Abstract
Notch signaling controls cell fate decisions of hematopoietic progenitors by inhibiting certain steps of differentiation and inducing either self-renewal or differentiation toward lymphoid or myeloid lineages. In addition, truncated Notch1 alleles could be associated with 10% of all cases of human T lymphoblastic leukemia and, when introduced into mouse bone marrow stem cells, cause T-cell neoplasms. However, functional links between the abundant expression of intact Notch1 and oncogenesis are still lacking. Here we show that Notch1 is highly expressed in B- and T-cell–derived tumor cells of Hodgkin and anaplastic large cell lymphoma. We demonstrate a novel mechanism for the oncogenic capacity of Notch1 by showing that the interaction between intact Notch1 on tumor cells and its ligand Jagged1 dramatically induces proliferation and inhibition of apoptosis in vitro. We further provide evidence that in Hodgkin and anaplastic large cell lymphoma, Jagged1 is expressed in malignant and in bystander cells colocalizing with Notch1-positive tumor cells. Notch1 signaling may therefore be activated in tumor cells by Jagged1 through homotypic or heterotypic cell–cell interactions, and it seems likely that these interactions contribute to lymphomagenesis in vivo. Thus, our data suggest that activated Notch1 signaling plays an important role in the pathobiology of Hodgkin and anaplastic large cell lymphoma and that it might be a potential new target for treatment.
Introduction
Hodgkin and Reed-Sternberg (HRS) cells represent clonal progeny of germinal center B cells in most cases of classical Hodgkin disease (cHD).1,2 It has further been demonstrated that HRS cells contain nonfunctional immunoglobulin (Ig) genes, suggesting that they are derived from germinal center cells that should have been negatively selected but were rescued from apoptosis by cellular transforming events.3 4
Our previous work has provided evidence that constitutive NF-κB activity is a survival factor for HRS cells.5-8 We have directly manipulated the NF-κB system and overexpressed a dominant-negative version of the inhibitor IκBα. HRS cells depleted of constitutive nuclear NF-κB reveal decreased proliferation rates, enhanced apoptotic response, and strongly impaired tumor growth in severe combined immunodeficient mice.7 To investigate molecular alterations of the NF-κB/IκB system that might be responsible for constitutive NF-κB activity, we have analyzed the IκBα gene.9 We and others have demonstrated mutations of the IκBα gene in a subset of HD cases that contribute to constitutive NF-κB activation and are involved in the pathogenesis of HD.9,10 To evaluate additional molecular mechanisms that lead to constitutive NF-κB activity in HRS cells, and because activated Notch signaling has been implicated in the regulation of NF-κB,11-13 we analyzed Notch1 gene expression in cultured and primary HRS cells.
Notch1 belongs to a family of transmembrane receptors that control cell proliferation and differentiation in response to extracellular ligands expressed on neighboring cells.14-18 Notch1 has been isolated as a translocation in human acute T-cell lymphoblastic leukemia–lymphoma,19 and its constitutively active form produces T-cell neoplasms in mice.20 These truncated Notch1 proteins have been implicated in the transformation of rat kidney cells through cooperation with adenoviral oncogene E1A.21 In addition, transgenic mice that express the Notch3 intracellular domain under lck promoter control develop aggressive T-cell lymphomas, with tumor cells showing constitutive NF-κB activation.11 However, a pathogenetic role for Notch1 in B-cell neoplasms is unknown.
Here we show that Notch1 is strongly expressed in B-cell–derived HRS cells and in tumor cells of T-cell–derived anaplastic large cell lymphoma (ALCL). Our data indicate that the activation of Notch1 signaling in tumor cells by its ligand Jagged1 regulates tumor cell growth and survival. We suggest that pharmacologic manipulation of the Notch1 system might have therapeutic potential in these lymphomas.
Materials and methods
Cell culture
Human cell lines analyzed in this study were as follows: HD cell lines L428, KM-H2, L1236, L540, HD-LM2; ALCL cell lines Karpas 299 and SU-DHL1; BL cell line Daudi and Namalwa; and myeloma cell line U266 (DSMZ, Braunschweig, Germany). Cell lines were maintained in RPMI 1640 (Biochrom, Berlin, Germany), 10% heat-inactivated fetal calf serum (Gibco, Karlsruhe, Germany). Peripheral blood lymphocytes were isolated from citrated venous blood from healthy donors. Mononuclear cells were separated by centrifugation on Ficoll gradients (Biochrom) for 30 minutes at 1200g. The interphase was harvested, and the cells were washed 3 times in phosphate-buffered saline (PBS) and were frozen at −80°C until use. CD19+ B cells and CD2+ T cells were enriched using the Dynabead system (Dynal, Hamburg, Germany). In brief, mononuclear cell suspension was incubated at 4°C for 30 minutes with Dynabeads coated with a monoclonal antibody specific for either the human B-cell–restricted membrane antigen CD19 or the human T-cell surface marker CD2. CD19+ and CD2+ cells were selected by a magnetic separation device and were resuspended in PBS.
HtTA-jag10 cells expressing human Jagged1 under tetracycline control, as described previously,13 (HeLa-derived cell line) were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum, hygromycin (225 U/mL; Calbiochem, Bad Soden, Germany), G418 (125 μg/mL; Gibco), and tetracycline (2 μg/mL; Sigma, Deisenhofen, Germany). For cocultivation assays HtTA-jag10 cells were plated in 10-cm dishes. Jagged1 expression was induced by washing HtTA-jag10 cells 3 times with DMEM without tetracycline 24 hours and 48 hours after plating. After 72 hours, cells were cocultured with lymphoma cell lines (2.0 × 106 cells/dish) for 48 hours. Lymphoma cells that were aggregated onto the HtTA-jag10 monolayer were harvested by firm tapping before RNA extraction.
Immunohistochemistry
Five-micrometer–thick sections of paraffin-embedded tissue blocks were immunostained using the immunoalkaline-phosphatase method. Primary antibodies were rat monoclonal human anti-Notch1 antibody bTAN 20 specific for the intracellular domain of Notch1 and human anti-Jagged1 antibody TS1-15H specific for the intracellular domain of Jagged1 (Developmental Studies Hybridoma Bank, Iowa City, IA). Antibodies were used in a concentration of 1:2 at 4°C overnight or 1:5 at room temperature for 1 hour. Antibodies were visualized with the immunoalkaline–phosphatase technique using new fuchsin as chromogen.
In situ hybridization
The cRNA probe was prepared by subcloning a humanJagged1 gene cDNA fragment (834 base pair [bp]) in the run-off transcription vector pGEM-T (Promega Biotec, Heidelberg, Germany). After linearization, run-off antisense transcripts with the incorporation of 35S-labeled UTP were generated using T7 RNA polymerases (Promega-Biotech, Madison, WI). In situ hybridization for the detection of Jagged1 transcripts was performed using microwave irradiation before the hybridization. Slides were hybridized with 4 × 105 cpm of labeled probes overnight at 50°C.
Immunoblotting
Cell extracts were prepared and quantitated as described.7 Proteins (20 μg) were resolved by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis and were transferred to nitrocellulose membranes. Protein load was normalized by Ponceau red staining. Membranes were incubated with rat monoclonal anti-Notch1 antibodies (bTAN 20) and anti-Notch2 antibodies (bhN6D), followed by goat antirat horseradish peroxidase–conjugated antibodies (Dianova, Hamburg, Germany), and were detected by enhanced chemiluminescence (Amersham Pharmacia, Freiburg, Germany).
Northern blot analysis
Total RNA preparations were performed using the guanidinium isothiocyanate–phenol chloroform method as described previously.8 For Northern blot analysis, 10 μg total RNA was subjected to gel electrophoresis on a 1.1% formaldehyde–1.2% agarose gel and was transferred to a nylon membrane (Appligene, Heidelberg, Germany). After UV cross-linking, the membrane was prehybridized (ExpressHyb hybridization solution; Clontech, Heidelberg, Germany) at 68°C for 1 hour. Blots were hybridized with a32P-random prime-labeled DNA probe overnight at 68°C. Probes were rat Hes-1 (723 bp of coding region) and human GAPDH (249 bp of coding region) cDNAs. Membranes were washed for 40 minutes at room temperature in 2 × SSC and 0.1% SDS and then for 40 minutes at 50°C in 0.5% SSC and 0.1% SDS.
Proliferation assay
HtTA-jag10 cells were plated in microtiter wells (2 × 103 cells/100 μL DMEM). Plates were washed with DMEM without tetracycline for the induction of Jagged1 after 24 and 48 hours. After 72 hours, HtTA-jag10 cells were irradiated with 100 Gy and were cocultured with lymphoma cells (2-4 × 104cells/well). Triplicate samples were cultured for 24 hours at 37 °C in the presence or absence of tetracycline. 3[H] thymidine (1μCi [3.7 MBq]/100 μL DMEM) was added to each well for 20 hours before a determination was made of radioisotope incorporation into DNA. Soluble human Jagged1 was kindly provided by Seiji Sakano (Asahi Kasei, Shizuoka, Japan). This ligand is a fusion protein in which the extracellular domain of Jagged1 was fused in frame to the Fc part of human IgG1 (hJagged1-IgG). To cluster soluble ligand molecules, we used mouse L cells, stably expressing Fc gamma RII/CDw32.22 Then 2 × 104 L cells were seated in 96-well microtiter plates and irradiated with 75 Gy, after which 10μg/mL human IgG (control treated) or soluble hJagged1-IgG was coated onto the L-cell monolayer for 1 hour at 37°C. Plates were washed with PBS, and lymphoma cells were cocultured with L cells. Twenty-four hours later, 3[H] thymidine (1μCi [3.7 MBq]/100 μL DMEM) was added to each well for another 20 hours before a determination was made of radioisotope incorporation into DNA.
Apoptosis assay
After the induction of apoptosis by 10 μM sodium arsenite (Sigma), cocultured cells were harvested and washed with PBS. CD30+ HRS cells were detected with human monoclonal anti-CD30 antibodies (DAKO, Glostrup, Denmark) and stained with F(ab)2 phycoerythrin (PE)-conjugated antispecies IgG (Dianova). Free-binding groups of the secondary antibody were blocked by incubating cells with 10% mouse serum. Apoptotic cells were stained for 15 minutes with fluorescein isothiocyanate–labeled annexin V (Bender MedSystems, Vienna, Austria). Data were collected and analyzed with Becton Dickinson FACScan and CellQuest software (Becton Dickinson, Heidelberg, Germany).
Results
Notch1 is highly expressed in tumor cells of Hodgkin and anaplastic large cell lymphoma
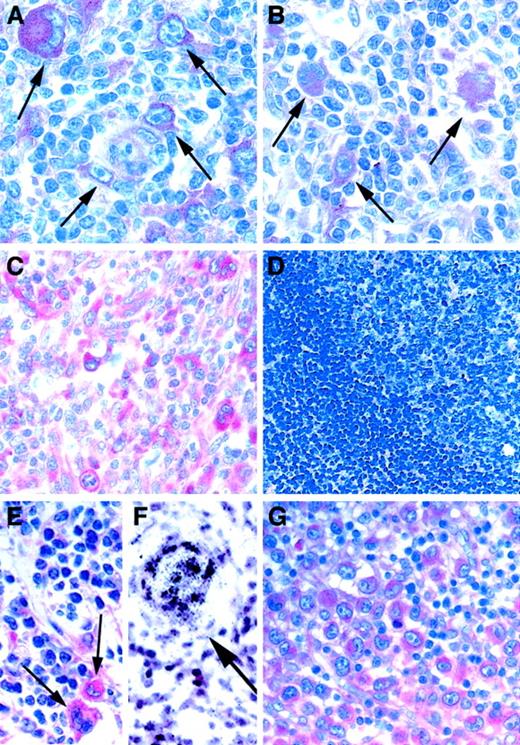
We analyzed Notch1 expression in 25 cases of B-cell–derived cHD using a monoclonal antibody specific for the intracellular domain of Notch1 (Table 1). Applying immunohistochemistry we observed intensive Notch1 staining in HRS cells in all 25 cases of cHD, including nodular sclerosis and mixed cellularity subtypes (Figure 1A-B), though we found low to undetectable levels of immunoreactivity for Notch1 in reactive lymphoid tissues that served as a negative control (Figure 1D). Because infection with Epstein-Barr virus (EBV) has been linked to the pathogenesis of HD23 and because EBV nuclear antigen 2 (EBNA-2) and activated Notch1 are regarded as functional homologs that deregulate B-cell–specific gene expression,24 we were interested to know whether the expression of Notch1 correlates with EBV infection. However, we could not observe any correlation between these parameters using our immunohistochemical data (Table 1). Interestingly, using immunohistochemistry and in situ hybridization, we found abundant protein and mRNA expression of the Notch1 ligand, Jagged1, in HRS cells (Figure 1E), in endothelial and smooth muscle cells (Figure 1F), and in epithelioid cells neighboring HRS cells. These data suggest that Jagged1-induced Notch1 signaling might contribute to the pathobiology of cHD.
Notch1 expression in human malignant B- and T-cell lymphoma entities
| No. cases . | Lymphoma entity . | Notch1 protein expression* . | Epstein-Barr-virus‡ . | ALK-protein† . | ||
|---|---|---|---|---|---|---|
| Absent . | Weak . | Strong . | ||||
| 8 | B-CLL1-153 | 3 | 5 | — | ND | ND |
| 9 | Mantle cell lymphoma | 5 | 4 | — | ND | ND |
| 10 | Follicle center lymphoma | 2 | 8 | — | ND | ND |
| 8 | Marginal zone B-cell lymphoma | 3 | 5 | — | ND | ND |
| 10 | Diffuse large B-cell lymphoma | — | 10 | — | ND | ND |
| 8 | Sporadic Burkitt lymphoma | — | 8 | — | — | ND |
| 25 | Classical HD | — | — | 25 | 14 | — |
| 12 | ALCL | — | — | 12 | — | 9 |
| No. cases . | Lymphoma entity . | Notch1 protein expression* . | Epstein-Barr-virus‡ . | ALK-protein† . | ||
|---|---|---|---|---|---|---|
| Absent . | Weak . | Strong . | ||||
| 8 | B-CLL1-153 | 3 | 5 | — | ND | ND |
| 9 | Mantle cell lymphoma | 5 | 4 | — | ND | ND |
| 10 | Follicle center lymphoma | 2 | 8 | — | ND | ND |
| 8 | Marginal zone B-cell lymphoma | 3 | 5 | — | ND | ND |
| 10 | Diffuse large B-cell lymphoma | — | 10 | — | ND | ND |
| 8 | Sporadic Burkitt lymphoma | — | 8 | — | — | ND |
| 25 | Classical HD | — | — | 25 | 14 | — |
| 12 | ALCL | — | — | 12 | — | 9 |
Rat monoclonal antibody against human Notch1.
Fusion of the nucleophosmin gene (NPM) to ALK.
Epstein-Barr virus–encoded RNA.
Patients with B-cell chronic lymphocytic leukemia. ND, not determined.
Lymph node biopsy specimens of cHD and ALCL.
(A-D) Paraffin-embedded sections immunostained for Notch1 and counterstained with hematoxylin. HRS cells (A, B; arrows) with bilobed or multilobed nuclei, huge inclusionlike nucleoli, and abundant cytoplasm and (C) large pleomorphic cells with prominent nucleoli, ALCL tumor cells, are intensely labeled by anti-Notch1 antibodies. Bystander cells and reactive lymphoid tissue (D) show low to undetectable immunoreactivity levels against Notch1. (E, G) Immunostaining for Jagged1. Jagged1 is expressed in HRS cells (E, arrows) and in tumor cells of ALCL (G). (F) Radioactive in situ hybridization of a case of cHD with probes specific for Jagged1. Endothelial and smooth muscle cells show abundant Jagged1 mRNA expression (arrow). Original magnification × 200 (A-C), × 100 (D), × 200 (E-G).
Lymph node biopsy specimens of cHD and ALCL.
(A-D) Paraffin-embedded sections immunostained for Notch1 and counterstained with hematoxylin. HRS cells (A, B; arrows) with bilobed or multilobed nuclei, huge inclusionlike nucleoli, and abundant cytoplasm and (C) large pleomorphic cells with prominent nucleoli, ALCL tumor cells, are intensely labeled by anti-Notch1 antibodies. Bystander cells and reactive lymphoid tissue (D) show low to undetectable immunoreactivity levels against Notch1. (E, G) Immunostaining for Jagged1. Jagged1 is expressed in HRS cells (E, arrows) and in tumor cells of ALCL (G). (F) Radioactive in situ hybridization of a case of cHD with probes specific for Jagged1. Endothelial and smooth muscle cells show abundant Jagged1 mRNA expression (arrow). Original magnification × 200 (A-C), × 100 (D), × 200 (E-G).
In contrast, in 8 cases of B-cell–derived sporadic Burkitt lymphoma (BL) associated with c-Myc deregulation, which is critical for malignant transformation and is known to collaborate with Notch1 for oncogenesis of murine T-cell tumors,25 tumor cells showed low to undetectable levels of Notch1 expression (Table 1). In addition, in most cases of other B-cell–derived non-Hodgkin lymphoma entities, we detected only weak immunoreactivity for Notch1 in tumor cells when compared to Notch1 highly expressed in HRS cells (Table 1).
Interestingly, the tumor cells of all cases of T-cell–derived ALCL were also intensely labeled by Notch1 antibodies (Figure 1C). CD30+ ALCL resembles HD because of the CD30+tumor cell immunophenotype and the presence of occasional RS cells.23 High expression levels of Notch1 in ALCL were independent of the presence or absence of the unique translocation t(2;5) (Table 1) that involves the fusion of the nucleophosmin geneNPM to an anaplastic lymphoma kinase (ALK) regarded as an important transforming event of most ALCLs.23 Using immunohistochemistry, we further showed that ALCL tumor cells were also positive for the Notch1 ligand Jagged1 in all cases tested (Figure 1G).
Notch1 signaling is activated by Jagged1 in cultured HRS and ALCL cells
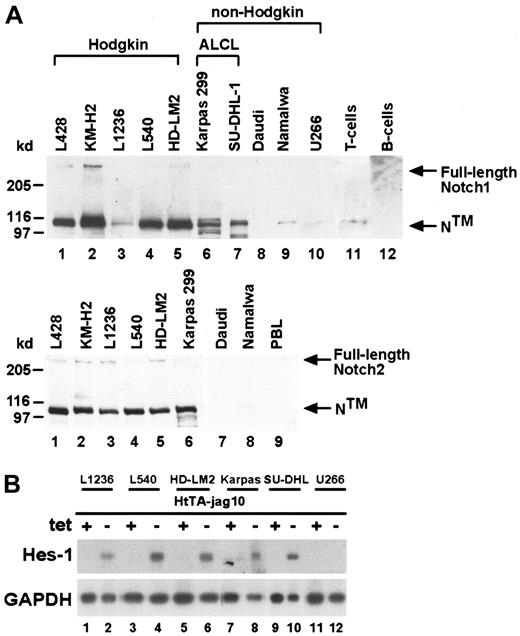
To verify high Notch1 expression in lymphoma-derived cell lines, we performed Western blot analysis using Notch1 antibodies directed against the intracellular domain of the receptor. Apart from the inactive full-length protein (300 kd), we detected as the predominant species a 110-kd fragment (Figure 2A, upper panel). This fragment represents the transmembrane and intracellular domains (NTM) and results, along with the extracellular fragment (NEC), from proteolytic cleavage of full-length Notch1. NTM and NEC are linked together and appear as the active ligand-accessible form of the receptor on the cell surface.26 In cultured HRS cells (Figure 2A; lanes 1-5), we observed high expression of NTMwhen compared with mature CD19+ B cells (Figure 2A; lane 12). In addition, both ALCL cell lines (Figure 2A; lanes 6,7) displayed higher amounts of NTM than did their normal counterparts (Figure 2A; lane 11). In contrast, neither the BL cell lines (Figure2A; lanes 8,9) nor the myeloma cell line (Figure 2A; lane 10) expressed significant levels of Notch1. Similarly, we observed higher Notch2 protein expression in HRS and ALCL cells than in BL cells and peripheral blood lymphocytes (Figure 2A, lower panel). These results are in accordance with a previous report showing by microarray experiments that in HD cell lines (L428, KM-H2, and HD-LM2), the expression of Notch2 mRNA is increased compared with lymphoblastoid EBV-infected B-cell lines.27 Our data here indicate that the cell lines derived from HD, ALCL, and BL used in this study resemble tumor cells in vivo with respect to Notch1 expression. They might represent a valuable model by which to assess Notch1-associated pathobiologic mechanisms in vitro. However, in contrast to primary HRS and ALCL cells, cultured tumor cells showed low to undetectable levels of the Notch1 ligand Jagged1 in Western blot analysis (data not shown).
High Notch1 and Notch2 expression in cultured HRS and ALCL cells.
(A) Upper panel: Western blot analysis of cell lysate proteins from Hodgkin (lanes 1-5) cell lines, non-Hodgkin (lanes 6-10) cell lines, mature CD2+ T cells (lane 11), and mature CD19+B cells (lane 12) showing bands of full-length Notch1 (300 kd) and 110-kd major cleavage product (NTM). Lower panel: Notch2 protein expression in cell lines indicated. (left margin) Size markers in kilodalton. (B) Cocultivation of HtTA-jag10 cells expressing the Notch1 ligand, Jagged1, under tetracycline control and lymphoma cells expressing various amounts of endogenous Notch1 receptors. Jagged1 expression in HtTA-jag10 cells was either uninduced (+tet) or induced (−tet). Northern blot analysis showing increased levels of endogenous Hes-1 transcripts in HRS (lanes 1-6) and ALCL cells (lanes 7-10) after activation by Jagged1. The myeloma cell line U266 served as a negative control (lanes 11, 12).
High Notch1 and Notch2 expression in cultured HRS and ALCL cells.
(A) Upper panel: Western blot analysis of cell lysate proteins from Hodgkin (lanes 1-5) cell lines, non-Hodgkin (lanes 6-10) cell lines, mature CD2+ T cells (lane 11), and mature CD19+B cells (lane 12) showing bands of full-length Notch1 (300 kd) and 110-kd major cleavage product (NTM). Lower panel: Notch2 protein expression in cell lines indicated. (left margin) Size markers in kilodalton. (B) Cocultivation of HtTA-jag10 cells expressing the Notch1 ligand, Jagged1, under tetracycline control and lymphoma cells expressing various amounts of endogenous Notch1 receptors. Jagged1 expression in HtTA-jag10 cells was either uninduced (+tet) or induced (−tet). Northern blot analysis showing increased levels of endogenous Hes-1 transcripts in HRS (lanes 1-6) and ALCL cells (lanes 7-10) after activation by Jagged1. The myeloma cell line U266 served as a negative control (lanes 11, 12).
To test whether Jagged1-induced Notch1 signaling directly activates downstream target genes,13 we cultured HRS and ALCL cells in the presence of Jagged1 to study mRNA expression of the mammalian basic helix–loop–helix transcription factor, hairy enhancer of split (Hes-1; Figure 2B). To that end we performed cocultivation assays using feeder cells expressing Jagged1 under tetracycline control (HtTA-jag10 cells)13 and Notch1-expressing lymphoma cells. Using Northern blot analysis, we demonstrated that in cultured HRS and ALCL cells, Hes-1–specific transcripts were up-regulated (Figure 2B, lanes 1-6; Figure 2C, lanes 7-10) after the induction of Jagged1 by tetracycline. This phenomenon was not observed in myeloma cells that did not express Notch1 (Figure 2B; lanes 11,12). Taken together, these data strongly suggest that intact Notch1 signaling of cultured HRS and ALCL cells can be induced by the ligand expressed on neighboring cells.
Activated Notch1 signaling accelerates growth and inhibits arsenite-induced apoptosis of lymphoma cells
As we observed the co-expression of Jagged1 and Notch1 in tumor cells and the colocalization of Jagged1-expressing bystander cells and Notch1-positive tumor cells on tumor biopsy specimens, we investigated the biologic significance of the functional interaction between Notch1 and Jagged1. One important role of the aberrant high expression of Notch1 in tumor cells of HD and ALCL may be to mimic a continuous growth-promoting signal. To study whether the activation of highly expressed Notch1 by Jagged1 would affect cell growth, we performed proliferation assays and measured 3[H] thymidine uptake using the cocultivation assay described above. Surprisingly, stimulation by Jagged1 dramatically increased the proliferation rates of HRS and ALCL cells up to 2- or even 3-fold in 20 hours compared with unstimulated controls (Figure 3A). Because the cell-cycle progression of these cell lines normally lasts between 40 and 48 hours, our data indicate an exponential increase in proliferation rates. These data were confirmed through comparison of the actual cell numbers of Jagged1-induced and -uninduced lymphoma cells (data not shown). In contrast, we could not induce a significant proliferative response in BL or myeloma cell lines. To further address whether the promotion of growth did not occur through the engagement of Notch on target cells but through Jagged1 on feeder cells—which also activated Notch signaling on the feeder cells and thereby changed the expression of other proteins—we used purified, clustered, soluble Jagged1 (Figure 3B). Stimulation of HD and ALCL cells by soluble Jagged1 increased their proliferation rates up to 3-fold compared to control treatment. These data confirm our results with Jagged1-expressing feeder cells and demonstrate that soluble Jagged1 is a potent growth factor for Notch-expressing lymphoma cells. We conclude that the interaction between Jagged1 and intact Notch1 strongly accelerates the proliferation of HRS and ALCL cells and may, therefore, contribute to a transformation process in vivo.
Activated Notch1 signaling promotes growth and survival of lymphoma cells.
(A) Proliferative responses of Notch1-expressing lymphoma cells after activation by Jagged1. After cocultivation for 24 hours, lymphoma cells were pulsed for another 20 hours with 3[H] thymidine and were harvested for scintillation counting. Results are shown as the mean of 3[H] thymidine incorporation (± SD) of 3 independent experiments. Counts of activated lymphoma cells (presence of Jagged1; −tet, black bars) are given relative to counts of nonactivated cells (absence of Jagged1; +tet, white bars) which were set arbitrarily at 100% for each cell line. (B) Proliferative responses of Notch1-expressing lymphoma cells after activation by soluble Jagged1. Counts of activated lymphoma cells (presence of soluble hJagged1; black bars) are given relative to counts of nonactivated cells (white bars), which were set arbitrarily at 100% for each cell line. *P < .05; **P < .001; n.s., not significant using the Student t test. (C) Activated Notch1 signaling inhibits the apoptosis of HRS cells. After cocultivation of lymphoma cells in the presence (−tet, black bars) or absence (+tet, white bars) of Jagged1, apoptosis was induced by 10 μM arsenite. Counts of apoptotic cells were determined and are given in percentages. *P < .05.
Activated Notch1 signaling promotes growth and survival of lymphoma cells.
(A) Proliferative responses of Notch1-expressing lymphoma cells after activation by Jagged1. After cocultivation for 24 hours, lymphoma cells were pulsed for another 20 hours with 3[H] thymidine and were harvested for scintillation counting. Results are shown as the mean of 3[H] thymidine incorporation (± SD) of 3 independent experiments. Counts of activated lymphoma cells (presence of Jagged1; −tet, black bars) are given relative to counts of nonactivated cells (absence of Jagged1; +tet, white bars) which were set arbitrarily at 100% for each cell line. (B) Proliferative responses of Notch1-expressing lymphoma cells after activation by soluble Jagged1. Counts of activated lymphoma cells (presence of soluble hJagged1; black bars) are given relative to counts of nonactivated cells (white bars), which were set arbitrarily at 100% for each cell line. *P < .05; **P < .001; n.s., not significant using the Student t test. (C) Activated Notch1 signaling inhibits the apoptosis of HRS cells. After cocultivation of lymphoma cells in the presence (−tet, black bars) or absence (+tet, white bars) of Jagged1, apoptosis was induced by 10 μM arsenite. Counts of apoptotic cells were determined and are given in percentages. *P < .05.
Moreover, there is substantial evidence that, apart from the involvement of Notch1 in proliferation events, programmed cell death can be affected by Notch1 signaling.14,15 Given that, we used the above-described cocultivation assay and tested whether activated Notch1 inhibits the susceptibility of HRS cells to arsenite-induced apoptosis. We used this compound because it can promote apoptosis through the inhibition of NF-κB activation.28 Consequently, we treated cocultured HRS cells, which are strongly protected against apoptosis by constitutive NF-κB activation. Interestingly, arsenite induced apoptosis in up to 40% of HRS cells, in contrast to various apoptotic stimuli that failed to cause programmed cell death.7 Using annexin V labeling, we observed that the activation of Notch1 signaling led to a 50% decrease in the frequency of apoptotic HRS cells compared with unstimulated controls (Figure 3C). We conclude that highly expressed Notch1 is a novel growth and survival factor for cultured HRS cells.
Discussion
In this study we analyzed Notch1 expression in human malignant B- and T-cell lymphoma entities. We show here that Notch1 protein is highly expressed in tumor cells of cHD and ALCL and that the Notch1 ligand, Jagged1, is expressed in primary HRS and ALCL cells and in bystander cells neighboring tumor cells. Although Notch and its ligands are often expressed on the same cell, it has been shown that Notch is activated primarily through binding to its ligand on adjacent cells.29 It seems conceivable, therefore, that Notch1 signaling can be activated by Jagged1 in primary HRS and ALCL cells through homotypic or heterotypic cell–cell interactions. Notch interactions cause a dramatic increase in growth rates of cultured HRS and ALCL cells and might also influence tumor biology.
HRS cells are known to produce cell surface receptors and cytokines to create their own reactive cell microenvironment, which contributes to stimulation of their growth and survival. We recently described that HRS cells induce fibroblasts by tumor necrosis factor-α to secrete eotaxin (CCL11), which recruits T cells and eosinophils into tumor tissue.7 T cells and eosinophils transmit through CD40 ligand (CD154) proliferative and antiapoptotic signals to CD40, a member of the tumor necrosis factor receptor superfamily, on HRS cells.30 CD40 is highly expressed in HRS cells and plays a critical role in the proliferation and inhibition of B-cell apoptosis.31,32 In addition, HRS cells express the CC chemokine TARC (CCL17), which may contribute to the characteristic T-cell infiltrate surrounding HRS cells. Furthermore, a recent study showed that in addition to other cytokines, interleukin-13, secreted by HRS cells, plays an important role in the stimulation of HRS cell growth, possibly by an autocrine mechanism.27
In addition, our data show that activated Notch1 signaling induces expression of the downstream target gene, Hes-1, in cultured HRS and ALCL cells. Given the colocalization of Jagged1-expressing cells and Notch1-positive tumor cells in malignant lymphoid tissue, it seems likely that this interaction also leads to activated Notch1 signaling in vivo. Our data suggest that Jagged1-induced Notch1 signaling might contribute to the pathobiology of cHD and ALCL.
Our study reveals a novel mechanism for potential oncogenic activity of Notch1 not only in T-cell but also in B-cell neoplasms. We have shown that Notch1 signaling, activated by its ligand Jagged1, dramatically accelerates the growth and inhibits the apoptosis of cHD and ALCL tumor cells. It has been shown that the increased expression of Notch1 is associated with the transformed phenotype of epithelial cells in cervical, lung squamous, and colon adenocarcinomas.33Furthermore, a series of recent studies revealed that Notch influences apoptosis and proliferation.11,14,34-37 Our data indicate that direct ligand receptor interactions can account for the oncogenic capacity of Notch1. It is, however, conceivable that Notch1-induced transformation of hematopoietic lineages requires the collaborative action of additional cellular oncogenes.38
Recently, Jagged1 has been identified as a novel growth factor of human stem cells.18 This study provides evidence that Jagged1 can maintain and expand primitive hematopoietic cells capable of multilineage reconstitution in vivo.18 Given the great potential of Jagged1 to enhance the proliferation and survival of HRS cells in vitro, it is feasible that Jagged1-expressing monolayers can facilitate the difficult generation of new Hodgkin cell lines from primary biopsy samples. Studies to prove this hypothesis are under way.
In normal hematopoiesis, activation of the Notch pathway can result in a block of differentiation.14-16 HRS cells originate from single clones of germinal center B cells because they have functional immunoglobulin gene rearrangements and carry high loads of somatic immunoglobulin mutations. However, despite the presence of clonal immunoglobulin rearrangements, immunoglobulin transcription is impaired, correlating with the down-regulated synthesis of the B-cell transcription factors BOB.1/OBF.1 and Oct2.39 Because Notch1 signaling down-regulates Igμ expression,24 40 it is possible that Notch1 interrupts the differentiation process of HRS cells, and this may act in concert with constitutively activated NF-κB to transform germinal center B cells.
Finally, this study suggests that the down-regulation of Notch1 signaling may be used to control proliferation and to enhance apoptosis of tumor cells of cHD and ALCL. Modulation of the Notch pathway may provide a valuable tool to manipulate the transformed state of malignant lymphatic cells, thereby potentially offering a novel therapeutic approach.
We thank Katharina Kley for excellent technical assistance; Dr Kurt Bommert for comments on the manuscript; Dr Hans-Dieter Foss for performing Jagged1-encoded in situ hybridization; and Dr Celine Gelinas from the University of Medicine and Dentistry of New Jersey for the gift of HtTA-jag10 cells. Soluble human Jagged1 was kindly provided by Seiji Sakano (Asahi Kasei, Shizuoka, Japan).
Supported by grant JU 426/1-1 from the Deutsche Forschungsgemeinschaft.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Franziska Jundt, Charité, Robert-Rössle-Klinik, Humboldt University of Berlin, D-13125 Berlin, Germany; e-mail: fjundt@mdc-berlin.de.

![Fig. 3. Activated Notch1 signaling promotes growth and survival of lymphoma cells. / (A) Proliferative responses of Notch1-expressing lymphoma cells after activation by Jagged1. After cocultivation for 24 hours, lymphoma cells were pulsed for another 20 hours with 3[H] thymidine and were harvested for scintillation counting. Results are shown as the mean of 3[H] thymidine incorporation (± SD) of 3 independent experiments. Counts of activated lymphoma cells (presence of Jagged1; −tet, black bars) are given relative to counts of nonactivated cells (absence of Jagged1; +tet, white bars) which were set arbitrarily at 100% for each cell line. (B) Proliferative responses of Notch1-expressing lymphoma cells after activation by soluble Jagged1. Counts of activated lymphoma cells (presence of soluble hJagged1; black bars) are given relative to counts of nonactivated cells (white bars), which were set arbitrarily at 100% for each cell line. *P < .05; **P < .001; n.s., not significant using the Student t test. (C) Activated Notch1 signaling inhibits the apoptosis of HRS cells. After cocultivation of lymphoma cells in the presence (−tet, black bars) or absence (+tet, white bars) of Jagged1, apoptosis was induced by 10 μM arsenite. Counts of apoptotic cells were determined and are given in percentages. *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/9/10.1182_blood.v99.9.3398/6/m_h80922466003.jpeg?Expires=1763472888&Signature=BYdSWi32bc8dOYTL4~vkJzHs5-CThHcr1sqDt3sOCKwlppMg8fmaZwoQlln9HpsiBf9L5lt9arRrUJqnB0fp861~078tIhIW8ORhujX~0F33JzHraCjDgOASvGyopuzlDNJoFcZGej9sASfDl~8pzHeetW-DxPSpC1j12tSiK7H2qbUxayHh4ok9tgN08pJkhswLriyFnRI9Uj73~TQWbEeb7qwq2WSycN-osEeBvAY2L8vVAqdwkt~Yfimzx~-uHbJg~bWtEGkRLNM~-V9XqIvh2yr4Q8CBzuTlsVp7UifPXxTMFOnvtoT1tW1Uuuqcz-yKf2P9MAwLvNZzylu6Kw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal