shown in Figure 1B, demonstrate that after 4 days of culture in SFM, erythrocytes did not express externalized PS. However, after 6 days of culture, there is a dramatic increase in annexin-V staining. At this stage, approximately 90% of the erythrocytes expressed PS on the outer leaflet of their plasma membrane. The timing of this PS exposure is immediately preceding the rapid decrease in cell number observed in erythrocytes cultured in SFM (Figure 1A). In contrast, cells cultured in the presence of PPP did not show an increase in cell-surface PS, again correlating with cell survival. To rule out the possibility that annexin V was simply getting into leaky erythrocytes, cells were stained at each time point with actin-binding phalloidin FITC. There was no increase in phalloidin FITC staining even under conditions where annexin-V staining increased dramatically (Figure 1B). Erythrocytes cultured in SFM were also observed to undergo membrane shape changes and display significant echinocytosis (data not shown). All together, these results demonstrate that the loss of viability of erythrocytes cultured in SFM is accompanied by the appearance of externalized PS on their cell surface, an event that has been well documented both as a feature of the apoptotic cell27 and as a marker of erythrocyte senescence.11
Bcl-XL and Bak are expressed in erythrocyte plasma membranes
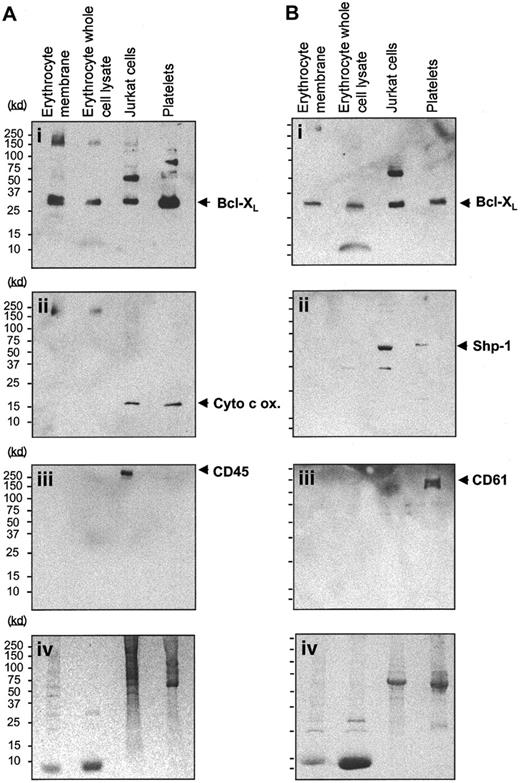
Having established that we could manipulate erythrocyte survival in culture, we next investigated what proteins may be involved in regulating cell survival or death in culture. We therefore investigated whether known regulators of cell death, such as the Bcl-2 family members and caspases, were expressed in erythrocytes. We first investigated the expression of Bcl-XL in both erythrocyte whole cell lysates and plasma membrane extracts by Western blotting compared with the Jurkat T-cell line and with platelets. As can be seen in Figure 2Ai,Bi, Bcl-XL is detected in all of the cells tested, with lower levels observed in erythrocytes than in Jurkat cells and platelets. Bcl-XL expression appears to be higher in erythrocyte plasma membrane extracts than in the whole cell lysates; this is consistent with its association with erythrocyte membranes. To rule out the possibility that Bcl-XL expression in erythrocytes was due to contamination from reticulocytes or leukocytes, the Western blot was reprobed with antibodies specific for the mitochondrial marker cytochrome c oxidase28 (Figure 2Aii) and for the leukocyte marker CD45 (Figure 2Aiii).29 In a separate experiment, an anti–Bcl-XL–stained Western blot was also reprobed with antibodies for hematopoietic cells (anti–SHP-1 phosphatase30; Figure 2Bii) and for the platelet marker CD 61 (Figure 2Biii).31 The total protein loaded for each lane is shown in duplicate Coomassie-stained gels (Figure 2Aiv,Biv). As can be seen in Figure 2Aii, cytochromec oxidase (17 kd), which is detected strongly in Jurkat cells and platelets, is not detected in erythrocyte whole cell or membrane extracts. The platelet and leukocyte markers CD61 and CD45 were detected only in platelets and Jurkat cells, respectively. In contrast, low levels of SHP-1 are detected in erythrocyte whole cell and membrane extracts (Figure 2Bii). This may be due to retention of this protein in mature erythrocytes following its function of regulating EPO-receptor levels in erythroid progenitors.32
Expression of Bcl-XL in terminally differentiated erythrocytes.
Bcl-XL is expressed in terminally differentiated erythrocytes. (A) Western blot analysis of the expression of Bcl-XL (Ai) in erythrocyte plasma membrane extracts, erythrocyte whole cell lysates, Jurkat T cells, and platelets. Blots were stripped of bound antibody and reprobed with the use of anti–cytochrome c oxidase as a mitochondrial marker (Aii) and CD 45 as a lymphocyte marker (Aiii). Panel Aiv shows an identical Coomassie-stained SDS-PAGE gel showing protein loading. (B) Western blots of erythrocyte plasma membrane and whole cell lysates, Jurkat T cells, and platelets that express Bcl-XL (Bi) were also stripped of bound antibody and reprobed for the expression of SHP-1, the hematopoietic cell marker (Bii), and the platelet-specific marker CD 61 (Biii). Panel Biv depicts an identical Coomassie-stained SDS-PAGE gel showing protein loading.
Expression of Bcl-XL in terminally differentiated erythrocytes.
Bcl-XL is expressed in terminally differentiated erythrocytes. (A) Western blot analysis of the expression of Bcl-XL (Ai) in erythrocyte plasma membrane extracts, erythrocyte whole cell lysates, Jurkat T cells, and platelets. Blots were stripped of bound antibody and reprobed with the use of anti–cytochrome c oxidase as a mitochondrial marker (Aii) and CD 45 as a lymphocyte marker (Aiii). Panel Aiv shows an identical Coomassie-stained SDS-PAGE gel showing protein loading. (B) Western blots of erythrocyte plasma membrane and whole cell lysates, Jurkat T cells, and platelets that express Bcl-XL (Bi) were also stripped of bound antibody and reprobed for the expression of SHP-1, the hematopoietic cell marker (Bii), and the platelet-specific marker CD 61 (Biii). Panel Biv depicts an identical Coomassie-stained SDS-PAGE gel showing protein loading.
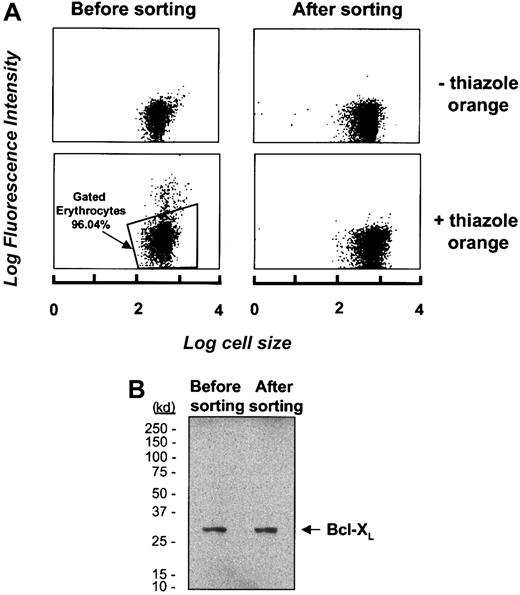
To rule out the possibility that Bcl-XL detected in erythrocytes is due to the presence of a subpopulation of reticulocytes and not due to erythrocytes per se, we sought to isolate a pure erythrocyte population by flow cytometric cell sorting. Erythrocytes that were purified by density gradient centrifugation through a Ficoll-hypaque gradient were incubated with the RNA-specific probe thiazole orange.26 With the use of flow cytometry, reticulocytes as well as any contaminating leukocytes or platelets were identified as those cells that displayed a fluorescence signal in excess of that of an unlabeled control; these were found to represent 3.96% of the entire cell population (Figure3A). The thiazole orange–negative cells were then collected from the erythrocyte sample by cell sorting and Bcl-XL expression was investigated in erythrocyte samples before and after cell sorting. As shown in Figure 3B, the levels of Bcl-XL expression are not different in unsorted erythrocytes compared with the thiazole-negative erythrocytes that do not include reticulocytes. From this result, taken together with the absence of cytochrome c oxidase and other leukocyte markers in these cells, we conclude that the Bcl-XL protein detected in erythrocyte whole cell or membrane extracts represents Bcl-XL expressed in mature erythrocytes.
Bcl-XL expression and isolation of contaminating reticulocytes.
(A) Erythrocytes were labeled with the RNA probe thiazole orange and analyzed by flow cytometry to identify the RNA-containing subset of cells prior to its depletion from the erythrocyte sample by cell sorting. The top panel represents unlabeled cells; thiazole orange–labeled cells are represented in the bottom panel. The erythrocytes selected for retention for further analysis are gated and represent 96.04% of the total cell population. (B) Western blot analysis of the expression of Bcl-XL in an erythrocyte sample before and after depletion of thiazole orange–staining cells.
Bcl-XL expression and isolation of contaminating reticulocytes.
(A) Erythrocytes were labeled with the RNA probe thiazole orange and analyzed by flow cytometry to identify the RNA-containing subset of cells prior to its depletion from the erythrocyte sample by cell sorting. The top panel represents unlabeled cells; thiazole orange–labeled cells are represented in the bottom panel. The erythrocytes selected for retention for further analysis are gated and represent 96.04% of the total cell population. (B) Western blot analysis of the expression of Bcl-XL in an erythrocyte sample before and after depletion of thiazole orange–staining cells.
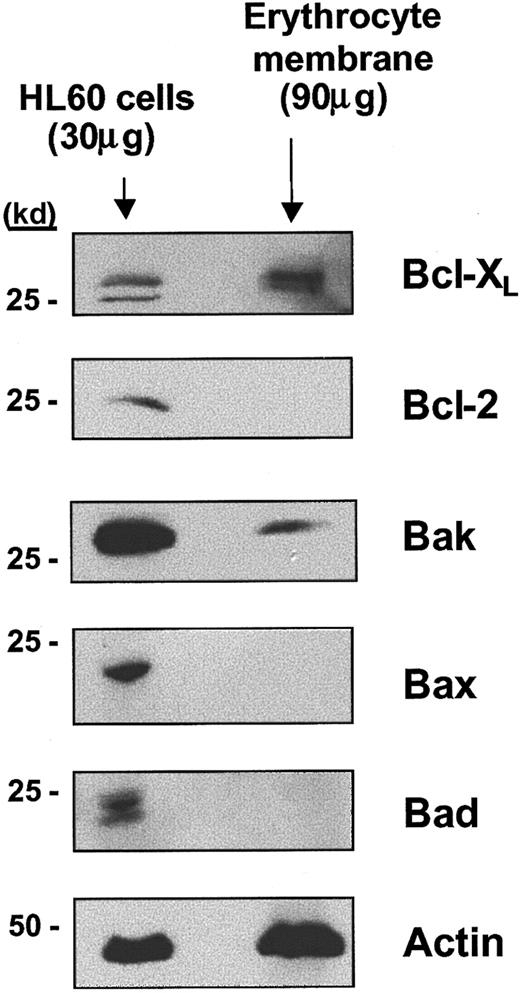
We next investigated expression of other Bcl-2 family members in erythrocyte membrane extracts. Bak expression could be detected (Figure 4) whereas neither Bcl-2 nor other proapoptotic members of the Bcl-2 family, including Bax and Bad, were expressed in erythrocytes. The levels of Bcl-XL and Bak expression were not observed to change over the lifespan of the erythrocyte in culture (data not shown). Western blotting using whole cell lysates or membrane extracts and appropriate controls for the expression of caspases 3, 7, and 8 demonstrated that none of these caspases could be detected in either total erythrocytes or erythrocyte membrane extracts (data not shown). Thus, Bcl-XL, an antiapoptotic protein, and its proapoptotic partner, Bak, are expressed in terminally differentiated erythrocytes.
Western blot analysis of the expression of Bcl-X, Bcl-2, Bax, Bad, and Bak in erythrocyte plasma membrane extracts.
Bcl-XL and Bak are expressed in terminally differentiated erythrocyte plasma membranes. As a positive control for the expression of these Bcl-2 family members, cell lysates were prepared from HL60 cells. Actin expression in erythrocyte membrane extracts is also shown as a control for the amount of erythrocyte protein present on the immunoblots.
Western blot analysis of the expression of Bcl-X, Bcl-2, Bax, Bad, and Bak in erythrocyte plasma membrane extracts.
Bcl-XL and Bak are expressed in terminally differentiated erythrocyte plasma membranes. As a positive control for the expression of these Bcl-2 family members, cell lysates were prepared from HL60 cells. Actin expression in erythrocyte membrane extracts is also shown as a control for the amount of erythrocyte protein present on the immunoblots.
Bak-BH3 peptides induce erythrocyte cell death
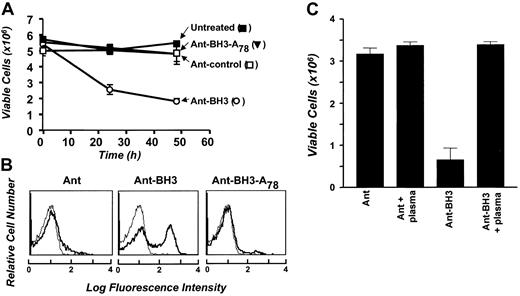
Bcl-XL and Bak have previously been shown to heterodimerize through the BH3 domain of Bak, and enhanced expression of Bak has been shown to antagonize the survival activity of Bcl-XL in different cellular systems.8Therefore, to investigate whether membrane-associated Bcl-XL plays a role in promoting the survival of erythrocytes, we sought to antagonize Bcl-XL function using synthetic BH3 peptides derived from the Bak protein. These BH3 peptides are fused to the internalization sequence of the Ant protein, which permits uptake of the peptides into cells via facilitated diffusion,25 and have been shown to effectively antagonize Bcl-XL function in tumor cells. Erythrocytes were cultured in SFM with the Ant-BH3 peptide, the Ant peptide alone, or a mutant form of the Ant-BH3 peptide, which has an alanine substitution for a leucine at position 78 of full-length Bak that prevents heterodimerization with Bcl-XL. As shown in Figure5A, erythrocytes cultured with the Ant-BH3 peptide undergo a significant decrease in viable cell number within 24 hours, and by 48 hours the cultures have decreased in viability by about 70%. This reduction in cell viability is accompanied by a translocation of PS to the outer leaflet of the plasma membrane in the Ant-BH3–treated cells (Figure 5B). Treatment of erythrocytes with the mutant Ant-BH3 peptide does not induce cell death (Figure 5A-B). Similarly, treatment of cells with a peptide of the Ant internalization sequence on its own has no effect on erythrocyte viability. This indicates that the induction of erythrocyte cell death is specific to the action of the Ant-BH3 peptide and that it requires a residue necessary for interaction with Bcl-XL to kill cells.
Bak-BH3 peptides and erythrocyte cell death.
Bak-BH3 peptides induce erythrocyte cell death. (A) First, 5 × 106 erythrocytes were cultured in SFM and treated with 50 μM Ant, Ant-BH3, or Ant-BH3-A78 or left untreated. Cell number was determined at 0, 24, and 48 hours for triplicate cultures and is represented as mean cell number ± SD. (B) Following 24 hours of treatment, samples were taken from panel A cultures for flow cytometric analysis of annexin-V FITC binding (bold). The thin line again represents erythrocyte autofluorescence in the absence of annexin-V FITC. (C) First, 5 × 106erythrocytes were cultured in SFM in the presence and absence of 10% PPP. Cells were then treated with 50 μM Ant or Ant-BH3. Cell number was determined at 0 and 24 hours, again for triplicate cultures. Results are presented as mean cell number ± SD and represent the 24-hour time point.
Bak-BH3 peptides and erythrocyte cell death.
Bak-BH3 peptides induce erythrocyte cell death. (A) First, 5 × 106 erythrocytes were cultured in SFM and treated with 50 μM Ant, Ant-BH3, or Ant-BH3-A78 or left untreated. Cell number was determined at 0, 24, and 48 hours for triplicate cultures and is represented as mean cell number ± SD. (B) Following 24 hours of treatment, samples were taken from panel A cultures for flow cytometric analysis of annexin-V FITC binding (bold). The thin line again represents erythrocyte autofluorescence in the absence of annexin-V FITC. (C) First, 5 × 106erythrocytes were cultured in SFM in the presence and absence of 10% PPP. Cells were then treated with 50 μM Ant or Ant-BH3. Cell number was determined at 0 and 24 hours, again for triplicate cultures. Results are presented as mean cell number ± SD and represent the 24-hour time point.
The ability of full-length Bak or Bak-BH3 peptides to antagonize Bcl-XL function can be inhibited by FBS.8Therefore, to further test whether Ant-BH3 killing in erythrocytes is similar to that in epithelial cells, we were next interested in determining whether plasma could block Ant-BH3–induced cell death. Erythrocytes were incubated with Ant-BH3 in SFM or medium supplemented with 10% PPP. As shown in Figure 5C, the BH3 peptide induces cell death in SFM, but this killing is blocked in the plasma-supplemented medium. This blocking could be due to survival factors in plasma protecting the cells from BH3-induced death, but could also be contributed to by the prevention of Ant-BH3 uptake by the cells because it also occurred to some degree in medium supplemented with BSA (not shown). Overall, we conclude that the Ant-BH3 peptide induces erythrocyte cell death via antagonizing Bcl-XL as it does in nucleated cells. Taken together with the loss of killing by the mutant peptide, the data suggest that Bcl-XL expression in erythrocyte membranes serves to promote the survival of these cells.
The Bak-BH3 peptide interacts with Bcl-XL in erythrocyte membrane extracts
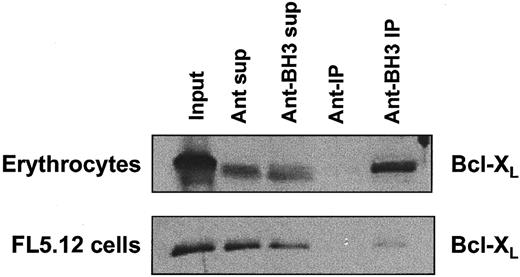
To confirm that the Ant-BH3 peptide could interact with Bcl-XL expressed in erythrocyte plasma membranes, we next tested whether a biotin-tagged version of the Ant-BH3 peptide could interact with Bcl-XL in erythrocyte plasma membranes. Extracts of erythrocyte membranes were incubated with biotinylated BH3 peptide (biotin-Ant–BH3) or the biotinylated internalization sequence (biotin-Ant) as a control. Streptavidin-coated agarose beads were used to “pull down” the peptides, and the presence of Bcl-XLin the “pulldowns” was investigated by Western blot analysis with anti–Bcl-XL antibodies. As demonstrated in Figure6, Bcl-XL is detected in the Western blot where lysates were incubated with the biotinylated BH3 peptide. This indicates that it can be pulled down by the BH3 peptide. In contrast, no Bcl-XL protein was pulled down with the biotinylated Ant internalization sequence alone. As a control for the interaction of biotin-Ant–BH3 and Bcl-XL, FL5.12 cells, which express endogenous Bcl-XL, were tested in parallel for these interactions. Again, the BH3 peptide selectively interacts with Bcl-XL in FL5.12 cell lysates (Figure 6). These data indicate that the interaction between the Bak-BH3 peptide and Bcl-XL in erythrocytes is specific. This supports the conclusion that a Bak-BH3 peptide acts to antagonize Bcl-XLfunction in killing erythrocytes and that Bcl-XL regulates cell death in erythrocytes.
Bak-BH3 interaction with erythrocyte Bcl-XL.
Bak-BH3 peptides directly interact with erythrocyte Bcl-XL. Erythrocyte membrane extracts were incubated with 50 μM biotin-Ant or biotin-Ant–BH3 overnight at 4°C. The biotinylated peptides and any bound protein were then pulled down by incubating the extracts with streptavidin-coated agarose beads, and the presence of Bcl-XL was determined by Western blotting. FL5.12 cells were used as positive control for the coimmunprecipitation.
Bak-BH3 interaction with erythrocyte Bcl-XL.
Bak-BH3 peptides directly interact with erythrocyte Bcl-XL. Erythrocyte membrane extracts were incubated with 50 μM biotin-Ant or biotin-Ant–BH3 overnight at 4°C. The biotinylated peptides and any bound protein were then pulled down by incubating the extracts with streptavidin-coated agarose beads, and the presence of Bcl-XL was determined by Western blotting. FL5.12 cells were used as positive control for the coimmunprecipitation.
Bak-BH3–induced erythrocyte cell death occurs independently of known caspases
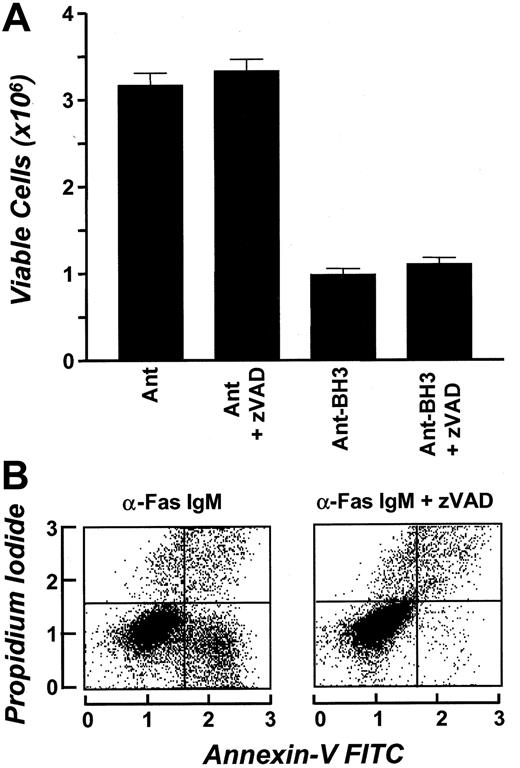
Bak-BH3–induced apoptosis has previously been described as being caspase mediated.25 As discussed above, we did not detect the expression of caspases 3, 7, and 8 in erythrocyte whole cell extracts by Western blotting. To determine if BH3-mediated erythrocyte cell death requires the activation of known caspases and to further investigate the possibility that caspase activity could exist in erythrocytes in the absence of detectable protein, we tested whether the broad-spectrum caspase inhibitor zVAD-fmk could block BH3-induced erythrocyte cell death. Erythrocytes were cultured in the presence of Bak-BH3 peptide in the presence or absence of zVAD, and cell viability was assessed 24 hours later. Results shown in Figure7A demonstrate that zVAD-fmk treatment has no effect on BH3-induced death (Figure 7A). The efficiency of the zVAD-fmk reagent in blocking cell death was confirmed by demonstrating its ability to block Fas-induced death in Jurkat cells (Figure 7B). These data indicate that BH3 induction of erythrocyte cell death does not require activation of known caspases. This suggests that Bcl-XL acts to regulate a caspase-independent death program in these cells.
Effect of known caspases on Bak-BH3–induced erythrocyte cell death.
Bak-BH3–induced erythrocyte cell death occurs independently of known caspases. (A) First, 5 × 106 erythrocytes were incubated in SFM in the presence or absence of 80 μM zVAD. Following 15 minutes of incubation, 50 mM Ant or Ant-BH3 was added to the cell suspension. Cell number was determined at 0 and 24 hours in triplicate. Results are expressed as mean cell number ± SD and represent the 24-hour time point. (B) The α-Fas IgM–treated Jurkat cells were used as a control for zVAD. The percentages 21% and 1% represent the percentage of cells that have bound annexin-V FITC in the absence and presence of zVAD, respectively.
Effect of known caspases on Bak-BH3–induced erythrocyte cell death.
Bak-BH3–induced erythrocyte cell death occurs independently of known caspases. (A) First, 5 × 106 erythrocytes were incubated in SFM in the presence or absence of 80 μM zVAD. Following 15 minutes of incubation, 50 mM Ant or Ant-BH3 was added to the cell suspension. Cell number was determined at 0 and 24 hours in triplicate. Results are expressed as mean cell number ± SD and represent the 24-hour time point. (B) The α-Fas IgM–treated Jurkat cells were used as a control for zVAD. The percentages 21% and 1% represent the percentage of cells that have bound annexin-V FITC in the absence and presence of zVAD, respectively.
Calcium accumulates in cultured erythrocytes, and the BH3 peptide increases erythrocyte intracellular calcium
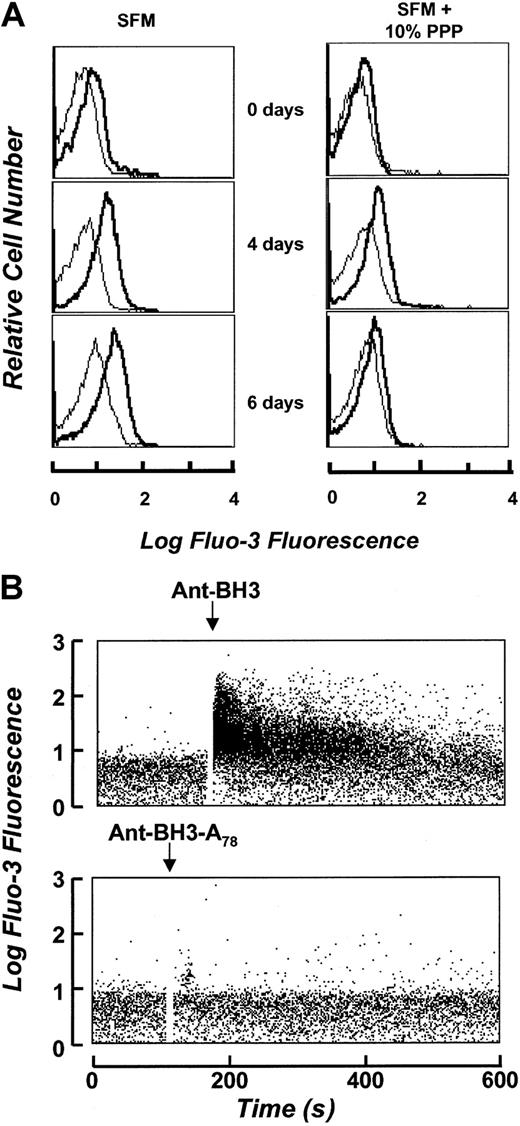
Alterations in intracellular calcium levels have been previously observed in aging erythrocytes,17 18 suggesting that calcium may have a role in regulating the events leading up to PS exposure. Therefore, we sought to determine if calcium played a role in Bak-BH3–induced erythrocyte cell death. We first measured calcium levels in erythrocytes under different culture conditions using the calcium probe Fluo-3 AM. As shown in Figure8A, erythrocytes displayed markedly increased intracellular calcium levels over time when cultured in SFM up to 6 days as compared with culture in medium containing 10% plasma. This correlates with the acquisition of annexin-V staining in Figure1B. This indicates that calcium levels are correlated with the survival of the cells. We next asked whether calcium levels might be altered in response to treatment with the Bak-BH3 peptide. Cells cultured in SFM were loaded with Fluo-3 AM and then analyzed by flow cytometry before, and immediately after, addition of the Ant-BH3 or Ant-BH3-A78 peptide. As shown in Figure 8B, treatment of erythrocytes with the Ant-BH3 peptide results in an immediate increase in erythrocyte intracellular calcium levels, whereas treatment with the mutant form of the peptide, Ant-BH3-A78, has no detectable effect on intracellular calcium. Therefore, the Ant-BH3 peptide, which is competent to interact with Bcl-XL, can influence calcium accumulation in the cells, whereas the mutant peptide, which cannot interact with Bcl-XL, does not have an effect on calcium accumulation. These data suggest that antagonization of Bcl-XL in erythrocyte membranes can alter calcium accumulation and suggests that Bcl-XL may function to control erythrocyte survival through regulation of calcium homeostasis.
BH3 peptide–induced cell death and intracellular calcium in erythrocytes.
Induction of cell death by the BH3 peptide increases erythrocyte intracellular calcium. (A) Intracellular calcium levels as determined by the Fluo-3 AM probe. Erythrocytes cultured in the presence and absence of plasma were loaded with the calcium probe Fluo-3 AM and analyzed by flow cytometric analysis immediately following initial cell culture and at 4 and 6 days respectively. The thin line represents erythrocyte autofluorescence. Fluo-3 fluorescence is represented in bold. (B) Intracellular calcium levels in the presence of Ant-BH3 and Ant-BH3-A78. Fluo-3 fluorescence is represented over time prior to and following addition of the peptides.
BH3 peptide–induced cell death and intracellular calcium in erythrocytes.
Induction of cell death by the BH3 peptide increases erythrocyte intracellular calcium. (A) Intracellular calcium levels as determined by the Fluo-3 AM probe. Erythrocytes cultured in the presence and absence of plasma were loaded with the calcium probe Fluo-3 AM and analyzed by flow cytometric analysis immediately following initial cell culture and at 4 and 6 days respectively. The thin line represents erythrocyte autofluorescence. Fluo-3 fluorescence is represented in bold. (B) Intracellular calcium levels in the presence of Ant-BH3 and Ant-BH3-A78. Fluo-3 fluorescence is represented over time prior to and following addition of the peptides.
Discussion
In this study, we investigated the mechanisms of erythrocyte survival and death by establishing an in vitro culture system whereby erythrocyte survival could be modulated. Supplementation of the cell-culture medium with PPP doubled the lifespan of the cultured cells and also significantly delayed the cell-surface appearance of the erythrocyte senescent marker PS. These findings suggest that plasma contains essential survival factors for erythrocytes that act to control their lifespan in vivo. Erythrocytes are enucleated cells and do not undergo cell division following terminal differentiation. Therefore, it is likely that plasma acts purely to provide survival signals that act via transcription-independent signaling pathways. Interestingly, plasma has also been found to promote the survival of platelets,24 and in a separate study, p38 signaling has been associated with platelet survival.21 Human plasma contains high levels of circulating IGFs that act to promote survival of erythroid progenitor cells and most other hematopoietic cells. However, we failed to block plasma survival activity with an anti–IGF-I receptor–blocking antibody that we have previously shown to block the survival activity of IGF-I/IGF-II in many cell types, including T cells33 (data not shown). This finding was supported by the observation that bovine serum, which provides survival and growth factors for most cell cultures, did not provide any survival advantage in erythrocyte cultures. Efforts are currently underway to further characterize the survival activity in plasma.
Expression of the prosurvival Bcl-XL protein in erythrocytes suggested a role for this Bcl-2 family member in erythrocyte survival. Expression of Bcl-XL in mature erythrocytes has not been previously detected, but use of several controls, including cytochrome c oxidase and SHP-1, as well as reticulocyte depletion of erythrocyte samples to detect potential cross-contamination by leukocytes or reticulocytes confirmed that the Bcl-XL protein detected in our erythrocyte preparations was expressed in mature erythrocytes. Given the importance of Bcl-XL in promoting the survival of erythroid progenitor cells during differentiation,15,34 it is not wholly surprising that it could also regulate the survival of the mature erythrocyte. Bcl-XL reaches maximum protein and transcript levels at the time of maximum hemoglobin synthesis.35 A role for Bcl-XL in erythrocyte survival is also supported by a conditional knockout of the murine Bcl-XL gene in the erythroid lineage that resulted in pronounced anemia, enhanced cell death in late erythroid progenitor cells, and possibly decreased lifespan of mature erythrocytes.36
To investigate whether Bcl-XL was active in promoting erythrocyte survival, we exposed erythrocytes to a known Bcl-XL antagonist, the BH3 domain of its binding partner Bak. The Bak-BH3 peptide fused to the internalization domain of the Drosophila antennapedia protein caused rapid induction of erythrocyte cell death. Neither the Ant peptide alone nor a mutant Ant-BH3 peptide that cannot bind to Bcl- XL25,37caused cell death. This Ant-BH3 peptide has previously been demonstrated to be rapidly internalized in HELA cells and to induce apoptosis in HELA cells via antagonization of Bcl-XLsurvival function.25 We therefore conclude that the BH3 peptide induces cell death in erythrocytes in a similar way through antagonism of Bcl-XL function. The corollary of this conclusion is that Bcl-XL acts to promote erythrocyte cell survival.
The mechanism of BH3-induced death in erythrocytes is of interest both in terms of understanding erythrocyte survival as well as in terms of understanding a caspase-independent death regulation by the Bcl-2 family in different cells. We found no evidence of caspase involvement in erythrocytes either by protein detection or by using the broad-spectrum inhibitor zVAD-fmk, although, these BH3 peptides have been demonstrated to depend on caspase activity in HELA cells.25 The BH3 peptide could interact with proteins other than Bcl-XL in erythrocytes. However, it has recently been shown that BH3-only proteins cannot modulate cell survival in the absence of Bak or Bax.38,39 This would suggest that the Ant-BH3 protein requires dimerization with Bak or Bax to kill erythrocytes. Caspase-independent mechanisms for BH3-peptide killing of erythrocytes would have to invoke suppression of the normal functions of Bcl-XL in these cells. In other cells, Bcl-XL is expressed in the mitochondrial membranes, where it is thought to regulate mitochondria homeostasis,40,41and in membranes of the endoplasmic reticulum, where its function is less clear.42,43 In mitochondrial membranes, Bcl-XL has been shown to directly interact with voltage-dependent anion channel (VDAC) channels and modulate their function and, possibly, also the function of the permeability transition pore (PTP).44 Several members of the Bcl-2 family, including Bcl-XL, have the ability to associate with themselves or other proteins to form pores or ion channels in membranes.6,7 Thus, it is possible that Bcl-XLforms a channel in the erythrocyte membrane. The rapid increase in intracellular calcium upon treatment with Ant-BH3 suggests that erythrocyte Bcl-XL may regulate calcium flux in the membrane. Therefore, the normal function of Bcl-XL in erythrocytes could be to regulate cell death at the level of a membrane ion channel by a mechanism analogous to its role in preserving membrane integrity and homeostasis in mitochondria. Antagonization of this function by forced expression of a BH3 protein could disrupt the regulation of calcium homeostasis. We also cannot rule out the possibility that Bcl-XL could regulate a large channel like mitochondrial VDAC or PTP that could control the transport of apoptosis-inducing factors (proteins). However, the rapid alteration in calcium flux and the rapid induction of PS exposure on erythrocytes by the BH3 peptide suggest that these 2 events are closely connected and may be sufficient for death. In the erythroleukemic cell line HEL, PS externalization has very recently been shown to depend on store-operated entry of calcium into the cells,45 and there is also considerable evidence to indicate that some of the enzymes involved in PS exposure require calcium.46 47
The expression of Bak in erythrocyte plasma membranes raises the question of whether Bak is expressed in mature or aging erythrocytes to regulate Bcl- XL function. In platelets, where Bcl-2 is thought to contribute to cell survival, levels of its antagonistic partner Bax have been shown to increase with age.23 We investigated whether Bak levels were altered in erythrocyte membranes upon aging in culture or in response to withdrawal of plasma, but could detect no significant changes (not shown). Thus, it is still not clear how Bcl-XL function might be regulated under physiological conditions in aging erythrocytes.
In conclusion, we have shown that the survival of mature erythrocytes in culture can be modulated by plasma and by antagonization of membrane-associated Bcl-XL. These findings support the hypothesis that, like other cells of the body, the survival and lifespan of erythrocytes in vivo is tightly regulated. Identification of some of the proteins and mechanisms involved in controlling erythrocyte survival opens possibilities for manipulation of cell survival under certain conditions, such as storage of red blood cells or in anemia.
We thank Claire O'Herlihy of Mercy Hospital, Cork, Ireland, and our donors for providing blood samples.
Supported by the Health Research Board and the Higher Education Authority of Ireland.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Rosemary O'Connor, Department of Biochemistry, Lee Maltings, National University of Ireland, Cork, Ireland; e-mail:r.oconnor@ucc.ie.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal