Abstract
From 1992 to 2000, we identified 23 lymph node biopsies with focal germinal centers (GCs) containing centrocytes staining strongly for bcl-2 protein, whereas most of the remaining lymph node showed bcl-2–negative follicular hyperplasia. We propose the designation in situ localization of follicular lymphoma (FL) for this phenomenon. In 2 additional cases, bcl-2+ follicles with features of in situ FL were identified in association with other low-grade B-cell lymphomas. To investigate the clonality of the bcl-2+follicles, we performed laser capture microdissection of bcl-2+ and bcl-2 follicles from the same lymph node in 5 cases, and analyzed them in parallel by polymerase chain reaction (PCR) amplification of immunoglobulin heavy chain (IgH) genes. In 4 of 5 cases the bcl-2+ follicles contained monoclonal IgH gene rearrangements, whereas the bcl-2− GCs exhibited a polyclonal ladder. A BCL2/JH gene rearrangement was detected in 6 of 14 (43%) evaluable cases. There were 5 patients with synchronous evidence of FL at another site. There were 13 patients who, without a prior diagnosis of FL, had clinical follow-up; one developed FL in an adjacent lymph node within one year, and 2 manifested FL at 13 and 72 months, respectively. There are 10 patients who have not yet shown other evidence of FL. These results suggest that at least close to half of these cases (8/18; 44%) represent homing to and early colonization of reactive GCs by FL. Other cases might represent FL at the earliest stage of development, or a preneoplastic event, requiring a second hit for neoplastic transformation. These findings provide insight into the pathophysiology of early FL, and illustrate the utility of immunohistochemistry for early diagnosis.
Introduction
Follicular lymphoma (FL) is the most common adult lymphoma in Western countries, comprising 35% to 45% of all non-Hodgkin lymphomas, and 75% to 80% of low-grade B-cell lymphomas.1-3 Despite its indolent nature, FL has remained largely an incurable disease, in part due to its disseminated nature at presentation.4 FL is a mature B-cell neoplasm thought to be derived from follicular center B lymphocytes.5Morphologically and phenotypically, FL closely resembles the normal germinal center (GC) of secondary lymphoid follicles.6 It contains 2 major types of cells normally found in the GCs, centrocytes and centroblasts, and has a follicular growth pattern. The cells express the immunophenotypic markers associated with GC B cells, including CD10 and bcl-6. The neoplastic cells are intimately associated with follicular dendritic cells and T cells, which also are components of the normal GC.
FLs are characterized by the t(14;18)(q32;q21),7,8 which juxtaposes the BCL-2 gene on chromosome 18 with the immunoglobulin heavy chain gene locus on chromosome 14, resulting in constitutive expression of bcl-2 protein.9-15 Bcl-2 protein is antiapoptotic, leading to the accumulation of follicular B cells that might otherwise be eliminated by programmed cell death.16-18 The BCL-2 gene is not expressed in normal follicle center cells; inappropriate expression of theBCL-2 oncogene is believed to be the initial event in malignant transformation to FL.19 Not only has molecular genetic analysis of the t(14;18) been used as a genetic marker for follicular lymphoma, but immunohistochemical staining for bcl-2 protein also provides a very useful tool for distinguishing between reactive and neoplastic lymphoid follicles, as neoplastic GCs in most follicular lymphomas express high levels of bcl-2 protein, whereas normal GCs are always negative.20-22
It has been postulated that the BCL-2/JH translocation occurs as a mistake in VDJ rearrangement during normal B-cell differentiation.10 23 Therefore, this molecular event has been proposed to take place at the pre–B-cell stage of differentiation in the bone marrow. This observation has been used as a partial explanation as to why most patients with FL present with disseminated stage III or IV disease with involvement of multiple nodal sites and bone marrow. When lymph node biopsies are performed, architectural effacement is generally observed, with uniform involvement of all follicles. The earliest events associated with lymph node involvement by FL have never been described. It is not known whether the neoplastic cells populate all of the follicles simultaneously, or whether only a very few follicles are initially involved.
In the present study, we describe rare lymph node biopsies that appear to represent early in situ localization of FL within isolated GCs based on immunohistochemical findings and molecular clonality analysis following laser capture microdissection. The 23 affected lymph nodes were detected largely on the basis of aberrant bcl-2 staining in affected follicles. We use the term “in situ localization of FL” because in each case only a very few follicles within the lymph node were involved, and the architectural and cytological features were otherwise benign. In 2 additional cases, in situ FL was identified in lymph nodes containing another low-grade B-cell lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), and lymphoplasmacytic lymphoma, respectively. The goals of our study were to use laser capture microdissection to investigate the clonal nature of the bcl-2+ follicles, their BCL-2 gene rearrangement status, and to compare bcl-2+ and bcl-2− follicles within a single lymph node. We also attempted clinical follow-up to ascertain the clinical significance of these findings.
Materials and methods
Case selection
From 1992 to 2000, approximately 15 000 biopsy specimens were referred to the Hematopathology Section at the National Institutes of Health for consultation, and a diagnosis of follicular hyperplasia was made in 900 cases. Cases of atypical follicular hyperplasia were examined by morphologic and immunohistochemical techniques, including staining for bcl-2 protein. Over this time period, we identified 23 cases that contained focal GCs strongly positive for bcl-2 protein, whereas most of the remaining lymphoid tissue showed bcl-2− follicular hyperplasia. In addition, 2 more cases were identified representing low-grade B-cell lymphomas of other types in which focal residual secondary follicles demonstrated immunophenotypic evidence of in situ FL. Clinical information and follow-up of these patients were obtained from the referring physicians.
Histology and immunohistochemistry
Only paraffin-embedded tissue was available from the diagnostic specimens. Immunohistochemical stains were performed with a panel of monoclonal and polyclonal antibodies (Table1), including CD20, CD3, CD10, MIB1, and bcl-2, using an automated immunostainer (Ventana Medical Systems, Tucson, AZ) according to the company's protocols, with minor modifications. Antigen retrieval was performed using a Tender Cooker (Nordicware, Minneapolis, MN) with citrate buffer. Stains for kappa and lambda light chains were usually performed, but were generally not informative as to the light chain expression by the atypical cells.
Primary antibodies and sources
| Antibody . | Source . | Dilution . |
|---|---|---|
| CD20 (L26) | Dako, Carpinteria, CA | 1:200 |
| CD3 | Dako | 1:100 |
| CD10 | Novacastra, Newcastle upon Tyne, United Kingdom | 1:40 |
| MIB1 | Dako | 1:50 |
| BCL-2 | Dako | 1:20 |
| Kappa | Dako | 1:25 000 |
| Lambda | Dako | 1:25 000 |
| CD5 | Novacastra | 1:50 |
| IgD | Dako | 1:800 |
| CD23 | The Binding Site, Birmingham, United Kingdom | 1:20 |
| Cyclin D1 | Novacastra | 1:10 |
| Bcl-6 | Dako | 1:20 |
| Antibody . | Source . | Dilution . |
|---|---|---|
| CD20 (L26) | Dako, Carpinteria, CA | 1:200 |
| CD3 | Dako | 1:100 |
| CD10 | Novacastra, Newcastle upon Tyne, United Kingdom | 1:40 |
| MIB1 | Dako | 1:50 |
| BCL-2 | Dako | 1:20 |
| Kappa | Dako | 1:25 000 |
| Lambda | Dako | 1:25 000 |
| CD5 | Novacastra | 1:50 |
| IgD | Dako | 1:800 |
| CD23 | The Binding Site, Birmingham, United Kingdom | 1:20 |
| Cyclin D1 | Novacastra | 1:10 |
| Bcl-6 | Dako | 1:20 |
All antibodies used in this study are monoclonal except CD3, kappa, and lambda.
Each case was scored to evaluate the number of bcl-2+follicles and their distribution within the affected lymph node. Based on the number of bcl-2+ follicles, the cases were divided into 3 groups. Cases in group 1 contained fewer than 5 bcl-2+ follicles on bcl-2–immunostained sections; group 2 contained 6 to10; and group 3 contained more than 10. The cases were also scored into 2 groups evaluating the total number of secondary follicles. Cases with less than half of the follicles positive for bcl-2 were scored as “a,” and cases with more than half of the follicles positive for bcl-2 were scored as “b.” Each case was assigned a composite score based on the total number and percentage of follicles involved (Table2, column 4).
Histologic scoring, clinical features, and follow-up
| Case no. . | Age, y/sex . | Site/in situ FL . | Degree of involvement by in situ FL* . | Site/FL . | Months to FL . |
|---|---|---|---|---|---|
| 10 cases with no other evidence of lymphoma | |||||
| 1 | 23/F | Femoral LN | 2/b | — | NEL (96) |
| 2 | 53/F | R axillary LN | 2/a | — | NEL (72) |
| 3 | 63/F | Thyroid | 1/a | — | NEL (58) |
| 4 | 52/F | L axillary LN | 2/b | — | NEL (19) |
| 5 | 28/F | Tonsil/grade III | 1/a | — | NEL (16) |
| 6 | 76/M | R groin LN | 1/a | — | NEL (15) |
| 7 | 37/M | L groin LN | 1/a | — | NEL (7) |
| 8 | 75/F | R axillary LN | 3/b | — | NEL (6) (died of MDS) |
| 9 | 52/F | R cervical LN | 3/b | — | NEL (5) |
| 10 | 41/F | L axillary LN | 1/a | — | NEL (2) median 15.5 |
| 8 cases with subsequent or coexisting FL | |||||
| 11 | 41/M | R axillary LN (1988) | 3/b | Soft tissue, L cheek/FL, grade I (1994) | 72 |
| L neck LN (1992) | 3/b | Paraspinal mass/FL (2000) | |||
| 12 | 55/F | L axillary LN | 3/a | R axillary LN/FL, grade II | 13 |
| 13 | 45/F | R groin LN | 1/a | R groin LN/FL, grade II | 3 |
| 14 | 42/M | R posterior cervical LN | 1/a | R posterior cervical LN/FL, grade II | 0 |
| 15 | 72/M | Cervical LN | 2/b | Parotid mass/FL, grade II | 0 |
| 16 | 55/F | R tonsil | 1/a | L tonsil, FL, grade III | 0 |
| 17 | 65/F | Intralobar LN | 3/b | Lung, FL, grade I | 0 |
| 18 | 58/F | R groin LN | 3/b | L supraclavicular LN/FL, grade II | 0 |
| 5 cases with no available follow-up | |||||
| 19 | 37/F | Retroperitoneal LN | 1/a | — | NA |
| 20 | 68/M | Inguinal LN | 3/b | — | NA |
| 21 | 46/M | Parotid gland | 1/a | — | NA |
| 22 | 44/M | Inguinal LN | 3/a | — | NA |
| 23 | 56/M | LN, site NOS | 3/b | — | NA |
| 2 cases with composite in situ FL and low-grade B-cell lymphoma | |||||
| 24 | 53/M | R & L pelvic LNs | 1/a | CLL/SLL | NA |
| 25 | 63/M | Scalene LN | 2/b | LPL | NA |
| Case no. . | Age, y/sex . | Site/in situ FL . | Degree of involvement by in situ FL* . | Site/FL . | Months to FL . |
|---|---|---|---|---|---|
| 10 cases with no other evidence of lymphoma | |||||
| 1 | 23/F | Femoral LN | 2/b | — | NEL (96) |
| 2 | 53/F | R axillary LN | 2/a | — | NEL (72) |
| 3 | 63/F | Thyroid | 1/a | — | NEL (58) |
| 4 | 52/F | L axillary LN | 2/b | — | NEL (19) |
| 5 | 28/F | Tonsil/grade III | 1/a | — | NEL (16) |
| 6 | 76/M | R groin LN | 1/a | — | NEL (15) |
| 7 | 37/M | L groin LN | 1/a | — | NEL (7) |
| 8 | 75/F | R axillary LN | 3/b | — | NEL (6) (died of MDS) |
| 9 | 52/F | R cervical LN | 3/b | — | NEL (5) |
| 10 | 41/F | L axillary LN | 1/a | — | NEL (2) median 15.5 |
| 8 cases with subsequent or coexisting FL | |||||
| 11 | 41/M | R axillary LN (1988) | 3/b | Soft tissue, L cheek/FL, grade I (1994) | 72 |
| L neck LN (1992) | 3/b | Paraspinal mass/FL (2000) | |||
| 12 | 55/F | L axillary LN | 3/a | R axillary LN/FL, grade II | 13 |
| 13 | 45/F | R groin LN | 1/a | R groin LN/FL, grade II | 3 |
| 14 | 42/M | R posterior cervical LN | 1/a | R posterior cervical LN/FL, grade II | 0 |
| 15 | 72/M | Cervical LN | 2/b | Parotid mass/FL, grade II | 0 |
| 16 | 55/F | R tonsil | 1/a | L tonsil, FL, grade III | 0 |
| 17 | 65/F | Intralobar LN | 3/b | Lung, FL, grade I | 0 |
| 18 | 58/F | R groin LN | 3/b | L supraclavicular LN/FL, grade II | 0 |
| 5 cases with no available follow-up | |||||
| 19 | 37/F | Retroperitoneal LN | 1/a | — | NA |
| 20 | 68/M | Inguinal LN | 3/b | — | NA |
| 21 | 46/M | Parotid gland | 1/a | — | NA |
| 22 | 44/M | Inguinal LN | 3/a | — | NA |
| 23 | 56/M | LN, site NOS | 3/b | — | NA |
| 2 cases with composite in situ FL and low-grade B-cell lymphoma | |||||
| 24 | 53/M | R & L pelvic LNs | 1/a | CLL/SLL | NA |
| 25 | 63/M | Scalene LN | 2/b | LPL | NA |
Each case was scored on bcl-2–stained sections to evaluate the number of bcl-2+ follicles. 1 = < 5 follicles; 2 = 5-10 follicles; 3 = > 10 follicles. Percent of bcl-2+ follicles of total follicles: a = < 50%; b = > 50%.
FL indicates follicular lymphoma; R, right; L, left; NEL, no evidence of lymphoma by means of physical examination, bone marrow biopsy, and whole-body CT-scan; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; and NA, not available.
Laser capture microdissection
Five formalin-fixed lymph node biopsies were available for microdissection. Other cases could not be analyzed by laser capture microdissection (LCM) because (1) deeper sections did not contain affected follicles; (2) only B5 blocks were available; or (3) paraffin blocks were no longer available from the original referring institution. LCM was performed on bcl-2–immunostained slides using a PixCell laser capture microscope (Arcturus Engineering, Santa Clara, CA) as previously described.24 25 Multiple bcl-2+ and bcl-2− follicles from the same lymph node were microdissected and pooled separately. The microdissection was repeated at least twice for each case. The microdissected cells were then immersed in 50 μL Tris buffer (pH 8.0) containing 0.5% Tween, 1 mM ethylenediaminetetraacetic acid (EDTA), and 400 μg/mL proteinase K, and digested overnight at 55°C. After digestion, the enzyme was heat-inactivated for 10 minutes at 95°C. The extract was then used directly for polymerase chain reaction (PCR).
Polymerase chain reaction
Both intact paraffin sections and microdissected tissue were used for PCR analysis. PCR amplification for detecting monoclonal immunoglobulin heavy chain gene rearrangement was performed using primers directed against consensus sequences of the IgH joining (J) region and framework 3 (FR III) variable region of the immunoglobulin heavy chain (IgH) genes, according to the method of Segal et at.26 DNA, extracted from either entire paraffin sections or from 10 μL to 20 μL of the crude extract from microdissected tissue as described above, was used as template. We performed 40 cycles of amplification consisting of denaturation at 94°C for 45 seconds, annealing at 56°C for 1 minute, and elongation at 74°C for 1 minute. The products were then analyzed on 16% polyacrylamide gels and stained with ethidium bromide.
Previously published primers for BCL-2 and JHwere used for the detection of t(14;18) translocation involving the major breakpoint region (MBR).27 The specificity of theBCL-2 amplification product was confirmed by Southern blotting and hybridization with a digoxigenin (DIG)-labeled oligonucleotide. Bound probe was detected with an alkaline phosphatase–labeled anti-DIG Fab fragment and CSPD as chemiluminescence substrate (Boehringer Mannheim, Indianapolis, IN). In all experiments, test samples were run in parallel with positive and negative controls. Approximately 50% to 60% of breakpoints in translocation-positive FL cluster in the MBR, with an overall detection rate in our laboratory and literature of 50%.28 29 Case no. 1 and case no. 19 were evaluated for BCL2/JH by the referring laboratory using PCR for the MBR or Southern blot, respectively.
Results
Histopathology and immunohistochemistry
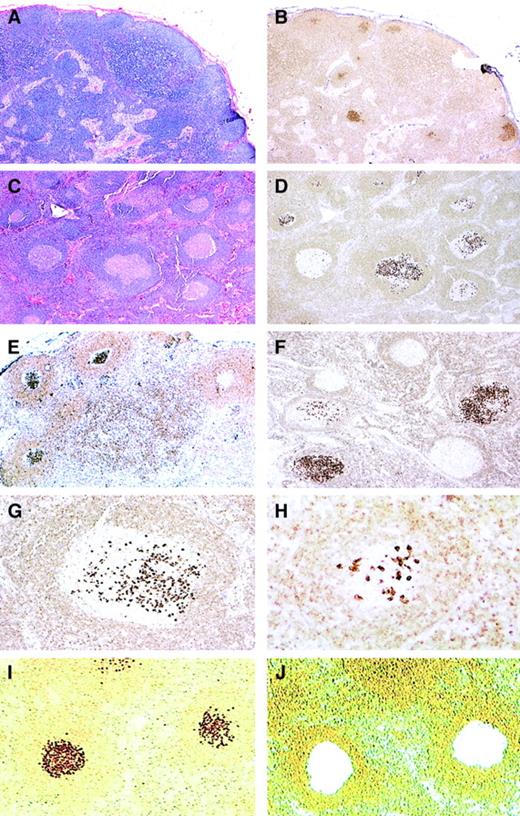
We identified 23 lymph node biopsies that showed atypical follicular hyperplasia on hematoxylin and eosin (H&E)–stained sections, but contained focal GCs strongly positive for bcl-2 by immunohistochemical staining (Figure 1). In these lymph nodes the remaining GCs in the same lymph node were bcl-2−. The affected follicles expressed CD10 and had a lower proliferation rate with MIB1, in comparison to the remaining reactive GCs in the same lymph node. Based on the morphologic and immunohistochemical findings, we termed this process in situ localization of FL.
Histopathology and bcl-2 immunohistochemistry of in situ follicular lymphoma.
(A-B) Low-power view of H&E-stained (A) and bcl-2–stained (B) sections showing preserved nodal architecture with open sinuses and prominent paracortex. In B, focal germinal centers (GCs) stain strongly positive for bcl-2, 25 × (case no. 4). (C-D) In this case, most follicles have sharply demarcated lymphoid cuffs resembling reactive GCs (C; H&E). A bcl-2 stain (D) reveals a variable number of cells strongly positive for bcl-2, with some GCs containing only rare bcl-2+ cells. Note that the intensity of the bcl-2+ cells is much higher than that of mantle zone cells and T-lymphocytes, 50 × (case no. 11). (E-F) Stains for bcl-2 show focal GCs strongly positive for bcl-2 protein with some adjacent GCs being completely negative. (immunoperoxidase, hematoxylin counterstain, 50 ×) (case nos. 6, 7). (G-H) At higher power, strongly bcl-2+ cells resemble centrocytes, and are seen amid a bcl-2− GC (immunoperoxidase, hematoxylin counterstain, 100 ×, 200 ×) (case no. 11). (I-J) An example of bcl-2–stained sections before (I) and after (J) microdissection of bcl-2+ follicles as viewed from the microdissection microscope (immunoperoxidase, no counterstain, 50 ×) (case no. 11).
Histopathology and bcl-2 immunohistochemistry of in situ follicular lymphoma.
(A-B) Low-power view of H&E-stained (A) and bcl-2–stained (B) sections showing preserved nodal architecture with open sinuses and prominent paracortex. In B, focal germinal centers (GCs) stain strongly positive for bcl-2, 25 × (case no. 4). (C-D) In this case, most follicles have sharply demarcated lymphoid cuffs resembling reactive GCs (C; H&E). A bcl-2 stain (D) reveals a variable number of cells strongly positive for bcl-2, with some GCs containing only rare bcl-2+ cells. Note that the intensity of the bcl-2+ cells is much higher than that of mantle zone cells and T-lymphocytes, 50 × (case no. 11). (E-F) Stains for bcl-2 show focal GCs strongly positive for bcl-2 protein with some adjacent GCs being completely negative. (immunoperoxidase, hematoxylin counterstain, 50 ×) (case nos. 6, 7). (G-H) At higher power, strongly bcl-2+ cells resemble centrocytes, and are seen amid a bcl-2− GC (immunoperoxidase, hematoxylin counterstain, 100 ×, 200 ×) (case no. 11). (I-J) An example of bcl-2–stained sections before (I) and after (J) microdissection of bcl-2+ follicles as viewed from the microdissection microscope (immunoperoxidase, no counterstain, 50 ×) (case no. 11).
On H&E-stained sections, the lymph nodes showed preservation of the nodal architecture with open sinuses, a relatively low density of GCs, intact mantle zones, and preserved paracortical regions. Prior to immunohistochemical studies, although most follicles were cytologically reactive, rare GCs appeared to be somewhat monotonous in appearance, composed predominantly of centrocytes and lacking mitotic figures and tingible body macrophages. However, these changes were usually subtle, and overall the morphologic features were most consistent with atypical follicular hyperplasia, which was generally the submitter's diagnosis. By comparison of parallel sections stained for H&E and bcl-2 protein, the bcl-2+ follicles were observed to be those that were cytologically atypical with the bcl-2+ cells resembling centrocytes.
The degree of lymph node involvement is summarized in Table 2. In more than half of the cases, the bcl-2+ follicles represented a minority of the secondary follicles within a given lymph node. When multiple follicles were identified as bcl-2+, the positive follicles tended to be adjacent to one another in the lymph node biopsy, although occasionally they were more widely scattered. In 11 cases, fewer than 5 bcl-2+ follicles were observed; in 5 cases, 6 to 10 bcl-2+ follicles were observed; and in 9 cases more than 10 bcl-2+ follicles were observed. In 15 of the 25 cases, fewer than half of the total GCs on bcl-2–stained sections were positive for bcl-2. Nevertheless, in each case the positive cells were confined only to GCs and were not seen in the interfollicular region or elsewhere in the lymph node.
There was also a spectrum in the number of the positive cells identified within affected follicles. Some of the involved GCs contained only a few positive cells in a background of bcl-2− centrocytes and centroblasts, whereas others were more extensively replaced by bcl-2+ centrocytes (Figure 1). In all cases, the bcl-2 staining in the abnormal follicles was notable for its high-level and uniform intensity. The intensity was much higher than that of admixed T-lymphocytes or mantle zone cells, which usually showed a weak level of expression.
There were 2 additional cases (case no. 24 and case no. 25) identified as composite low-grade B-cell lymphomas with in situ FL. In these 2 cases, whereas most of the lymph node showed involvement by either CLL/SLL (case no. 24) or lymphoplasmacytic lymphoma (LPL) (case no. 25), focal residual GCs showed morphologic and immunophenotypic evidence of in situ FL. Immunophenotypically, the CLL/SLL component in case no. 24 was positive for CD20, CD5 and IgD, weakly positive for bcl-2, but negative for CD23 and cyclin D1. The LPL component in case no. 25 was positive for CD20 and IgD and expressed monoclonal kappa light chain, but was negative for CD10 and bcl-6. In both cases, the abnormal follicles strongly overexpressed bcl-2 protein and in addition were positive for CD10 and bcl-6, indicating that the abnormal cells overexpressing bcl-2 were of GC origin. In case no. 24, many bcl-2− follicles were also observed in the same lymph node. In addition in case no. 25, a CD10+ B-cell population was identified by flow cytometry expressing monoclonal lambda light chain in contrast to expression of monoclonal kappa light chain by the LPL, further confirming the presence of 2 phenotypically different lymphomas.
Clonality analysis of microdissected bcl-2+ and bcl-2− follicles by PCR
Initial IgH FRIII-PCR analysis of DNA obtained from the whole tissue sections from these cases failed to reveal evidence of monoclonality. However, this is not an unexpected result since the bcl-2+ cells represented less than 1% of the total B-cell population. There were 5 cases with non–B5-fixed and paraffin-embedded tissue blocks available for microdissection. We microdissected the bcl-2+ follicles and bcl-2− follicles from the same lymph node, and analyzed them in parallel by IgH FRIII-PCR and BCL2/JH PCR. The results are shown in Figure2 and Table 3.
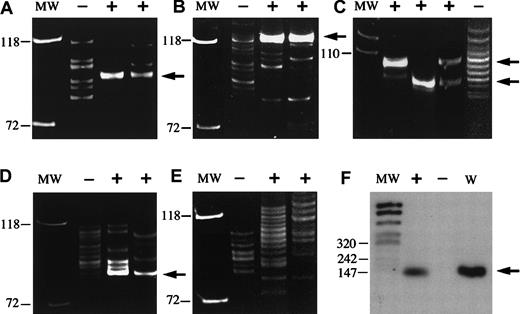
PCR of microdissected bcl-2+ and bcl-2−follicles from 5 cases.
(A-E) IgH− FRIII PCR. (F) BCL2/JHPCR. (A,F) Case no. 17. (B) Case no. 8. (C) Case no. 11. (D) Case no. 6. (E) Case no. 2. MW indicates molecular weight standard; +, microdissected bcl-2+ follicles; –, microdissected bcl-2− follicles; W, whole intact tissue section without microdissection. In case nos. 17, 8, 11, and 6, reproducible single or double discrete clonal bands (arrows) with or without a background ladder were generated from bcl-2+ GCs, whereas a polyclonal ladder was generated from bcl-2− GCs. Case no. 11 (C) contained 2 separate distinct clonal bands. Case no. 2 (E) showed a polyclonal ladder pattern from both bcl-2+ and bcl-2− follicles. (F) A BCL-2 gene rearrangement at the major breakpoint region was identified from microdissected bcl-2+ GCs (+) and the whole (W) intact tissue section without microdissection, but was negative in bcl-2− (−) GCs (case no. 17).
PCR of microdissected bcl-2+ and bcl-2−follicles from 5 cases.
(A-E) IgH− FRIII PCR. (F) BCL2/JHPCR. (A,F) Case no. 17. (B) Case no. 8. (C) Case no. 11. (D) Case no. 6. (E) Case no. 2. MW indicates molecular weight standard; +, microdissected bcl-2+ follicles; –, microdissected bcl-2− follicles; W, whole intact tissue section without microdissection. In case nos. 17, 8, 11, and 6, reproducible single or double discrete clonal bands (arrows) with or without a background ladder were generated from bcl-2+ GCs, whereas a polyclonal ladder was generated from bcl-2− GCs. Case no. 11 (C) contained 2 separate distinct clonal bands. Case no. 2 (E) showed a polyclonal ladder pattern from both bcl-2+ and bcl-2− follicles. (F) A BCL-2 gene rearrangement at the major breakpoint region was identified from microdissected bcl-2+ GCs (+) and the whole (W) intact tissue section without microdissection, but was negative in bcl-2− (−) GCs (case no. 17).
Clonality and BCL2/JH rearrangement analysis by PCR
| Case no. . | Clonal IgH rearrangement . | BCL2/JH rearrangement . | ||||
|---|---|---|---|---|---|---|
| Entire paraffin section . | Microdissected . | Entire paraffin section . | Microdissected . | |||
| bcl-2−follicles . | bcl-2+ follicles . | bcl-2−follicles . | bcl-2+ follicles . | |||
| 2 | − | − | − | − | − | − |
| 6 | − | − | + | − | − | − |
| 8 | − | − | + | − | − | − |
| 11 | − | − | + | − | − | − |
| 17 | − | − | + | + | − | + |
| Case no. . | Clonal IgH rearrangement . | BCL2/JH rearrangement . | ||||
|---|---|---|---|---|---|---|
| Entire paraffin section . | Microdissected . | Entire paraffin section . | Microdissected . | |||
| bcl-2−follicles . | bcl-2+ follicles . | bcl-2−follicles . | bcl-2+ follicles . | |||
| 2 | − | − | − | − | − | − |
| 6 | − | − | + | − | − | − |
| 8 | − | − | + | − | − | − |
| 11 | − | − | + | − | − | − |
| 17 | − | − | + | + | − | + |
Figure 1 (I,J) shows bcl-2–stained sections before and after LCM of bcl-2+ follicles. DNA was prepared from microdissected GCs and used for PCR analysis. Amplifiable DNA was obtained from all 5 cases. In 4 of 5 cases, DNA isolated from bcl-2+follicles showed reproducible single or double discrete clonal bands, with or without a background ladder, whereas a polyclonal ladder pattern was generated from bcl-2−follicles. Interestingly, one of the 4 cases contained 2 separate distinct clonal bands. The fifth case showed a polyclonal ladder pattern from both bcl-2+ and bcl-2−follicles.
Additional molecular studies using PCR also were performed to detect the BCL-2/JH translocation using primers to the MBR, both with and without microdissection. In one of the 5 cases with microdissection, a BCL-2 gene rearrangement at the MBR was identified from bcl-2+ GCs, but not from bcl-2− GCs. In this case, a BCL2/JH gene rearrangement was also detected in DNA from whole (W) tissue sections.
In all, the BCL2/JH translocation was evaluated in 14 of 25 cases. There were 6 cases (43%) positive for BCL2/JH by PCR studies of whole tissue sections (case nos. 10, 17, 18, 19, and 25), or in one case by Southern blot analysis (case no. 1) (data not shown). In case no. 18 the in situ FL (groin LN) and the FL (supraclavicular LN) were analyzed separately. Rearranged bands of identical sizes were identified from both lymph nodes by BCL2/JH PCR, suggesting that they were clonally related. PCR studies for BCL2/JHwere negative in case nos. 2, 4, 6, 8, 11, 12, 13, and 24. The overall PCR detection rate of in situ FL cases was 5 of 13 (38%), a rate slightly less than that expected when using only the MBR probe.28 29 The remaining cases could not be analyzed, either because the lymph nodes were fixed in B5, or because additional involved tissue sections could not be obtained.
Clinical features
Clinical features and follow-up are summarized in Table 2. There were 23 patients who presented with in situ localization of FL; 14 females and 9 males. Their ages ranged from 23 years to 76 years, with a median age of 52.5 years. There were 5 patients found to have synchronous FL at another site. Following a provisional diagnosis of in situ FL, the remaining 18 patients were further evaluated. Clinical follow-up was available in 13 cases. In 10 cases (76%), there was no other evidence of lymphoma from 2 months to 96 months (median, 15.5 months) after the initial diagnosis of in situ FL. Of these 10 cases, 8 had negative bone marrow examinations, and 4 had negative CT scans. In 3 cases (23%), FL was identified by biopsy of adjacent or distant lymph nodes at 3 months, 13 months, and 72 months following an initial diagnosis of in situ FL. Case no. 11 first presented in 1988 with in situ FL involving an axillary LN. In 1992, the patient developed an enlarged cervical LN, which also showed only an in situ pattern of involvement by FL. In 1994, 6 years after initial presentation, a biopsy of a cheek mass showed extensive involvement by FL. Clinical follow-up for the 2 patients with composite in situ FL and other B-cell lymphomas was not available.
Discussion
This study describes immunophenotypic, genotypic, and morphologic changes that appear to represent early microscopic involvement of FL within selected GCs in otherwise reactive lymph nodes. We propose the term “in situ localization of follicular lymphoma” for this phenomenon. It was detected based on the presence of focal GCs containing centrocytes strongly positive for bcl-2 protein, whereas most of the remaining GCs within the same lymph node were bcl-2−. A recent study by Su et al30 also observed cases of FL in which bcl-2+ cells were seen within a reactive GC background. However, their cases were selected from patients with established diagnoses of FL, and thereby differ from the cases described in this study. By microdissecting the bcl-2+ and bcl-2− follicles and analyzing them in parallel by PCR, we demonstrated the monoclonal nature of the bcl-2+ follicles and the polyclonal nature of bcl-2− follicles in 4 of 5 cases analyzed. IgH FRIII-PCR has a false-negative rate of up to 50% in detecting clonality in FLs, mainly due to the high somatic mutation of the immunoglobulin gene in follicular lymphoma. Thus, the demonstration of monoclonality within nearly all affected follicles is suggestive of a neoplastic process.
In addition, a BCL-2 gene rearrangement was detected in 6 of 14 cases (43%) tested, with or without microdissection. Approximately 50% to 60% of BCL-2 rearrangements in FL occur at the MBR,11,31,32 10% to 20% of cases in the minor cluster region (mcr)33 and in a few other cases at the 5′ end of BCL-2 gene. In approximately 15% of cases, no t(14;18) can be detected by cytogenetics, Southern blot analysis, or PCR techniques.34 PCR analysis using primers for only the MBR, as utilized in this study, will detect the t(14;18) in approximately 50% of cases of FL.28 The detection rate observed in this study is within expected limits for usual FL, although other mechanisms of bcl-2 overexpression cannot be ruled out.
We postulated that these immunophenotypic findings were an indication of in situ localization by FL. However, to ascertain their clinical significance, further clinical evaluation and follow-up were obtained. There were 5 patients who had evidence of synchronous FL. Of the remaining 18 patients, 13 had available follow-up information, and in 3 of 13 patients FL was diagnosed 3 months, 13 months, and 72 months after the initial lymph node biopsy. The identification of synchronous or subsequent FL in this subgroup indicates that the in situ FL identified is truly neoplastic and is most likely the result of spreading and homing of neoplastic cells to reactive GCs of adjacent or distant lymph nodes. This hypothesis is supported by the identification of bands of similar molecular weight from the in situ FL (groin LN) and the FL (supraclavicular LN) in case no. 18. The bcl-2+cells identified within GCs were generally centrocytes, regardless of the histologic grade of the FL observed at other lymph node sites. This observation is similar to the pattern of FL involvement in bone marrow biopsies, in which centrocytes typically predominate, despite variations in grade in primary lymph node biopsies.
In one patient, FL was not clinically evident until 6 years after an initial diagnosis of in situ FL. This interesting case (case no. 11) was a 41-year-old male who had an axillary lymph node biopsy in 1988 and a neck lymph node biopsy in 1992, both showing only in situ FL. Clinical evaluation at that time did not reveal other sites of disease. However, in 1994, 6 years after the initial diagnosis of in situ FL, a soft tissue mass of the cheek displayed FL, grade I. FL was diagnosed again from a paraspinal mass in 2000. Thus, in this particular case, clinically disseminated FL developed after a long latent period following the initial diagnosis of in situ FL. The in situ FL in this case may be the result of homing of neoplastic cells from an undetected occult FL at other sites, although in situ origination within the involved lymph nodes cannot be ruled out.
In 10 of 13 patients without concurrent FL, clinical follow-up showed no FL from 2 months to 96 months (median, 15.5 months) after the initial diagnosis of in situ FL. Of these patients, 8 had negative bone marrow biopsies and 4 had negative CT scans. However, given the indolent nature of FL, longer follow-up is needed to determine the clinical significance of these findings. One may reasonably assume that in some patients FL will become clinically evident at other sites over time. However, it is also possible that the bcl-2+cells in these cases may represent a preneoplastic process, which requires a “second hit” for true neoplastic transformation. Prior studies have identified cells carrying theBCL2/JH in normal peripheral blood and tonsil using nested PCR amplification.35-38 However, morphologic or immunophenotypic changes in lymph nodes or tonsils associated with this molecular phenomenon have not been described. Unlike other traditional oncogenes, the bcl-2 gene does not promote cell proliferation; it inhibits apoptosis leading to accumulation of B cells which are likely to become the target of additional mutations necessary for neoplastic transformation. Therefore, t(14;18) may be necessary for the development of FL, but is not, by itself, sufficient for malignant transformation.17 19 If a second hit does not occur the patient will not develop FL despite the presence of cells with t(14;18).
The degree of involvement by in situ FL was scored in each case to correlate with the presence or absence of concurrent or subsequent FL (Table 2). The degree of involvement by in situ FL and the presence of occult FL at other sites showed some correlation. In 4 of 8 cases (50%) with evidence of FL at other sites, there was a relatively high degree of involvement by in situ FL (score 3b), whereas only 2 of 10 cases (20%) with no other evidence of FL had a score of 3b. This score indicates more than 50% of follicles were involved and more than 10 bcl-2+ follicles were identified within the lymph node. However, biopsies from 3 patients had the lowest score (1a), and yet manifested evidence of FL concurrently in another biopsy.
The findings from this study provide insight into the biology and pathogenesis of early FL. The favored hypothesis of FL lymphomagenesis has been that the clonogenic cell containing t(14;18) arises at the pre–B-cell stage in the bone marrow as an error of D-J or V-DJ joining. The B cell with the t(14;18) translocation then enters the circulation, homes to LNs, and eventually seeds a lymphoid follicle. It may undergo clonal expansion upon antigenic stimulation. If the second hit does not occur, the patient will not develop FL, despite the presence of cells with t(14;18).
The bcl-2+ centrocytes observed with the GCs showed unusually strong staining for bcl-2 protein. The intensity of the staining contrasted with the weak staining of normal lymphoid cells made it possible to speculate on the migration of these cells at a single cell level. Only rare, strongly bcl-2+ cells could be seen in the interfollicular areas and in the mantle zones, and these cells probably represent rare trafficking cells, moving into or out of the follicle. In some cases, several positive follicles could be identified, each containing only a few bcl-2+ cells. This observation suggests that these follicles are being colonized from an outside source, rather than from a primary event occurring within the GCs.
It is interesting that composite in situ FL and other low-grade B-cell lymphomas were identified in 2 cases. We have previously reported on other cases of composite B-cell lymphoma confirmed at the molecular and immunophenotypic levels.39 In our prior report, FL represented one of the components in 2 of 3 cases. The demonstration of a BCL2/JH rearrangement in 1 of the 2 composite lymphoma cases in this study provides additional evidence that the follicular component is not the result of follicular colonization by the other low-grade malignancy. In addition, the 2 components could be readily distinguished by their distinctive phenotypes in each case. Although phenotypically different, the clonal relationship of the in situ FL and the low-grade B-cell lymphomas or any possibility of a shared common pathogenesis by 2 distinct lymphomas is not clear as molecular studies of microdissected regions were not performed. Nonetheless, our previous studies of low-grade composite lymphomas have indicated separate clonal processes in such cases.39
This study further underscores the diagnostic utility of bcl-2 immunohistochemical staining in the evaluation of atypical follicular hyperplasia. If in situ localization of FL is recognized, we recommend careful clinical evaluation for other sites of disease, including bone marrow examination. However, if clinical and pathologic evaluations are negative, a conservative approach without further therapy seems advisable. Although the clinical significance of the cases without other evidence of lymphoma is as yet unknown, long-term follow-up of these patients may provide further insight into the biology and pathogenesis of early FL.
This work was presented in part at the American Society of Hematology, 42nd Annual Meeting, San Francisco, CA, December 1-5, 2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Elaine S. Jaffe, Bldg 10, Rm 2N202, 10 Center Dr MSC-1500, Bethesda, MD 20892-1500; e-mail: elainejaffe@nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal