Abstract
The role of angiogenesis in the growth and metastasis of solid tumors is well established. However, the role of angiogenesis in hematologic malignancies was only recently appreciated. We show that HTLV-I–transformed T cells, but not HTLV-I–negative CD4+T cells, secrete biologically active forms of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) and, accordingly, induce angiogenesis in vitro. Furthermore, fresh ATL leukemic cells derived from patients with acute ATL produce VEGF and bFGF transcripts and proteins. The viral transactivator Tax activates the VEGF promoter, linking the induction of angiogenesis to viral gene expression. Angiogenesis is associated with the adhesion of HTLV-I–transformed cells to endothelial cells and gap junction–mediated heterocellular communication between the 2 cell types. Angiogenesis, cell adhesion, and communication likely contribute to the development of adult T-cell leukemia–lymphoma and represent potential therapeutic targets.
Introduction
HTLV-I–associated adult T-cell leukemia–lymphoma (ATL)1 carries a poor prognosis because of an intrinsic resistance of leukemic cells to chemotherapy and to an associated severe immunosuppression.2,3 Although the combination of zidovudine (AZT) and interferon (IFN)–α produces a high response rate in patients with ATL,4-7 most of the patients eventually have relapses, which underscores the need for novel therapeutic approaches such as retinoids,8 monoclonal antibodies,9,10 and arsenic trioxide.11-13
Angiogenesis, the formation of new blood vessels from existing ones, is a prerequisite for the growth and metastasis of many solid tumors.14-16 Angiogenesis is predominantly mediated by vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF)17,18 secreted by tumor or stromal cells. Recently, a role of angiogenesis in the pathophysiology of hematologic malignancies has been demonstrated. The most compelling evidence is associated with multiple myeloma (MM), in which a correlation between the extent of bone marrow angiogenesis and of plasma cell proliferative index and disease progression has been described.19,20More significantly, the antiangiogenic drug thalidomide resulted in a high response rate in patients with MM resistant to chemotherapy.21 Similarly, in non-Hodgkin lymphoma, a correlation between the degree of angiogenesis and the stage of the lymphoma was reported22 as was a direct correlation between the pretreatment level of angiogenic factors and prognosis.23,24 In leukemia, increased angiogenesis in the bone marrow and increased level of proangiogenic factors were demonstrated in children with acute lymphoblastic leukemia25 and in adults with acute and chronic myeloid leukemia, myelodysplastic syndromes,26-28 or chronic lymphocytic leukemia.29 30
Cells influence their immediate microenvironment either through paracrine mechanisms or direct cell–cell interaction, such as adhesion and gap junction–mediated communication. Tumor cells produce, directly or through accessory cells, proangiogenic factors such as VEGF and bFGF that induce endothelial cells to hydrolyze their basement membranes, proliferate, and migrate.31-34 In turn, endothelial cells release growth factors that enhance tumor cell proliferation and metastatic potential. Tumor cell adhesion to endothelial cells can also trigger cellular responses. However, gap junctions represent an efficient and specific conduit to deliver molecules up to 1 kd that are characteristically membrane impermeable, produced in low concentration, or have a short half-life. These include cell metabolites and second messengers such as cyclic nucleotides, inositol triphosphate, and calcium.35 Gap junctions are clusters of trans-membranous aqueous channels composed of structurally related proteins known as connexins (Cx),36 which play an important role in tissue organization and cellular differentiation.37-39 There is evidence for the existence of functional gap junctions in endothelial cells40,41 and normal lymphocytes.42,43However, though the endothelium interacts with many cell types, including leukocytes,44-47 astrocytes,48smooth muscle cells,49 and cancer cells,50 51communication of neoplastic lymphocytes with the endothelium has never been described before.
In this study, we show that HTLV-I–transformed cells secrete VEGF and bFGF proteins and induce angiogenesis in vitro. Moreover, adhesion and gap-junction formation between HTLV-I–transformed cells and endothelial cells are demonstrated, representing the first evidence for heterocellular communication in leukemias.
Materials and methods
Cells and antibodies
HuT-102, MT-2, C91-PL, and C8166 cells are HTLV-I–transformed CD4+ T-cell lines that constitutively express the HTLV-I virus (gift from A. Gessain, Pasteur Institute, Paris, France). HTLV-I–negative CD4+ T-cell lines CEM, Jurkat, and Molt-4 were used as control cells. Cells were grown in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum and antibiotics. Cell concentration of 2 × 105 cells/mL was chosen for seeding for all experiments, unless otherwise stated. The human endothelial cell line ECV-304 and HeLa cells were grown in RPMI containing 10% heat-inactivated fetal calf serum in the presence of penicillin and streptomycin, and human aortic endothelial cells (HAECs) (Clonetics, San Diego, CA) were grown in the manufacturer's provided medium at an initial cell concentration of 4 × 105/mL.
After informed consent was obtained, peripheral blood mononuclear cells were extracted from diluted venous blood from 3 patients with acute ATL and 4 healthy controls by Ficoll-Hypaque centrifugation (Lymphoprep, Nyegaard, Norway). A lymphocyte-enriched cell population was achieved by preplating peripheral blood mononuclear cells.
Rabbit polyclonal antibodies to VEGF and bFGF (Santa Cruz Biotechnology, CA) that recognize all isoforms of human VEGF and human bFGF, respectively, were used. Rabbit polyclonal antibodies to Cx43 and its blocking peptide were purchased from Zymed (San Francisco, CA). Rabbit antiactin (Santa Cruz) antibodies were used to assess protein loading.
RNA isolation and reverse transcription–polymerase chain reaction
cDNA was synthesized from 100 or 500 ng total cellular RNA, isolated by Trizol-reagent (Gibco-BRL, Paisley, United Kingdom), using reverse transcriptase (Superscript II; Gibco-BRL). The cDNA was then amplified by polymerase chain reaction (PCR) using Taq DNA polymerase (Gibco-BRL). We used PCR primers that recognize all VEGF and bFGF isoforms, universal Cx primers and Cx43-specific primers, and β-actin–specific primers. PCR conditions used to amplify VEGF, bFGF, and β-actin consisted of a precycle of 95°C for 3 minutes followed by 35 cycles consisting of 95°C for 1 minute, 55°C for 1 minute, and 72°C for 1 minute. A final extension at 72°C for 7 minutes was then performed. Primer sequences are presented in Table1. Amplification of the housekeeping gene, β-actin, was used to verify RNA quality and reverse transcription (RT)–PCR techniques. PCR-amplified products were electrophoresed in 1.5% agarose gel and visualized with ethidium bromide staining.
Primer sets used in PCR experiments
| Protein . | Sense primer . | Antisense primer . |
|---|---|---|
| β-Actin | CGCCTGCGCCTGGTCGTCGACA | GTCACGCACCGATTTCCCGCT |
| VEGF | TCGGGCCTCCGGAAACCATGA | CCTGGTGAGAGATCTGGTTC |
| bFGF | GGTCCTGTTTTGGATCCA | AGAGAGAGGAGTTGTGTC |
| Cx43 | CTACCGGACTCAGATCTCATGGGTGACTGGAGT | CTTGAGCTCGAGATCTGAAATCTCCAGGTCATC |
| Cx universal | GGCTGTAAAAATGTCTGCTATGAC | TGGGACTGGAAATGAAGCAGT |
| Protein . | Sense primer . | Antisense primer . |
|---|---|---|
| β-Actin | CGCCTGCGCCTGGTCGTCGACA | GTCACGCACCGATTTCCCGCT |
| VEGF | TCGGGCCTCCGGAAACCATGA | CCTGGTGAGAGATCTGGTTC |
| bFGF | GGTCCTGTTTTGGATCCA | AGAGAGAGGAGTTGTGTC |
| Cx43 | CTACCGGACTCAGATCTCATGGGTGACTGGAGT | CTTGAGCTCGAGATCTGAAATCTCCAGGTCATC |
| Cx universal | GGCTGTAAAAATGTCTGCTATGAC | TGGGACTGGAAATGAAGCAGT |
Western blot analysis
Approximately 107 cells were solubilized at 4°C in lysis buffer consisting of 0.125 M Tris-HCl (pH 6.8), 2% sodium dodecyl sulfate, 2.5% β-mercaptoethanol, and 10% glycerol. Samples were loaded onto a 12% sodium dodecyl sulfate–polyacrylamide gel, subjected to electrophoresis, and transferred onto nitrocellulose membranes. After blocking of the membrane in 5% skimmed milk in Tris-buffered saline containing 0.05% Tween-20, the blots were incubated with specific antibodies. Then they were washed, and protein bands were visualized using chemiluminescence (Amersham, Buckinghamshire, United Kingdom).
Enzyme-linked immunosorbent assay
Cells were seeded at a concentration of 0.2 × 106cells/mL. The cell-free supernatant harvested after 72 hours of culture was tested for the presence of soluble VEGF and bFGF proteins by enzyme-linked immunosorbent assay (ELISA) using a kit from R&D Systems (Minneapolis, MN) as recommended by the manufacturer. Absorbance of each well was measured spectrophotometrically at 450 nm. The amount of VEGF and bFGF proteins in the samples was calculated using a reference plot established from serial dilutions of rhVEGF or rhbFGF proteins as provided. All experiments were performed in triplicate and repeated at least 3 times.
Transient transfection and luciferase assays
VEGF-Luc construct in pGL-2 (provided by Bertrand Knebelmann, INSERM U507, Hôpital Necker, Paris, France) or HTLV-I LTR–Luc construct (provided by Christophe Nicot, National Cancer Institute, Bethesda, MD) corresponding to the luciferase gene under the control of a 2.6-kb fragment (−2361 to +298) of the VEGF promoter or the HTLV-I promoter, respectively, were cotransfected into Jurkat cells with the internal control PSV β-galactosidase and either pCMV-Tax or empty vector using Lipofectamine Plus (Gibco BRL) according to the manufacturer's recommendations. The total amount of transfected DNA of expression vectors was kept constant in all experiments by the addition of pCDNA3 plasmid. Luciferase activity was quantified 24 hours later using the Luciferase Assay System (Promega, Madison, WI). Values were normalized with the β-galactosidase activity.
HeLa cells were transfected with either pCMV-Tax or pCMV control vector using Lipofectamine plus (Gibco BRL) according to the manufacturer's recommendations. Cellfree supernatant harvested after 72 hours of culture was tested for the presence of soluble VEGF protein using ELISA, as detailed earlier.
Matrigel-induced capillary tube formation
The Matrigel assay has been widely used as an in vitro measure of angiogenesis and was performed as described previously.52 Briefly, 24-well plates were coated with 200 μL/well growth factor–reduced Matrigel (Becton Dickinson, San Jose, CA) and were allowed to stand for 30 minutes at 37°C to form a gel layer. HAECs (4 × 105 cells) were cultured for 18 hours, then incubated at 37°C for 48 hours with supernatant of HTLV-I–positive (HuT-102, C8166) or –negative (CEM, Jurkat) T-cell lines or with supernatantfree HuT-102 cells. Cells were then stained with Hoechst (Molecular Probes, Eugene, OR) (0.5 μg/mL for 10 minutes). Plates were observed by microscopy and were photographed at different time intervals under light and fluorescence illumination.
Functional assay of adhesion and communication
Calcine labeling of cells.
Cells were labeled with the membrane-permeable dye calcine-am (Molecular Probes) as previously described.44 50 Briefly, labeling was achieved with 1 μM calcine in complete culture medium. On entry of the dye into the cell, intracellular esterases rapidly cleave the molecule to the fluorescent membrane–impermeable, gap junction–permeable acid form. Labeled cells were washed twice with serumfree medium, incubated in complete growth medium for 30 minutes at 37°C to allow any non–de-esterified dye to leave the cells, washed, and used immediately in dye transfer experiments.
FACS analysis of dye transfer.
Confluent monolayers of endothelial cells grown in 35-mm Petri dishes were seeded with different amounts of calcine-labeled HTLV-I–positive (HuT-102) or –negative (Molt-4, CEM) T-cell lines and were cocultured for various periods of time at 37°C. Unbound cells were removed by a single wash. Endothelial cells and adhered lymphocytes were then released from the growth surface with trypsin-EDTA in Hanks balanced salt solution, washed once with Hanks balanced salt solution, fixed in 4% formaldehyde in phosphate-buffered saline, and analyzed by flow cytometry (FACScan; Becton Dickinson).
Results
HTLV-I–transformed cells secrete angiogenic factors
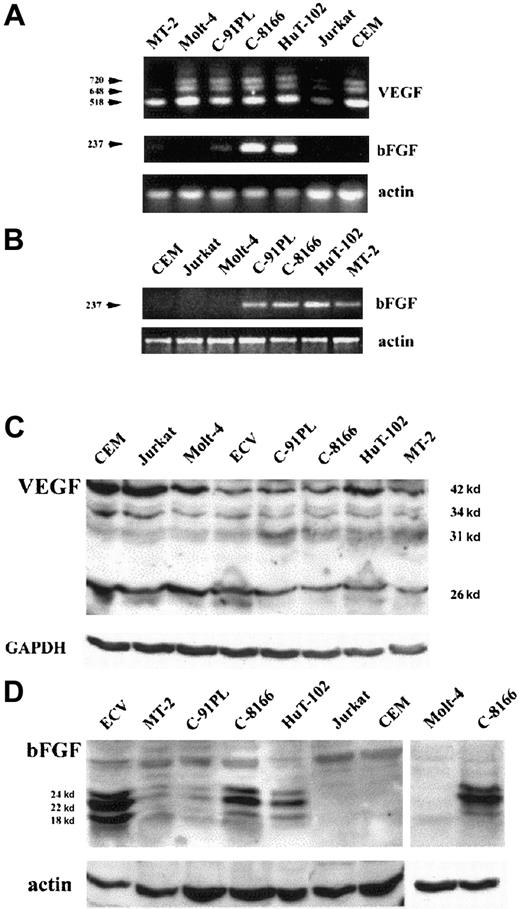
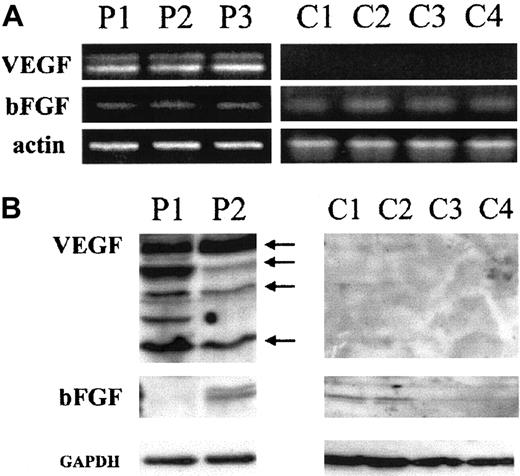
Expression of the 2 major angiogenic factors, VEGF and bFGF, was investigated at the mRNA and protein levels. Amplification of 100 ng total RNA by RT-PCR shows that HTLV-I–positive (HuT-102, MT-2, C8166, C91PL) and –negative cell lines (CEM, Jurkat, Molt-4) express 3 VEGF transcripts recognized as PCR products 518, 648, 720 bp (Figure1A), whereas 2 VEGF transcripts (518 and 648 bp) were detected in fresh leukemic cells from 3 patients with ATL (Figure 2A). In contrast, VEGF transcripts were undetectable in freshly isolated peripheral blood lymphocytes from 4 healthy controls (Figure 2A). Expression of the bFGF transcript (237 bp) was only detected in the HTLV-I–positive cell lines with a significant expression in HuT-102 and C8166 cells and to a lesser expression in MT-2 and C91PL, whereas CEM, Jurkat, and Molt-4 were completely negative (Figure 1A). When 500 ng total RNA was used, the bFGF transcript was detectable in all tested HTLV-I–positive cell lines (HuT-102, MT-2, C8166, C91PL) (Figure 1B), in fresh leukemic cells from 3 patients with ATL, and in freshly isolated peripheral blood lymphocytes from 4 healthy controls (Figure 2A), but the HTLV-I–negative cell lines (CEM, Jurkat, and Molt-4) were negative (Figure 1B).
HTLV-I–positive cells produce VEGF and bFGF.
(A,B) Analysis of VEGF and bFGF mRNA isoforms. HTLV-I–transformed cells (HuT-102, MT-2, C8166, C91PL) and HTLV-I–negative cells (Molt-4, Jurkat, CEM) were analyzed for VEGF and bFGF mRNA expression by RT-PCR using 100 ng total RNA (A). bFGF mRNA expression was also assessed by RT-PCR using 500 ng total RNA (B). (C,D) Detection of cell-associated VEGF and bFGF proteins. Total cell extracts from HTLV-I–transformed cells, HTLV-I–negative cells, and endothelial cells (ECV-304) were analyzed by Western blot using anti-VEGF– (C) and anti-bFGF– (D) specific antibodies. Antiactin and anti-GAPDH antibodies were used for assessment of protein loading.
HTLV-I–positive cells produce VEGF and bFGF.
(A,B) Analysis of VEGF and bFGF mRNA isoforms. HTLV-I–transformed cells (HuT-102, MT-2, C8166, C91PL) and HTLV-I–negative cells (Molt-4, Jurkat, CEM) were analyzed for VEGF and bFGF mRNA expression by RT-PCR using 100 ng total RNA (A). bFGF mRNA expression was also assessed by RT-PCR using 500 ng total RNA (B). (C,D) Detection of cell-associated VEGF and bFGF proteins. Total cell extracts from HTLV-I–transformed cells, HTLV-I–negative cells, and endothelial cells (ECV-304) were analyzed by Western blot using anti-VEGF– (C) and anti-bFGF– (D) specific antibodies. Antiactin and anti-GAPDH antibodies were used for assessment of protein loading.
Fresh ATL leukemic cells express VEGF and bFGF.
(A) Analysis of VEGF and bFGF mRNA isoforms. Fresh leukemic cells from 3 patients with acute ATL (P1, P2, and P3) and fresh lymphocytes from 4 healthy controls (C1, C2, C3, and C4) were analyzed for VEGF and bFGF mRNA expression by RT-PCR using 500 ng total RNA. (B) Detection of cell-associated VEGF and bFGF proteins. Total cell extracts from 2 patients with acute ATL (P1 and P2) and fresh lymphocytes from 4 healthy controls (C1, C2, C3, and C4) were analyzed by Western blot using anti-VEGF– and anti-bFGF–specific antibodies. Anti-GAPDH antibodies were used for assessment of protein loading.
Fresh ATL leukemic cells express VEGF and bFGF.
(A) Analysis of VEGF and bFGF mRNA isoforms. Fresh leukemic cells from 3 patients with acute ATL (P1, P2, and P3) and fresh lymphocytes from 4 healthy controls (C1, C2, C3, and C4) were analyzed for VEGF and bFGF mRNA expression by RT-PCR using 500 ng total RNA. (B) Detection of cell-associated VEGF and bFGF proteins. Total cell extracts from 2 patients with acute ATL (P1 and P2) and fresh lymphocytes from 4 healthy controls (C1, C2, C3, and C4) were analyzed by Western blot using anti-VEGF– and anti-bFGF–specific antibodies. Anti-GAPDH antibodies were used for assessment of protein loading.
Western blot analysis revealed that all cell lines significantly expressed at least one of the reported VEGF protein isoforms (Figure1C). High levels of VEGF protein were also detected in fresh ATL cells derived from 2 patients with acute ATL, but not in freshly isolated peripheral blood lymphocytes from 4 healthy controls (Figure 2B). On the other hand, a high level of protein expression of 3 isoforms of bFGF (24, 22, 18 kd) was observed, mainly in HuT-102 and C-8166 cell lines (Figure 1D). Consistent with the RT-PCR studies, no bFGF could be detected in HTLV-I–negative cell lines. Fresh ATL cells from 2 patients with acute ATL and freshly isolated peripheral blood lymphocytes from 4 healthy controls showed a moderate expression of 2 isoforms of bFGF in one patient with ATL and one isoform in 2 healthy controls (Figure 2B).
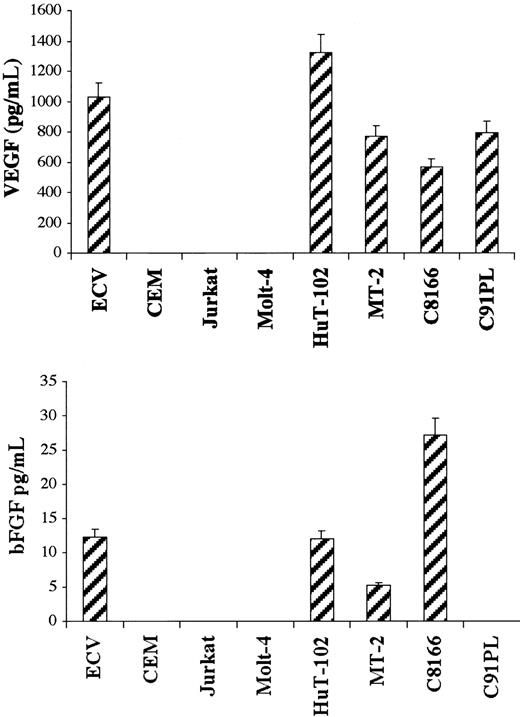
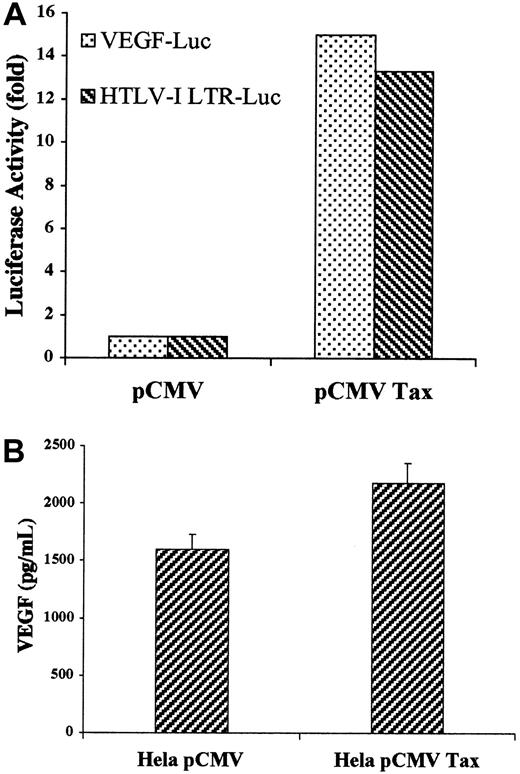
Secretion of VEGF and bFGF in cellfree culture supernatant was determined by ELISA. HTLV-I–positive cells produced variable levels of VEGF ranging from 570 pg/mL in the C-8166 cell line to 1320 pg/mL in the HuT-102 cell line, whereas ECV-304 cells produced 1000 pg/mL (Figure 3). In sharp contrast and despite the fact that these cells make VEGF (Figure 1), CEM, Jurkat, and Molt-4 cell lines were below the assay detection level (4 pg/mL). Similarly, bFGF was secreted into the culture medium of 3 of the 4 tested HTLV-I–positive cell lines—5.2 pg/mL in the MT-2 cell line, 12.1 pg/mL in the HuT-102 cell line, and 27.2 pg/mL in the C-8166 cell line—whereas the C91PL cell line did not reach the sensitivity of the assay (4 pg/mL) (Figure 3). ECV-304 cells produced 12.3 pg/mL. CEM, Jurkat, and Molt-4 cell lines consistently did not reach the sensitivity of the assay. The role of HTLV-I viral genes in the production of active angiogenic factors by HTLV-I–transformed cell lines was investigated by expressing the Tax viral transactivator in HTLV-I–negative cells. Jurkat cells were cotransfected with Tax and a reporter plasmid in which the VEGF promoter or the HTLV-I LTR controlled the luciferase cDNA. Luciferase assays showed that Tax induced a 15-fold increase in VEGF promoter activity compared with a 13.3-fold increase in HTLV-I LTR promoter activity (Figure4A). Furthermore, Tax-transfected HeLa cells showed a significant increase in VEGF secretion (Figure 4B). These results demonstrate that HTLV-I–positive cells synthesize and secrete VEGF and bFGF, presumably as the result of Tax-induced transcriptional activation, at least for the VEGF gene. Although moderate bFGF protein expression is observed in fresh leukemic cells from some patients with ATL and freshly isolated lymphocytes from some healthy controls, the demonstration that fresh ATL leukemic cells but not control lymphocytes produce VEGF transcripts and significant protein levels suggests that angiogenesis might play an important role in ATL pathogenesis.
HTLV-I–positive cells secrete VEGF and bFGF.
ELISA measurement of secreted VEGF and bFGF. HTLV-I–transformed cells, HTLV-I–negative cells, and endothelial cells were cultured for 72 hours. Levels of VEGF and bFGF in the cell supernatant were measured using a commercial ELISA kit. The amount of VEGF and bFGF proteins in the samples was calculated using a reference curve established from serial dilutions of rhVEGF and rhbFGF proteins. All experiments were performed in triplicate and were repeated a minimum of 3 times.
HTLV-I–positive cells secrete VEGF and bFGF.
ELISA measurement of secreted VEGF and bFGF. HTLV-I–transformed cells, HTLV-I–negative cells, and endothelial cells were cultured for 72 hours. Levels of VEGF and bFGF in the cell supernatant were measured using a commercial ELISA kit. The amount of VEGF and bFGF proteins in the samples was calculated using a reference curve established from serial dilutions of rhVEGF and rhbFGF proteins. All experiments were performed in triplicate and were repeated a minimum of 3 times.
Effect of Tax transfection on VEGF promoter activity and secretion.
(A) VEGF-Luc or HTLV-I LTR-Luc constructs in pGL-2 corresponding to the luciferase gene under the control of the VEGF promoter or the HTLV-I promoter, respectively, was cotransfected into Jurkat cells with the internal control PSV β-galactosidase and either pCMV-Tax or empty vector. Luciferase activity was quantified 24 hours later and was normalized with the β-galactosidase activity. (B) HeLa cells were transfected with pCMV-Tax or empty vector. Levels of VEGF in the cell supernatant were measured using a commercial ELISA kit.
Effect of Tax transfection on VEGF promoter activity and secretion.
(A) VEGF-Luc or HTLV-I LTR-Luc constructs in pGL-2 corresponding to the luciferase gene under the control of the VEGF promoter or the HTLV-I promoter, respectively, was cotransfected into Jurkat cells with the internal control PSV β-galactosidase and either pCMV-Tax or empty vector. Luciferase activity was quantified 24 hours later and was normalized with the β-galactosidase activity. (B) HeLa cells were transfected with pCMV-Tax or empty vector. Levels of VEGF in the cell supernatant were measured using a commercial ELISA kit.
HTLV-I–transformed cells induce endothelial cell tube formation in vitro
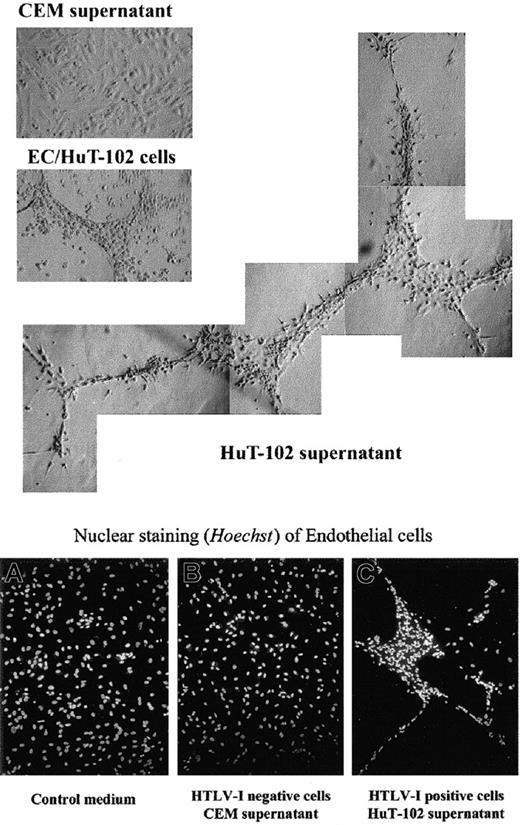
The functional consequence of VEGF and bFGF secretion by HTLV-I–transformed cells on endothelial cells was then evaluated. HAECs were plated onto growth factor–reduced Matrigel and were incubated with HTLV-I–positive (HuT-102, C8166 cells) or their cellfree supernatant for 48 hours. Cellfree supernatant from the HTLV-I–negative cells (CEM, Jurkat) was used as control. In the presence of HuT-102 cells or cell supernatant (Figure5) or of C8166 cell supernatant (data not shown), HAECs formed a network of capillarylike tubules with multicentric junctions. No effect was seen with CEM (Figure 5) or Jurkat supernatant (data not shown). These experiments demonstrated that the angiogenic factors released from HTLV-I–infected cells were biologically active.
Effect of HTLV-I–positive cells or cell supernatant on Matrigel-induced tube formation of endothelial cells in vitro.
Endothelial cells (ECs) were plated on growth factor-reduced Matrigel in the presence of culture medium, HuT-102 cells, HuT-102 cellfree supernatant, and CEM cellfree supernatant. Plates were photographed using light and fluorescence microscopy (magnification × 20) and Hoechst nuclear staining.
Effect of HTLV-I–positive cells or cell supernatant on Matrigel-induced tube formation of endothelial cells in vitro.
Endothelial cells (ECs) were plated on growth factor-reduced Matrigel in the presence of culture medium, HuT-102 cells, HuT-102 cellfree supernatant, and CEM cellfree supernatant. Plates were photographed using light and fluorescence microscopy (magnification × 20) and Hoechst nuclear staining.
HTLV-I–transformed cells adhere to and communicate with endothelial cells through gap junctions
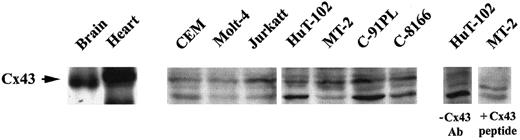
Coculture experiments between leukemic cells and the endothelium revealed that HTLV-I–transformed cells adhere to and communicate with endothelial cells much more efficiently than noninfected transformed lymphocytes. Indeed, coculture at a 1:1 ratio of HAECs with calcine-labeled HuT-102 cells resulted in a progressive transfer of fluorescence from the leukemic cells to the HAECs (Figure 6). As early as 15 minutes, 30% of labeled HuT-102 cells adhered to the endothelial cell monolayer, resulting in an increase in mean fluorescence intensity (MFI) of HAECs from 2 to 28 (Figure 6B). At 2 hours, 50% of HuT-102 cells adhered to endothelial cells, resulting in an increase in the MFI of HAECs to 70. Increasing the ratio of HuT-102 cells to HAECs resulted in a progressive increase in dye transfer. This reflects dye transfer through gap junctions from the leukemic cells because the addition of gap junction–inhibitor 18-alpha glycyrrhetinic acid (18αG)53 sharply reduced endothelial cell fluorescence (Figure 6B). Moreover, incubating endothelial cells with the supernatant of the washing step of labeled HuT-102 cells or with calcine acid at 0.5 μM did not significantly increase the MFI of HAECs, ruling out a non–gap junction-mediated dye transfer (data not shown). Adhesion and dye transfer to HAECs were also observed with HTLV-I–negative cells (Molt-4 [Figure 6] and CEM [data not shown]), albeit to a much lesser extent. The coculture of calcine-labeled Molt-4 cells with HAECs for 1 hour at a ratio of 1:1 resulted in a minimal adhesion of added Molt-4 cells (10%) associated with an increase of HAEC MFI to 8 (Figure 6). Furthermore, the MFI of endothelial cells increased to 20 only at a labeled Molt-4–to–HAEC ratio of 2:1 (Figure 6B). Consistent with the heterologous cell-to-cell transfer, Western blot analysis showed that all tested HTLV-I–infected cell lines express a basal level of Cx43 protein (Figure7). In addition, RT-PCR analysis using Cx-specific primers showed that these cell lines expressed mRNA for at least one other type of Cx (many exhibited multiple forms) (data not shown).
Adhesion and dye transfer from HTLV-I–transformed cells to endothelial cells.
Flow cytometry analyses of cocultures of unlabeled HAECs (ECs) with calcein-labeled HTLV-I–positive cells (*HuT-102) or control cells (*Molt-4). (A) The relative height of the peak of HuT-102 or Molt-4 cells in comparison with that of HAECs is a measure of cell adhesion. The increase in the MFI of endothelial cells reflects dye transfer through gap junctions from the leukemic cells. Endothelial cells were cocultured for 1 hour with calcine-labeled tumor cells at a ratio of 1:1. (B) Histogram analysis of MFI of endothelial cells under different coculture conditions. Cells were cocultured at different endothelial cell–tumor cell ratios (EC = TC) and time intervals as indicated.
Adhesion and dye transfer from HTLV-I–transformed cells to endothelial cells.
Flow cytometry analyses of cocultures of unlabeled HAECs (ECs) with calcein-labeled HTLV-I–positive cells (*HuT-102) or control cells (*Molt-4). (A) The relative height of the peak of HuT-102 or Molt-4 cells in comparison with that of HAECs is a measure of cell adhesion. The increase in the MFI of endothelial cells reflects dye transfer through gap junctions from the leukemic cells. Endothelial cells were cocultured for 1 hour with calcine-labeled tumor cells at a ratio of 1:1. (B) Histogram analysis of MFI of endothelial cells under different coculture conditions. Cells were cocultured at different endothelial cell–tumor cell ratios (EC = TC) and time intervals as indicated.
Detection of connexin-43 protein expression.
Total cell extracts from HTLV-I–transformed cells (HuT-102, MT-2, C8166, C91PL) and HTLV-I–negative cells (Jurkat, CEM, Molt4) were analyzed by Western blot using anti-Cx43–specific antibodies. Rat brain and heart extracts were used as positive control. Specificity was demonstrated by competition with Cx43 peptide or omission of anti-Cx43 primary antibody.
Detection of connexin-43 protein expression.
Total cell extracts from HTLV-I–transformed cells (HuT-102, MT-2, C8166, C91PL) and HTLV-I–negative cells (Jurkat, CEM, Molt4) were analyzed by Western blot using anti-Cx43–specific antibodies. Rat brain and heart extracts were used as positive control. Specificity was demonstrated by competition with Cx43 peptide or omission of anti-Cx43 primary antibody.
Discussion
We show that HTLV-I–transformed cells, but not HTLV-I–negative cell lines, secrete high levels of the angiogenic factors VEGF and bFGF, presumably as the result of Tax-dependent transcriptional activation, at least for the VEGF gene, and induce angiogenesis in vitro. Fresh ATL leukemic cells derived from patients with acute ATL produce VEGF and bFGF transcripts and proteins, suggesting an important role for angiogenesis in ATL pathogenesis. Gap junction–mediated heterocellular communication between HTLV-I–transformed cells and endothelial cells is also demonstrated, representing the first evidence for heterocellular communication in leukemia.
Angiogenesis is critical for the growth of solid tumors14,15 and of some hematologic malignancies.19-30,54,55 Our findings support a potential role for angiogenesis in HTLV-I–associated diseases because HTLV-I–transformed CD4+ T cells, but not the 3 tested uninfected cell lines, secrete active VEGF and bFGF and induce endothelial cell angiogenesis in vitro, though one HTLV-I–positive cell line (C91PL) secretes VEGF only. This hypothesis is further supported by the fact that fresh ATL leukemic cells derived from patients with acute ATL produce VEGF and bFGF transcripts and proteins, whereas freshly isolated peripheral blood lymphocytes produce bFGF but not VEGF. We present evidence for a transcriptional activation of the VEGF promoter by Tax associated with increased VEGF secretion, suggesting that VEGF may be one of the cellular Tax targets and that the viral transactivator may be the so-called angiogenic switch.56-58 Although it is difficult to detect Tax expression in vivo, recently it has been shown that Tax is expressed in vivo; however, Tax-expressing cells are rapidly eliminated by Tax-specific cytotoxic lymphocytes.59 However, a contribution of the other viral proteins cannot be ruled out.
We also demonstrate a direct cell–cell communication through gap junctions. Gap-junctional coupling of tumor cells facilitates their invasion of normal tissue.60 We have also demonstrated their role for heterocellular communication between blood-borne cancer cells and endothelium of the target organ of metastasis.50We report here the first evidence for gap junction–mediated heterocellular communication between endothelial cells and hematologic malignant cells, particularly HTLV-I–infected T lymphocytes. Angiogenic factors up-regulate the ability of endothelial cells to communicate through gap junctions and, therefore, greatly enhance the significance of heterocellular communication with tumor cells.61 62 Although Cx43 is expressed in HTLV-I–positive and –negative cell lines, gap junction–mediated heterocellular communication requires specific adhesion to endothelial cells, which we show to be significantly more important for HTLV-I–positive cells (Figure 6). Through the production of angiogenic factors, HTLV-I–positive cells stimulate the proliferation of endothelial cells, which increases the gap-junction–mediated communication. Hence, heterocellular communication with endothelial cells is much more significant for HTLV-I–positive cells than for transformed CD4+ T lymphocytes, with almost a 10-fold difference between the 2 cell types (Figure 6B, MFI).
The maintenance of ATL cells in culture is extremely difficult, and most, if not all, cell lines from patients with ATL are derived from the minor population of nonleukemic, HTLV-I–positive polyclonal T lymphocytes. This suggests that ATL cells may require a survival factor present in vivo but not in the culture medium. A recent abstract demonstrates the possibility of growing ATL cells in vitro in the presence of stromal cells.63 It is conceivable that interaction with endothelial cells through paracrine or direct cell–cell communication may facilitate the growth of HTLV-I–infected cells in vivo and, hence, may play a role in the development of HTLV-I–associated diseases.
In conclusion, angiogenesis, cell adhesion, and communication likely contribute to the development of adult T-cell leukemia–lymphoma, and they represent potential therapeutic targets.
We thank Drs Kamal Badr, Ghassan Dbaibo, and Fadia Homaidan for their critical reading of this manuscript. We also thank the personnel of the Core Laboratory Facilities of the American University of Beirut for their expert assistance.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ali Bazarbachi, Department of Internal Medicine, American University of Beirut, PO Box 113-6044; Marwan E. El-Sabban, Department of Human Morphology, Faculty of Medicine, American University of Beirut, PO Box 11-0236, Beirut, Lebanon; e-mail:bazarbac@aub.edu.lb; me00@aub.edu.lb.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal