Abstract
MDM2 protein is thought to exhibit tumorigenic activity by binding to the p53 tumor-suppressor protein and inhibiting its function. Alternatively, MDM2 may have oncogenic roles other than those resulting from p53 interactions. Here we report that MDM2 can induce expression of the p65 subunit of NF-κB, which is an anti-apoptotic factor expressed in certain neoplastic cells in response to chemotherapy. Initially, we noted that the overexpression of MDM2 protein in leukemic bone marrow cells of patients with B-cell precursor acute lymphoblastic leukemia (BCP-ALL), and an ALL cell line (EU-4) transfected with theMDM2 gene was associated with elevated expression of p65 and in vitro resistance to doxorubicin (Adriamycin). By cotransfection of the MDM2 gene and p65-promoter-reporter constructs into EU-4 cells, we found that transient and high-level MDM2 expression induced p65 promoter activity. In the presence of wild-type (wt) p53, MDM2 increased p65 promoter activity by reversing p53-mediated suppression of p65. In the absence of p53, MDM2 directly increased p65 promoter activity. Deletion and mutation analysis of the p65 promoter indicated that the region between nt −575 and −178, which contains the first and second Sp1-binding sites, was required for activation by MDM2. Further studies using chromatin immunoprecipitation (CHIP) and electrophoretic mobility shift assay (EMSA) showed that MDM2 was able to directly bind to the Sp1 site of the p65 promoter. Our findings suggest that by inducing p65 expression, MDM2 has a p53-independent role in tumorigenesis, which may further elucidate the association between MDM2 overexpression and resistant disease in childhood ALL.
Introduction
MDM2 is considered a new type of proto-oncogene because of the ability of its product to inhibit p53 tumor-suppressor function.1 However, several lines of evidence suggest that MDM2 can be oncogenic without complexing with p53. MDM2 is overexpressed independently of p53 in a number of human sarcomas and carcinomas, where it occurs as an alternatively spliced form lacking a p53 binding domain, and transfection of p53-negative cells with these MDM2 variants results in cell transformation.2 A recent study by Lundgren et al3 showed that overexpression of MDM2 in the mammary gland leads to uncontrolled entry into S-phase, polyploidy, and tumor formation in p53-null and in wt-p53 mice, further suggesting a p53-independent role for MDM2. Because the primary amino acid sequence of the MDM2 protein contains an acidic domain and zinc-finger motifs, it is possible that MDM2 acts as a transcription factor to directly modulate the expression of other genes involved in cell cycle regulation and transformation.4-7
Growing evidence indicates that the NF-κB family of transcription factors plays an important role in controlling cellular transformation8,9 and regulating apoptosis.10NF-κB proteins are present in many cells as an inactive cytoplasmic precursor complexed with the inhibitory subunit IκB. Activation of NF-κB in response to stimuli such as tumor necrosis factor (TNF)-α and interleukin-1 results from the degradation of IκB and the release of an active NF-κB, which then translocates to the nucleus.11,12 Interestingly, constitutive nuclear expression of NF-κB has been reported in certain neoplastic cells, such as breast and pancreatic cancer cells and lymphomas (but not in their normal counterparts), contributing to their survival and tumor progression.13-15 Many cancer cells protect themselves against treatment (TNF-α, ionizing radiation, and chemotherapeutic compounds) by activating NF-κB, which augments resistance to apoptosis through unknown mechanisms.16-19 The typical and most abundant form of NF-κB is a heterodimer composed of a 50-kd (p50) and a 65-kd (p65 or RelA) subunit, though other forms of homodimers and heterodimers of the NFκB family (c-Rel, p52, and RelB) also exist.20 The p65 subunit of NF-κB appears to play a central role in NF-κB–induced gene transcription, which in turn mediates cellular transformation and apoptosis. When translocated to the nucleus, p50 and p65 homodimers and p50/p65 heterodimers bind to a common DNA motif; however, only p65 regulates the transcriptional activity of its target genes.21,22 Mice deficient in the p65 subunit of NF-κB die prenatally because of massive hepatocyte apoptosis23 that appears related to the cytotoxic effect of TNF-α.24 The role of the p65 subunit in protecting against TNF-induced apoptosis was further studied by Beg16and Van Antwerp.18 In their studies, the treatment of p65(−/−) embryo fibroblasts with TNF-α caused marked reduction in viability, whereas p65(+/+) or p50(−/−) cells were resistant to this treatment. Moreover, the aberrant expression of p65 in human cells appears to be associated with the neoplastic phenotype. A previous study has demonstrated that p65 protein was constitutively overexpressed in thyroid carcinoma cells, and anti-p65 antisense greatly reduced the growth rate of these cells.25
NF-κB as a transcription factor regulating the expression of other genes in oncogenesis and apoptosis and in immune and inflammatory responses has been extensively studied.10,26-28 However, the regulation of NF-κB gene expression itself, including the expression of its p65 subunit, is little understood. In addition to the translocation of NF-κB to the nucleus in response to certain stimuli, the up-regulation of protein synthesis of the NF-κB subunits occurs through their promoter transactivation. For example, cytomegalovirus infection can increase the expression of p65 and p50 transcriptionally through the binding site for Sp1,29 which is a transcription factor, and it binds to specific GC-rich elements known as GC boxes.30 Similar transcriptional regulation of gene expression through Sp1-binding sites has been observed. For instance, oncogenes Ras and c-Jun can regulate their target gene p21/Waf-1 cyclin kinase inhibitor through the Sp1 binding sites in the promoter of p21/Waf-1.31,32 Because the promoter of the p65 subunit of NF-κB is GC rich and contains Sp1-binding sites,33 the expression of p65 might be transcriptionally activated by other cellular proteins through a similar mechanism.
The current study was prompted by our observation that MDM2 overexpression was associated with constitutively elevated levels of p65 and resistance to doxorubicin (Adriamycin) in several ALL cell lines established from pediatric patients. Furthermore, our finding in this study of an association between overexpression of MDM2 and elevated p65 levels in ALL cells, even in the absence of wt p53 protein, led us to examine whether MDM2 might directly regulate the expression of p65. Using ALL lines EU-4 and UOC-B11 as a model system, we performed a series of tests including cotransfection, gene reporter, chromatin immunoprecipitation (CHIP) and electrophoretic mobility shift assay (EMSA) assays. Our results demonstrate that MDM2 protein can transcriptionally up-regulate p65 expression through the Sp1-binding site in the p65 gene promoter.
Materials and methods
Patient cells and cell line
Bone marrow aspirates were studied from 10 patients with BCP-ALL, diagnosed by standard immunologic, morphologic, and cytochemical criteria. Mononuclear cells were separated by centrifugation on Ficoll-Hypaque (1.077 g/mL), washed twice in phosphate-buffered saline, and resuspended at 106/mL in RPMI 1640 containing 10% fetal bovine serum (FBS). Cells were incubated on plastic Petri dishes for 1 hour at 37°C to remove monocytes, and nonadherent cells were recovered by gently washing the dishes. All specimens for studies of gene expression and sensitivity to doxorubicin contained more than 90% blasts after purification.
The EU-4 cell line was established in our laboratory from pediatric ALL with myeloid-antigen expression. Cultured cells resembled the primary leukemic cells in lacking p53 expression and expressing low levels of MDM2.34 UOC-B11, a BCP-ALL cell line, was obtained from Dr S. D. Smith (University of Kansas). This line expressed more than 100-fold higher MDM2 than did EU-4 cells, as detected in our previous study.34 EU-4 and UOC-B11 cells were grown in standard culture medium (RPMI 1640 containing 10% FBS, 2 mM L-glutamine, 50 U penicillin, and 50 μg/mL streptomycin) at 37°C in 5% CO2 in air.
Plasmids
The MDM2 expression plasmid (pCMV-MDM2) and the wt p53 expression plasmid (pc53-SN3) were provided by Dr B. Vogelstein (Johns Hopkins University). To construct an MDM2–antisense cDNA expression vector as a control, pCMV-MDM2 was digested with BamHI, and 2 DNA fragments (2.0 and 4.6 kb) were isolated from agarose gel. The 4.6-kb fragment, containing the replication origin and ampicillin-resistance gene, was treated with alkaline phosphatase to remove 5′ phosphate residues. After phenol extraction, this fragment was ligated to the 2.0-kb MDM2 cDNA fragment. Then the new construct was transfected into Escherichia coli for propagation. The plasmid containing MDM2 in the antisense orientation was selected by restriction enzyme digestion (as compared with sense orientation). The p65 expression plasmid (pCMVp65) was provided by Dr N. H. Colburn (National Cancer Institute). Full-length p65 promoter-CAT construct (pKBCAT) was provided by Dr K. Ueberla (Harvard Medical School). The p65 promoter deletion and mutation constructs (p65F1CAT, p65F3CAT, p65F5CAT, p65F6CAT, p65F1xxCAT) have been previously described29 and were provided by Dr A. D. Yurochko (University of North Carolina). E-selectin promoter-CAT construct, in which a fragment containing the region −578 to +35 of the E-selectin promoter is subcloned into the reporter plasmid pCAT3,35was provided by Dr T. Collins (Harvard Medical School).
Transfection and CAT assay
For stable MDM2 gene transfection, EU-4 cells in exponential growth were transfected with pCMV-MDM2 or pCMV-neo control plasmid by electroporation at 300 V, 950 μF using a Gene Pulser II System (Bio-Rad, Hercules, CA). Cells were seeded 48 hours after transfection into culture dishes for the selection of G418-resistant colonies. Colonies were grown in methylcellulose medium containing G-418 (500 μg/mL) for 2 to 3 weeks, and clones were picked and grown in RPMI medium with or without G-418 for the duration of the experiments. To examine the effect of MDM2 on p65 promoter activity, EU-4 cells were cotransfected with full-length or varying length deletion and mutated p65 promoter constructs plus different doses of MDM2 or wt p53 expression plasmids. To test the effect of MDM2 on p65-directed gene expression, EU-4 cells were cotransfected with E-selectin promoter-CAT and p65 expression plasmid plus different amounts of MDM2 or wt-p53 plasmids. MDM2 antisense plasmid served as a control. Electroporation was performed as described above. Transfected cells were resuspended in 10 mL RPMI containing 10% FBS. At 48 hours after transfection, cell extracts were prepared, and CAT activity was evaluated with the CAT enzyme-linked immunosorbent assay (ELISA) kit (Boehringer Mannheim, Mannheim, Germany).
Western blot assay
Purified primary bone marrow leukemic cells and EU-4 cells stably transfected with the MDM2 gene were lysed in a buffer composed of 150 mM NaCl, 50 mM Tris (pH 8.0), 5 mM EDTA, 1% (vol/vol) Nonidet P40, 1 mM phenylmethylsulfonyl fluoride, 20 μg/mL aprotinin, and 25 μg/mL leupeptin for 30 minutes at 4°C. After clarification, equal amounts of protein extracts were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and were transferred to nitrocellulose paper. After blocking with buffer containing 20 mM Tris-HCl (pH 7.5) and 500 mM NaCl–5% nonfat milk for 1 hour at room temperature, the filter was incubated with specific antibodies to MDM2 (Calbiochem, San Diego, CA), p65 (Upstate Biotechnology, Lake Placid, NY), Sp1, cIAP1, cIAP2 (Santa Cruz Biotechnology, Santa Cruz, CA), XIAP (BD Company, San Diego, CA), and caspase-3 and PARP (PharMingen, San Diego, CA) for 2 hours at room temperature, followed by horseradish peroxidase–labeled secondary antibody. Blots were developed using a chemiluminescent detection system (ECL; Amersham Life Science, Buckinghamshire, England). After stripping, the filter was reprobed with an antiactin antibody (Calbiochem) as a control for equal protein loading and protein integrity.
Northern blot assay
Expression of MDM2 mRNA in gene-transfected EU-4 cells was detected by Northern blot assay. Total cellular RNA was extracted from cells with an RNA isolation kit (Biotex Laboratories, Houston, TX). Ten micrograms total RNA was electrophoresed per lane using a 1% agarose–6% formaldehyde gel and then transferred to a nylon filter. An MDM2 cDNA probe was prepared by randomized labeling using32P-dCTP. After hybridization and washing, the filter was autoradiographed for 24 hours.
XTT assay
The effect of doxorubicin on fresh bone marrow ALL cells or MDM2-transfected ALL line EU-4 was determined by the XTT cytotoxicity assay. Cleavage of XTT (a tetrazolium salt) by mitochondrial-associated dehydrogenase enzymes in metabolically active cells yielded a colored formazan product that could be measured spectrophotometrically. The concentration of the products was directly proportional to the viability of the cells. After treatment with different concentrations of doxorubicin, cells were cultured in 96-well microtiter plates and were incubated for 44 hours. XTT (25 μg/well) was then added, and cells were incubated for an additional 4 hours. Optical density of the wells was read with a microplate reader at a test wavelength of 450 nm and a reference wavelength of 620 nm. Appropriate controls lacking cells were included to determine background absorbance.
CHIP assay
The CHIP assay, as described previously,36 was performed to analyze the DNA-binding activity of transfected MDM2 protein. EU-4 cells were cotransfected with 10 μg MDM2 expression plasmid and 10 μg p65F1CAT or p65F1xxCAT using electroporation as described above. After 48 hours, formaldehyde was added at 1% to the culture media and cells were incubated at room temperature for 10 minutes with mild shaking to cross-link MDM2 protein to the p65 promoter. Then cells (1 × 106) were washed twice with cold phosphate-buffered saline and were resuspended in lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCI, pH 8.1) with 1 mM phenylmethylsulfonyl fluoride, 1 μg/mL aprotinin, and 1 μg/mL pepstatin A. After brief sonication, lysates were cleared by centrifugation and were diluted 10-fold with dilution buffer (0.01% SDS, 1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCl, pH 8.1, and 167 mM NaCl) containing protease inhibitors as above. Anti-MDM2 antibody was added at 4°C overnight with rotation. Immunoprecipitated complexes were collected by protein A/G plus agarose. Precipitants were sequentially washed with low-salt wash buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.1, 150 mM NaCl), high-salt wash buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.1, 500 mM NaCl), and LiCl wash buffer (0.25 M LiCl, 1% NP40, 1% deoxycholate, 1 mM EDTA, 10 mM Tris-HCl, pH 8.1), once, followed by 2 washes with 1 × TE. After the final wash, 250 μL elution buffer (1% SDS, 0.1 M NaHCO3) was added and was incubated at room temperature for 15 minutes with rotation. Then 5 M NaCl was added to reverse the formaldehyde cross-linking by heating at 65°C for 4 hours. After precipitation with ethanol, the pellets were resuspended and treated with proteinase K. DNA was recovered by phenol–chloroform extraction and ethanol precipitation. Pellets were resuspended in TE buffer and subjected to polymerase chain reaction (PCR) amplification using forward and reverse primers (5′-CATCTAGATTGGGGTGGGTA-3′ and 5′-CGCTGCCGGGGGTCGGGGCC-3′) to the p65F1 fragment. The resultant product measured 379 bp and contained the first and second Sp1-binding sites of p65 promoter. The PCR product was separated by agarose gel electrophoresis.
Electrophoretic mobility shift assay
Sequence-specific DNA-binding activity of MDM2 protein was assayed by EMSA. Nuclear protein extraction was prepared using a kit (NE-PER; Pierce, Rockford, IL). Nuclear protein (5 μg) from MDM2, control plasmid-transfected EU-4 cells, and UOC-B11 cells were incubated for 15 minutes in binding buffer (10 mM Tri-HCl, 50 mM NaCl, 0.5 mM EDTA, 10% glycerol, 1 mM dithiothreitol, 7.5 mM MgCl) plus 0.1 μg poly (dI-dC) carrier and a 32P-labeled double-stranded oligonucleotide probe (5′-CTGCGCGGGGCGGGCGCCGC-3′) containing the first Sp1-binding site (−382 to −363) in the p65 promoter or a mutant (5′-CTGCGCGGGTTGGGCGCCGC-3′) probe. Anti-MDM2 (Sigma, St Louis, MO) and anti-Sp1 (Santa Cruz Biotechnology) as a positive control were used to supershift the specific complexes of interest by pretreating the extract for 15 minutes at 4°C with antibodies. Samples were electrophoresed on a 5% polyacrylamide gel, dried, and developed with intensifier screen at −70°C.
Results
MDM2 overexpression is associated with elevated expression of p65 and resistance to doxorubicin in ALL cells
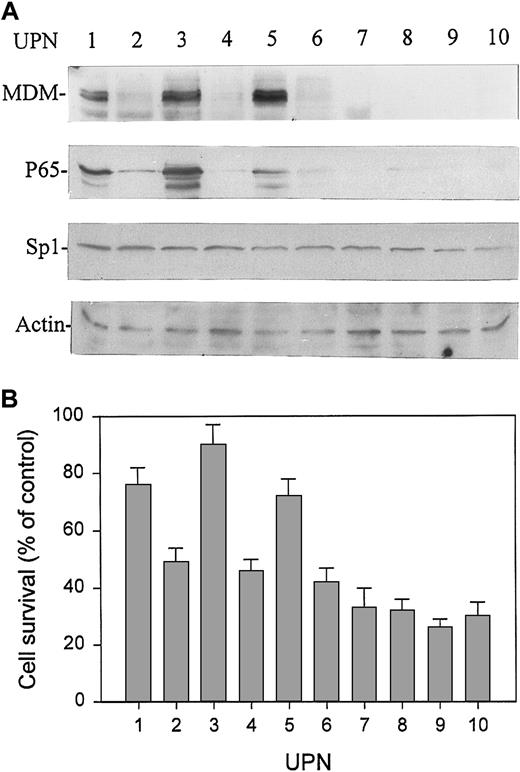
We expanded our previous study37 to evaluate the association of MDM2 expression and sensitivity of cells to doxorubicin in 10 pediatric patients with BCP-ALL. In these 10 patients, we also examined the expression of p65 and Sp1 protein in leukemic cells by Western blot assay. Intriguingly, we found a close correlation between the expression of MDM2 and p65 proteins in these specimens. Three of 10 patients expressed high levels of MDM2 and p65, and no increased p65 expression was detected in samples that expressed no or low levels of MDM2. All 10 patients expressed low and equal levels of Sp1 (Figure1A). The increased expression of p65 also represents an enhanced binding activity as tested by EMSA in the above 3 patients (data not shown). Furthermore, all 3 patients showing high levels of MDM2 and p65 also showed resistance to doxorubicin in vitro compared to those without overexpression of MDM2. As shown in Figure1B, after treatment with doxorubicin at 1 μM for 48 hours, a significantly higher percentage of mean cell survival was seen in patients with MDM2 and p65 overexpression than in those without MDM2 and p65 overexpression.
Expression of MDM2, p65, and Sp1 proteins and sensitivity to doxorubicin in primary leukemia cells from 10 randomly selected patients with BCP-ALL.
(A) Expression of MDM2, p65, and Sp1 proteins in marrow mononuclear cells (more than 90% leukemic blasts) from pediatric patients with ALL was analyzed by Western blot assay. (B) Response to doxorubicin of leukemic cells from the same samples examined for MDM2, p65, and Sp1 protein expression. Cells were cultured with doxorubicin at 1 μM for 48 hours, and cell survival (viability) was determined by XTT assay. Viability was expressed as percentage of control (ie, cultures without doxorubicin). Data represent mean ± SD detected in triplicate experiments. UPN, unique patient number.
Expression of MDM2, p65, and Sp1 proteins and sensitivity to doxorubicin in primary leukemia cells from 10 randomly selected patients with BCP-ALL.
(A) Expression of MDM2, p65, and Sp1 proteins in marrow mononuclear cells (more than 90% leukemic blasts) from pediatric patients with ALL was analyzed by Western blot assay. (B) Response to doxorubicin of leukemic cells from the same samples examined for MDM2, p65, and Sp1 protein expression. Cells were cultured with doxorubicin at 1 μM for 48 hours, and cell survival (viability) was determined by XTT assay. Viability was expressed as percentage of control (ie, cultures without doxorubicin). Data represent mean ± SD detected in triplicate experiments. UPN, unique patient number.
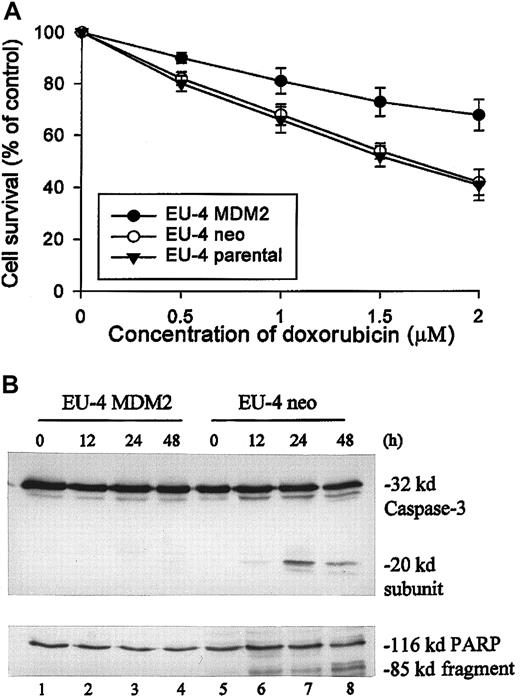
Moreover, we studied the effect of transfected MDM2 in EU-4 cells on the expression of p65. After transfection by electroporation, stable expression of ectopic MDM2 mRNA and protein in EU-4 cells was detected by Northern blot (Figure 2A) and Western blot (Figure 2B) assays. The expression of p65 protein was significantly increased in EU-4 cells transfected with MDM2 compared with that in parental EU-4 cells and in cells transfected with control vector (Figure 2B). These results in transfected EU-4 cells are consistent with the observations in primary ALL cells, in which high levels of p65 expression were directly correlated with MDM2 overexpression. We also examined the expression of Sp1 and several NFκB-regulated gene products—cIAP1, cIAP2,38,39 and XIAP40 41—which are members of the inhibitor of apoptosis protein (IAP) family. Expression of XIAP—but not of Sp1, cIAP1, and cIAP2—was significantly up-regulated in EU-4 cells expressing ectopic MDM2 compared with control cells (Figure 2B). Furthermore, EU-4 cells expressing ectopic MDM2 showed higher resistance to doxorubicin than did parental EU-4 cells and CMV-neo (control)-transfected cells. As shown in Figure 3A, a significant difference was noted in mean cell survival between MDM2-transfected and control-transfected cells at doxorubicin concentrations of 1 μM and greater (P < .05; Student t test). In addition, increased resistance to apoptosis induced by doxorubicin in MDM2-transfected cells was detected by examining the activation of caspase-3 and the cleavage of its substrate PARP. Significant cleavage of caspase-3 and PARP was detected at 24 hours after doxorubicin treatment in control cells, whereas cleavage of these 2 proteins was not observed even 48 hours after doxorubicin treatment in MDM2-transfected cells (Figure 3B).
Stable expression of transfected (ectopic) MDM2 and its effect on the expression of p65, Sp1, and NFκB-regulated gene products cIAP1, cIAP2, and XIAP.
MDM2 mRNA in EU-4 cells was detected by Northern blot assay (A), and the protein levels of MDM2, p65, Sp1, cIAP1, cIAP2, and XIAP in MDM2-transfected and control cells were detected by Western blot assay (B). Total RNA and protein were extracted simultaneously from parental EU-4 cells (lane 1) and EU-4 cells stably transfected with either pCMV plasmid-neo alone (lane 2) or pCMV-MDM2 (lanes 3-6). Stably transfected cells were derived from G418-resistant single clones maintained in culture for 3 months. Northern blot and Western blot analyses were performed as described in “Materials and methods.”
Stable expression of transfected (ectopic) MDM2 and its effect on the expression of p65, Sp1, and NFκB-regulated gene products cIAP1, cIAP2, and XIAP.
MDM2 mRNA in EU-4 cells was detected by Northern blot assay (A), and the protein levels of MDM2, p65, Sp1, cIAP1, cIAP2, and XIAP in MDM2-transfected and control cells were detected by Western blot assay (B). Total RNA and protein were extracted simultaneously from parental EU-4 cells (lane 1) and EU-4 cells stably transfected with either pCMV plasmid-neo alone (lane 2) or pCMV-MDM2 (lanes 3-6). Stably transfected cells were derived from G418-resistant single clones maintained in culture for 3 months. Northern blot and Western blot analyses were performed as described in “Materials and methods.”
Comparison of sensitivity to doxorubicin between MDM2-transfected EU-4 cells and control cells.
(A) Parental EU-4 cells and cells stably transfected with either CMV-MDM2 plasmid (EU-4 MDM2 from clone shown in Figure 2, lane 6) or CMV-neo plasmid (EU-4/neo) were cultured with different concentrations of doxorubicin for 48 hours, and cell survival was determined by XTT assay. (B) Results of Western blot assay for activation of caspase-3 and cleavage of its substrate, PARP, in EU-4 MDM2 and EU-4 neo cells that were treated with doxorubicin at 1.5 μM for indicated times. Anti–caspase-3 antibody recognized the 32-kd unprocessed proprotease and the 20-kd subunit. Anti-PARP detected an 85-kd fragment cleaved from the 116-kd PARP holoenzyme.
Comparison of sensitivity to doxorubicin between MDM2-transfected EU-4 cells and control cells.
(A) Parental EU-4 cells and cells stably transfected with either CMV-MDM2 plasmid (EU-4 MDM2 from clone shown in Figure 2, lane 6) or CMV-neo plasmid (EU-4/neo) were cultured with different concentrations of doxorubicin for 48 hours, and cell survival was determined by XTT assay. (B) Results of Western blot assay for activation of caspase-3 and cleavage of its substrate, PARP, in EU-4 MDM2 and EU-4 neo cells that were treated with doxorubicin at 1.5 μM for indicated times. Anti–caspase-3 antibody recognized the 32-kd unprocessed proprotease and the 20-kd subunit. Anti-PARP detected an 85-kd fragment cleaved from the 116-kd PARP holoenzyme.
Activation of p65 promoter by MDM2 is p53 dependent and p53 independent
Because the overexpression of MDM2 in primary ALL cells and EU-4 cells expressing ectopic MDM2 was correlated with increased p65 expression, we investigated whether MDM2 could transcriptionally activate the p65 gene promoter by cotransfecting the full-length pKBCAT and MDM2 expression plasmid into EU-4 cells. Given that EU-4 cells do not express wt p53, a wt p53 expression plasmid was included in the cotransfection to investigate whether the effect of MDM2 on p65 promoter activity is direct or indirect through interaction with p53. From the data presented in Figure 4, it can be seen that p53 suppressed p65 promoter activity (Figure 4, lanes 2-4). This inhibitory effect was reversed by MDM2 in a dose-dependent manner (Figure 4, lanes 5-7). Furthermore, increasing amounts of MDM2 in the absence of p53 resulted in an even greater dose-dependent stimulation of p65 promoter activity (Figure 4, lanes 8-10). As a control, we constructed an MDM2 antisense expression vector in the pCMV plasmid and transfected this into EU-4 cells. No effect on p65 promoter-CAT activity was observed with this construct (Figure 4, lane 11).
Effect of MDM2 on activation of p65 promoter in transient transfection assays.
EU-4 cells were cotransfected with 10 μg pKBCAT (containing the p65 promoter) and either increasing amounts (1, 5, and 10 μg) of wt p53 (lanes 2-4) or increasing amounts (1, 5, and 10 μg) of MDM2-sense in the presence (lanes 5-7) or absence (lanes 8-10) of a fixed amount (10 μg) of wt-p53. The pKBCAT was cotransfected with 10 μg MDM2-antisense plasmid (lane 11) as control. Total concentration of DNA was adjusted to 30 μg per transfection with empty pCMV-neo-Bam vector. Electroporation was performed at 300 V and 950 μF. At 48 hours after transfection, cell extracts were analyzed for CAT protein expression with a CAT ELISA kit. Data represent mean ± SD of 3 independent experiments. A value of 1 was assigned to CAT expression from the transfection with pKBCAT plasmid alone.
Effect of MDM2 on activation of p65 promoter in transient transfection assays.
EU-4 cells were cotransfected with 10 μg pKBCAT (containing the p65 promoter) and either increasing amounts (1, 5, and 10 μg) of wt p53 (lanes 2-4) or increasing amounts (1, 5, and 10 μg) of MDM2-sense in the presence (lanes 5-7) or absence (lanes 8-10) of a fixed amount (10 μg) of wt-p53. The pKBCAT was cotransfected with 10 μg MDM2-antisense plasmid (lane 11) as control. Total concentration of DNA was adjusted to 30 μg per transfection with empty pCMV-neo-Bam vector. Electroporation was performed at 300 V and 950 μF. At 48 hours after transfection, cell extracts were analyzed for CAT protein expression with a CAT ELISA kit. Data represent mean ± SD of 3 independent experiments. A value of 1 was assigned to CAT expression from the transfection with pKBCAT plasmid alone.
MDM2 activates p65 promoter through Sp1-binding sites
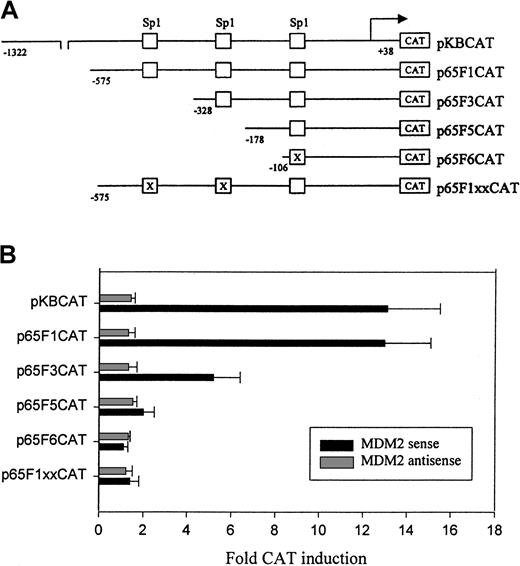
To find possible regions and elements in the p65 promoter responsible for activation by MDM2, we investigated the effect of MDM2 on a set of p65 promoter deletion and mutation constructs (Figure5A). Our results showed that the Sp1 DNA-binding sites in the p65 promoter played an important role in the regulation of the promoter by MDM2. As shown in Figure 5B, cotransfected MDM2 showed an effect on deletion construct p65F1CAT, which contained 3 Sp1-binding sites, that was similar to that of the full-length promoter pKBCAT. There was an approximately two-thirds reduction in the ability of MDM2 to activate construct p65F3CAT with deletion of the first Sp1 site. MDM2 had little effect on the p65F5CAT and p65F6CAT constructs, which contained only the third Sp1 site in either wt or mutant form. These results indicated that the regions containing the first and second Sp1 sites in the p65 promoter contributed to MDM2-mediated activation. The effect of MDM2 on p65F1CAT activation was completely abolished when the first 2 documented Sp1 sites were mutated, as shown in Figure 5B; MDM2 had no effect on the p65F1xxCAT construct with mutations in the first and second Sp1 sites. These results suggest that MDM2 mediates p65 promoter activation specifically through the Sp1 sites.
Deletion and mutation analysis of the p65 promoter activity.
(A) Schematic representation of p65 promoter-CAT reporter plasmids as described previously29: pKBCAT containing the full-length promoter; p65F1CAT, p65F3CAT, and p65F5 CAT containing a series of deleted promoters; p65F6CAT containing deleted promoter with the third Sp1-binding site mutated by site-directed mutagenesis; P65F1xxCAT, the p65F1CAT in which the first 2 Sp1 sites were mutated by site-directed mutagenesis. (B) Transient transfection and CAT assay. EU-4 cells were cotransfected with 10 μg MDM2 sense or antisense plasmid plus 10 μg p65 promoter constructs. Electroporation and CAT assays were performed as described in Figure 4. Fold CAT induction shows the comparison of cotransfection of MDM2 plasmid and promoter-CAT construct with transfection of individual promoter-CAT construct alone.
Deletion and mutation analysis of the p65 promoter activity.
(A) Schematic representation of p65 promoter-CAT reporter plasmids as described previously29: pKBCAT containing the full-length promoter; p65F1CAT, p65F3CAT, and p65F5 CAT containing a series of deleted promoters; p65F6CAT containing deleted promoter with the third Sp1-binding site mutated by site-directed mutagenesis; P65F1xxCAT, the p65F1CAT in which the first 2 Sp1 sites were mutated by site-directed mutagenesis. (B) Transient transfection and CAT assay. EU-4 cells were cotransfected with 10 μg MDM2 sense or antisense plasmid plus 10 μg p65 promoter constructs. Electroporation and CAT assays were performed as described in Figure 4. Fold CAT induction shows the comparison of cotransfection of MDM2 plasmid and promoter-CAT construct with transfection of individual promoter-CAT construct alone.
MDM2 binds to the Sp1-binding elements in the p65 promoter
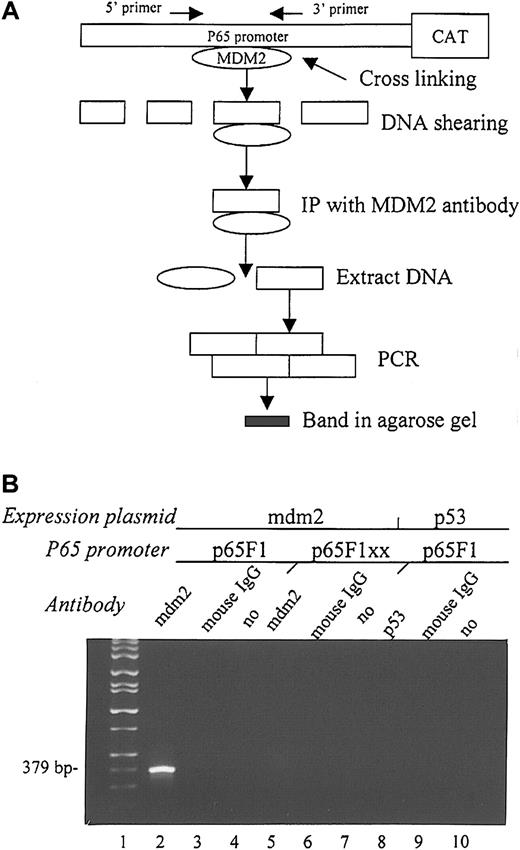
To further determine the effect of MDM2 on the Sp1 sites of the p65 promoter, we performed the CHIP assay to examine the ability of transfected MDM2 to bind to the region containing the first 2 Sp1 sites of the p65 promoter. This assay determines the ability of a protein to bind to a specific DNA sequence. We cotransfected MDM2 with either p65F1CAT or p65F1xxCAT into EU-4 cells and performed the CHIP assay as outlined in Figure 6A. After cross-linking and immunoprecipitation with anti-MDM2 antibody, PCR using a pair of primers to the fragment that contains the first 2 Sp1 sites in the p65F1CAT construct showed a DNA band in the sample cotransfected with MDM2 and p65F1CAT (Figure 6B, lane 2), but not in the sample cotransfected with MDM2 and p65xxCAT (Figure 6B, lane 5). For negative controls, we performed immunoprecipitations using normal mouse IgG and in the absence of antibody (Figure 6B, lanes 3-4 and 6-7, respectively). Furthermore, we performed a CHIP assay by cotransfecting p65F1CAT with a wt p53 expression plasmid and using anti-p53 antibody for immunoprecipitation. There was no binding of p53 to p65F1, as shown by the absence of a band in Figure 6B, lane 8.
CHIP assay to detect the ability of MDM2 to bind to the p65 promoter in vivo.
(A) Flow chart of the experimental design. (B) Agarose gel electrophoresis shows the PCR results from each CHIP assay. MDM2 expression plasmid and either wt p65F1 (lane 2) or mutant p65F1xx (lane 5) were cotransfected into EU-4 cells and precipitated with anti-MDM2 antibody. The p53 expression plasmid and wt p65F1 were also cotransfected into EU-4 cells and precipitated with anti-p53 antibody (lane 8) to detect the ability of p53 to bind to the p65 promoter. For negative control, immunoprecipitation was performed using a normal mouse IgG, or it was performed in the absence of antibody (no) in each experiment (lanes 3, 6, 9 and 4, 7, 10, respectively). Lane 1 shows DNA size markers. PCR product (379 bp) in lane 2 contains the first 2 Sp1-binding sites in the p65 promoter.
CHIP assay to detect the ability of MDM2 to bind to the p65 promoter in vivo.
(A) Flow chart of the experimental design. (B) Agarose gel electrophoresis shows the PCR results from each CHIP assay. MDM2 expression plasmid and either wt p65F1 (lane 2) or mutant p65F1xx (lane 5) were cotransfected into EU-4 cells and precipitated with anti-MDM2 antibody. The p53 expression plasmid and wt p65F1 were also cotransfected into EU-4 cells and precipitated with anti-p53 antibody (lane 8) to detect the ability of p53 to bind to the p65 promoter. For negative control, immunoprecipitation was performed using a normal mouse IgG, or it was performed in the absence of antibody (no) in each experiment (lanes 3, 6, 9 and 4, 7, 10, respectively). Lane 1 shows DNA size markers. PCR product (379 bp) in lane 2 contains the first 2 Sp1-binding sites in the p65 promoter.
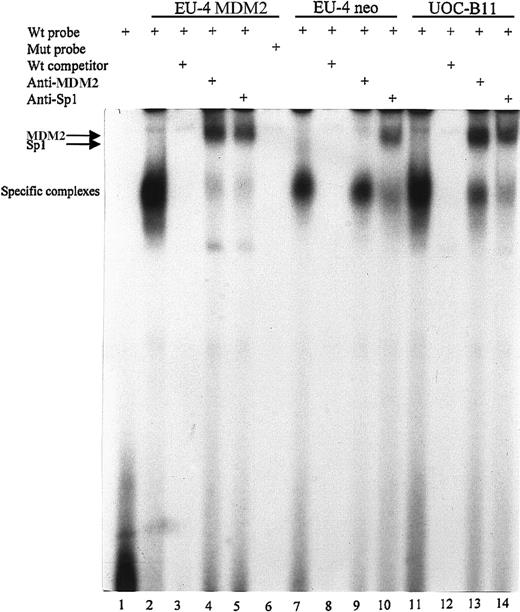
To further test whether MDM2 actually binds to the Sp1 site, EMSA was carried out with nuclear extracts from UOC-B11 cells with overexpression of endogenous MDM2, as well as MDM2 and control plasmid–transfected EU-4 cells. Binding reactions were performed using wt or mutated oligonucleotides corresponding to the first Sp1-binding site in the p65 promoter. In MDM2-transfected EU-4 cells (EU-4/MDM2) and UOC-B11 cells, one large DNA–protein complex specific for the wt probe was observed (Figure 7, lanes 2 and 11), which was effectively competed with wt cold oligonucleotide (Figure 7, lanes 3 and 12), but it was not observed using mutant probe (Figure 7, lane 6). The same specific band for wt oligonucleotide was also seen in control plasmid–transfected EU-4 (EU-4/neo) cells, but the density of the band was weaker than that in EU-4/MDM2 and UOC-B11 cells (Figure 7, lane 7). Because the sizes of Sp1 and MDM2 proteins are similar (approximately 90 kd on gel electrophoresis), to determine whether the specific band contained both MDM2 and Sp1 DNA–protein complexes, we incubated nuclear extracts with anti-MDM2 and anti-Sp1 antibodies to supershift the complex. In EU-4/MDM2 and UOC-B11 cells, the addition of either MDM2 or Sp1 antibody led to a supershift of the complex (Figure 7, lanes 4-5, 13-14), indicating the presence of MDM2 and Sp1 proteins in this complex. In EU-4/neo cells, a supershifted band was seen only with the addition of Sp1 antibody (Figure 7, lane 10), not with the addition of MDM2 antibody (Figure 7, lane 9), indicating the absence of MDM2 protein in the complex because of low levels of MDM2 expression in EU-4/neo cells.
EMSA to examine the binding of MDM2 to the first Sp1 site of the p65 promoter.
Nuclear extracts from UOC-B11 cells and MDM2 and control plasmid-transfected EU-4 cells (EU-4 MDM2 and EU-4 neo) were incubated in binding reactions with annealed and 32P-labeled wt or mutant (mut) oligonucleotide probe containing the first Sp1-binding site of the p65 promoter. Samples were run on a nondenaturing 5% polyacrylamide gel and were imaged by autoradiography. Lane 1, labeled wt probe without nuclear extracts; lanes 2 to 5, labeled wt probe incubated with nuclear extracts of EU-4 MDM2; lane 6, labeled mut probe with nuclear extracts of EU-4 MDM2; lanes 7 to 10, labeled wt probe with nuclear extracts of EU-4 neo cells; lanes 11 to 14, labeled wt probe with nuclear extracts of UOC-B11 cells. In reactions depicted in lanes 3, 8, and 12, 25-fold molar excess of nonlabeled wt oligonucleotide was added. In reactions depicted in lanes 4, 9, and 13, cell extracts were preincubated with 2 μg anti-MDM2 antibody for 1 hour at 4°C before probes were added. In reactions depicted in lanes 5, 10, and 14, cell extracts were preincubated with 2 μg anti-Sp1 antibody. Specific protein–DNA complexes and supershift with anti-MDM2 and anti-Sp1 antibodies are indicated.
EMSA to examine the binding of MDM2 to the first Sp1 site of the p65 promoter.
Nuclear extracts from UOC-B11 cells and MDM2 and control plasmid-transfected EU-4 cells (EU-4 MDM2 and EU-4 neo) were incubated in binding reactions with annealed and 32P-labeled wt or mutant (mut) oligonucleotide probe containing the first Sp1-binding site of the p65 promoter. Samples were run on a nondenaturing 5% polyacrylamide gel and were imaged by autoradiography. Lane 1, labeled wt probe without nuclear extracts; lanes 2 to 5, labeled wt probe incubated with nuclear extracts of EU-4 MDM2; lane 6, labeled mut probe with nuclear extracts of EU-4 MDM2; lanes 7 to 10, labeled wt probe with nuclear extracts of EU-4 neo cells; lanes 11 to 14, labeled wt probe with nuclear extracts of UOC-B11 cells. In reactions depicted in lanes 3, 8, and 12, 25-fold molar excess of nonlabeled wt oligonucleotide was added. In reactions depicted in lanes 4, 9, and 13, cell extracts were preincubated with 2 μg anti-MDM2 antibody for 1 hour at 4°C before probes were added. In reactions depicted in lanes 5, 10, and 14, cell extracts were preincubated with 2 μg anti-Sp1 antibody. Specific protein–DNA complexes and supershift with anti-MDM2 and anti-Sp1 antibodies are indicated.
MDM2 activates p65-mediated gene expression
In addition to the inhibitory effect of wt p53 on p65 promoter activity as described above, recent studies have demonstrated that wt p53 represses the ability of the p65 protein to activate the transcription of down-stream genes (ie, functional inhibition).42-44 Thus, we further investigated whether MDM2 could up-regulate p65 function either indirectly through the inhibition of p53 or directly in a p53-independent manner. Because the expression of E-selectin is p65 dependent,35 we cotransfected EU-4 cells with the E-selectin promoter-CAT construct and with p65 and MDM2 expression plasmids in the presence or absence of p53. As predicted, p65 increased the promoter activity of E-selectin (Figure 8, lane 2). P53 suppressed p65-directed transactivation (Figure 8, lanes 3-5), and MDM2 reversed this inhibitory effect (Figure 8, lanes 6-8). Importantly, MDM2 alone remarkably increased p65-mediated transactivation of the E-selectin promoter in a dose-dependent manner (Figure 8, lanes 9-11). MDM2 did not show any direct effect on E-selectin promoter activity in the absence of p65 (Figure 8, lane 12). Moreover, MDM2 antisense had no effect on either E-selectin promoter activity or p65-mediated transcription (Figure 8, lanes 13 and 14, respectively).
Effect of MDM2 on p65-dependent transcription.
EU-4 cells were cotransfected with 2 μg E-selectin promoter-CAT reporter construct or 5 μg p65 expression plasmid alone (lane 2), either with increasing amounts (1, 5, and 10 μg) of wt p53 (lanes 3-5) or with increasing amounts (1, 5, and 10 μg) of MDM2-sense in the presence (lanes 6-8) or absence (lanes 9-11) of a fixed amount of wt p53 (10 μg). MDM2 sense and antisense alone (10 μg), respectively, were cotransfected with E-selectin promoter-CAT as controls (lanes 12, 13). Controls also included cotransfection with E-selectin, p65, and MDM2 antisense (lane 14). Total concentration of DNA was adjusted to 27 μg per transfection with empty pCMV-neo-Bam vector. Transfection and CAT ELISA assay were as described in Figure 4.
Effect of MDM2 on p65-dependent transcription.
EU-4 cells were cotransfected with 2 μg E-selectin promoter-CAT reporter construct or 5 μg p65 expression plasmid alone (lane 2), either with increasing amounts (1, 5, and 10 μg) of wt p53 (lanes 3-5) or with increasing amounts (1, 5, and 10 μg) of MDM2-sense in the presence (lanes 6-8) or absence (lanes 9-11) of a fixed amount of wt p53 (10 μg). MDM2 sense and antisense alone (10 μg), respectively, were cotransfected with E-selectin promoter-CAT as controls (lanes 12, 13). Controls also included cotransfection with E-selectin, p65, and MDM2 antisense (lane 14). Total concentration of DNA was adjusted to 27 μg per transfection with empty pCMV-neo-Bam vector. Transfection and CAT ELISA assay were as described in Figure 4.
Discussion
Constitutive expression of MDM2 has been reported in 15% to 25% of ALL patients at diagnosis. Furthermore, the overexpression of MDM2 is associated with poor response to induction therapy, early relapse, and resistance to doxorubicin.45,46 Similarly, activation of the p65 subunit of NF-κB in several murine and human cell types after treatment with various apoptosis-inducing agents (including TNF-α, anthracyclines such as doxorubicin and daunorubicin, and ionizing radiation) protects these cells from apoptosis.16-19 However, p65 expression level and function in primary pediatric BCP-ALL cells has not previously been reported.
In evaluating the expression of p65 and MDM2 proteins in leukemic cells from 10 pediatric patients with ALL, we noted a high degree of correlation between overexpression of MDM2 and elevated expression of p65. This led us to investigate whether these 2 proteins are mutually regulated in ALL cells. First, we examined the possible regulation of p65 by MDM2. We transfected an MDM2 expression plasmid into an ALL line that was negative for MDM2 and p53 expression and found that MDM2 enhanced the expression of endogenous p65 and resistance of these cells to doxorubicin. Furthermore, using cotransfection and gene reporter assays, we found that MDM2 can enhance p65 promoter activity in a p53-dependent and p53-independent manner. In the presence of wt p53, MDM2 increased p65 promoter activity by reversing p53-mediated suppression of p65. In the absence of p53, MDM2 directly increased p65 promoter activity.
As a transcription factor, p53 can positively or negatively regulate the transcription of a particular gene promoter, depending on whether the promoter has a p53-binding site. Genes negatively regulated by p53 usually lack a p53-binding element in their promoter.47,48In our study, p53 significantly repressed p65 promoter activity and was unable to bind to the promoter, as demonstrated in p65-promoter CAT and CHIP assays. P53 represses the promoter activity of genes through binding to and inhibiting the function of the TATA-binding protein.49 Alternatively, p53 negatively regulates transcription, possibly by sequestering co-activators such as transcription factor Sp1.50 For example, p53 is able to protect the Sp1-binding sites in the HIV-LTR promoter, resulting in the repression of Sp1 DNA binding and HIV-LTR–directed transcription.50 Because the p65 promoter contains 3 Sp1-binding sites,33 wt p53 may down-regulate the transcription of p65 through this mechanism. The down-regulating effect of p53 on the p65 promoter is, however, reversed by MDM2.
In the absence of p53, MDM2 acts as a transcription factor to directly activate p65 gene expression. Using several deleted and mutated p65 promoter fragments, our results demonstrated that the region containing the first and second Sp1-binding sites in the p65 promoter plays an important role in its activation by MDM2. Additional studies using CHIP and EMSA experiments demonstrated that MDM2 can bind to the Sp1 sites, in particular the first Sp1 (−377) site, in the p65 promoter and can activate transcription. Previous studies have shown that Sp1 protein, a housekeeping transcription factor, strongly activates p65 expression.29 Sp1 levels are limiting within the cells, and, with the exception of infection by virus such as simian or human cytomegalovirus,29,51,52 little evidence exists documenting an increase in Sp1 levels during stimulation. Although Sp1 protein expression was observed in ALL cells, the samples tested expressed equal and low levels of this protein; no elevated expression was noted in patients with overexpression of MDM2 and p65 (Figure 1A). Moreover, the transfection of MDM2 did not increase Sp1 expression in cells (Figure 2B), suggesting that unlike cytomegalovirus infection, in which up-regulation of p65 results from the induction of Sp1,29 up-regulation of p65 by MDM2 is Sp1-independent. Furthermore, results from a recent study demonstrate that MDM2 physically binds to Sp1 protein and inhibits Sp1-mediated transcription.53 According to this finding, if the expression of p65 regulated by MDM2 is through interaction with Sp1 protein, there would be decreased expression of p65 in MDM2-overexpressing cells. Our results showed an increased expression of p65 in primary cells and in EU-4 cells overexpressing MDM2, suggesting that elevated expression of p65 by MDM2 is Sp1-independent. In fact, our results provide evidence that MDM2 directly binds to Sp1 sites of the p65 promoter, as demonstrated in CHIP and EMSA experiments.
Our results support and extend previous findings that wt p53 can repress the ability of p65 to activate transcription.42-44Using COS-7 monkey cells and cotransfection with an E-selectin promoter-CAT construct and p65 expression plasmid, Wadgaonkar et al44 reported that the activity of the p65-dependent E-selectin promoter was inhibited by p53. When an MDM2 expression plasmid was added to the cotransfection, the inhibitory effect of p53 on p65-dependent transcription was reversed. In the current study of human leukemia cells, we have found that ectopic wt p53 suppressed p65-dependent E-selectin promoter activation in p53-null ALL cells and that this suppression was alleviated by MDM2. We have further shown that MDM2 could directly augment the function of p65 in the absence of wt p53, as shown by the increased E-selectin promoter CAT activity assay in p53-null ALL cells cotransfected with MDM2 and p65 expression plasmids. This result was consistent with that obtained from a study in monkey cells in which MDM2 alone increased p65-dependent gene expression.44 However, we cannot rule out the possibility that increased E-selectin promoter activity resulted from increased production of p65 in the cotransfection, because the CMV promoter of the p65 expression construct contained Sp1-binding sites that could have been bound and activated by MDM2.
The ability of MDM2 to directly induce p65 transcription in the absence of p53 represents a novel finding of the current study. Previously, MDM2 has been shown to act in a p53-dependent manner by directly binding the wt p53 protein and inhibiting p53 function.1,54,55 However, p53-independent functions for MDM2 have been proposed based on the ability of alternatively spliced forms of MDM2 lacking the p53-binding domain to transform p53-null NIH3T3 cells. Possible p53-independent mechanisms for transformation of MDM2 include the ability of MDM2 to bind pRb and its associated transcription factor, E2F1/DP1, preventing the induction of pRb-mediated G1 cell cycle arrest and stimulating S-phase progression.6 7 We identified an additional mechanism whereby MDM2 may exert an antiapoptotic effect in the absence of p53 by directly up-regulating the expression of p65 in p53-null ALL cells. Specifically, ectopic MDM2 was able to increase p65-promoter activity (as detected in a p65 promoter-CAT assay) and to increase levels of p65 protein expression in these cells (as detected by Western blotting). MDM2–up-regulated p65 protein was present in an active form, as shown by the enhanced transcription of E-selectin from the p65-dependent E-selectin promoter in cells that had been cotransfected with MDM2 and p65 expression vectors. MDM2 was able to up-regulate the transcription of E-selectin in the absence of p53 but not p65, providing evidence for a direct effect of MDM2 on p65.
The p65 subunit of NF-κB plays a critical role in the regulation of apoptosis. Recent studies have demonstrated that NF-κB functions as a cellular protective factor against the apoptotic effects of TNF-α and chemotherapeutic drugs such as daunorubicin in rat embryonic fibroblasts, and this protective effect is dependent on the presence of p65.16-19 Treatment with TNF-α resulted in a dramatic decrease of viability in p65−/− embryonic fibroblasts.16 18 In contrast, TNF-α treatment had no substantial effect on the viability of p65+/+ or p50−/− embryonic fibroblasts, indicating that only the p65 subunit of NFκB has an antiapoptotic function.
The mechanisms by which p65 inhibits apoptosis are largely unknown. Several recent studies suggest that NFκB-mediated protection of cells from apoptosis is associated with the up-regulation of certain downstream target genes of NFκB such as cIAP1, cIAP2,38,39 and XIAP,40,41 which are members of the IAP family. These gene products have been shown to inhibit apoptosis by interacting with specific caspases required for the cleavage of certain proteins involved in the disassembly of the cell during apoptosis.56 In the current study, increased expression of XIAP was observed in the MDM2-transfected cell line compared with the control cells. We do not have evidence to conclude whether increased expression of XIAP is induced by p65 or is directly regulated by MDM2. However, increased expression of XIAP could be one mechanism for resistance of MDM2-transfected cells to apoptosis induced by doxorubicin.
MDM2-mediated up-regulation of p65 is of clinical significance. We previously reported that MDM2 was overexpressed in BCP-ALL cells from approximately one third of pediatric patients who had relapses and that BCP-ALL cells overexpressing MDM2 were highly resistant to doxorubicin in vitro and to conventional chemotherapy in vivo.37Moreover, MDM2 overexpression has been reported to confer resistance to doxorubicin and other chemotherapeutic drugs and to be associated with poor prognosis in other human cancers such as breast carcinoma,57 glioblastoma,58lymphoma,59 and soft tissue sarcoma.60Up-regulation of p65 function by MDM2 in BCP-ALL cells, or possibly in other cell types, suggests that this may be an important additional mechanism for MDM2-mediated resistance to chemotherapy-induced apoptosis.
We thank Drs B. Vogelstein, N. H. Colburn, K. Ueberla, A. D. Yurochko, and T. Collins for providing us with expression plasmids used in this work. We also thank Prof Stephen Lauer for his assistance in collecting clinical bone marrow samples and to Prof William G. Woods for helpful criticism, advice, and support for this project.
Supported by grants from the National Cancer Institute/National Institutes of Health (R01 CA82323, R29 CA72020), CURE Childhood Cancer, Children's Healthcare of Atlanta, and the University Research Committee of Emory University.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Muxiang Zhou, Division of Pediatric Hematology/Oncology/BMT, Emory University School of Medicine, 2040 Ridgewood Dr NE, Atlanta, GA 30322; e-mail: mzhou@emory.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal