Abstract
The human monoclonal antibody mAb-LE2E9 partially inactivates human factor VIII (FVIII), leaving approximately 10% residual activity. The antithrombotic efficacy of the antibody was evaluated in mouse models of inferior vena cava thrombosis. Thrombi were induced in wild-type mice given either the antibody or saline. No thrombi occurred in any of 8 mice treated with mAb-LE2E9, whereas 6 of 8 control mice developed thrombi (P = .007). Treatment with mAb-LE2E9 did not result in a severe bleeding phenotype: a tail-cutting experiment that resulted in death of C57BL/6 FVIII-deficient (FVIII−/−) mice did not cause hemorrhagic death in mice treated with mAb-LE2E9. To evaluate the antithrombotic effect of mAb-LE2E9 in presence of human FVIII, thrombus formation was induced in FVIII−/− mice reconstituted intravenously with recombinant human FVIII (rhFVIII) or rhFVIII preincubated with mAb-LE2E9. Only 1 of 9 mice produced a thrombus in the rhFVIII/antibody complex–treated group, compared with 7 of 9 in the control group (P = .015). FVIII−/− mice were also reconstituted with rhFVIII and then injected with either mAb-LE2E9 or saline. One of 14 mice in the group treated with the antibody developed a thrombus, compared with 10 of 14 in the control group (P = .001). The thrombi occurring in antibody-treated animals were smaller than in controls (P < .01). All animals survived, and there were no bleeding complications. Thus, the mAb-LE2E9 antibody inhibits thrombosis without causing an overt bleeding tendency.
Introduction
Venous thromboembolic disease remains a major health issue, with an incidence of 1 to 3 per 1000 individuals per year and a high early mortality rate.1,2 Current antithrombotic therapies primarily consist of heparin (or low molecular weight heparins) and vitamin K antagonists. All carry a significant risk of bleeding,3 which limits both the dose and duration of treatment and may require regular monitoring.4 5 Safer and more efficient methods for the prevention and treatment of venous thromboembolic diseases are therefore desirable.
Factor VIII (FVIII), an essential cofactor of blood coagulation, is a 330-kd glycoprotein produced by the liver, which circulates in the form of a complex with von Willebrand factor (VWF). Upon cleavage by thrombin, activated FVIII (FVIIIa) dissociates from VWF and assembles with activated factor IX (FIXa) on negatively charged phospholipids to form the “tenase” complex, resulting in a 100 000-fold increased activation of factor X.6 Besides its key role in hemostasis, FVIII has been associated with the development of venous thrombosis,7-10 suggesting that FVIII could constitute an appropriate target for antithrombotic therapy.
The human high-affinity monoclonal antibody mAb-LE2E9 inhibits the procoagulant activity of FVIII specifically, but only partially (around 90%), even in large molar excess.11 Here, we hypothesized that administration of mAb-LE2E9 might have an antithrombotic effect without the risk of overdosing or spontaneous hemorrhage. The aim of this study was to evaluate the pharmacokinetics and potential antithrombotic effect of mAb-LE2E9 in a novel model of venous thrombosis.
Materials and methods
Reagents
Recombinant human FVIII (rhFVIII) was obtained from Wyeth/Genetics Institute (Cambridge, MA). The human anti-FVIII monoclonal antibody, mAb-LE2E9, was produced in vitro by the lymphoblastoid cell line LE2E9.11 Antibodies were purified from cell culture supernatant by adsorption on immobilized protein A (high-TRAP Protein A, Pharmacia, Uppsala, Sweden). FVIII-deficient mice (FVIII−/−) in C57BL/6 genetic background were kindly provided by S. Antonarakis (Université de Genève, Switzerland) and bred at the Specific Pathogen Free facility of the Molecular Cardiovascular Medicine group of the University of Leuven, Belgium.
FVIII assays
FVIII activity was assessed in plasma of different species by a chromogenic substrate assay (Dade FVIII Chromogenic assay, Dade AG, Switzerland). Inhibition of plasma FVIII activity was measured by the Bethesda method.12 Briefly, equal volumes of citrated human, rat, rabbit, or mouse plasma and mAb-LE2E9 solution at different concentrations were mixed and incubated at 37°C for 2 hours. Residual FVIII was measured in a chromogenic substrate assay.
In vitro inhibition of rhFVIII was also measured when various concentrations of rhFVIII were incubated with mAb-LE2E9 at a fixed concentration and in excess over FVIII. Thus, rhFVIII at final concentrations of 1, 2, 4, 8, and 16 IU/mL was added to plasma preincubated for 2 hours at 37°C with 1 or 20 μg/mL mAb-LE2E9 or control buffer. Following a further 2-hour incubation period at 37°C, residual FVIII activity was measured in a chromogenic substrate assay.
Pharmacokinetics of mAb-LE2E9 in wild-type mice
The plasma disappearance rate of 100 μg intravenously or subcutaneously injected mAb-LE2E9 (which constitutes at least 100-fold molar excess over murine FVIII) and its effect on circulating murine FVIII levels were measured in blood samples obtained by cardiac puncture in groups of 3 mice taken at intervals up to 3 weeks after injection. Levels of mAb-LE2E9 were measured by enzyme-linked immunosorbent assay and expressed in percentage of the value obtained 20 minutes after intravenous injection and 24 hours after subcutaneous administration.
Pharmacokinetics of rhFVIII, mAb-LE2E9, and mAb-LE2E9/rhFVIII complex in FVIII−/− mice
The recovery and plasma disappearance rate of rhFVIII in 20 to 26 g FVIII−/− mice were determined following intravenous injection of (1) 7.5 IU rhFVIII alone, (2) 7.5 IU rhFVIII complexed with 10 μg mAb-LE2E9, and (3) 7.5 IU rhFVIII followed 15 minutes later by 10 μg mAb-LE2E9.
Bolus tail vein injections of 100 μL were used. Levels of rhFVIII were measured in blood samples collected by cardiac puncture at 5, 10, 15, 30, 45, 260, and 420 minutes after injection (n = 3 mice per time point).
Mouse model of vena cava thrombosis in wild-type mice
Thrombus was produced in the inferior vena cava of adult male wild-type mice (weight, 18-31 g; age, 8-10 weeks) using a modification of the previously described model of vena cava thrombosis in the rat.13 Mice were anesthetized with isoflurane, the inferior vena cava was exposed below the renal veins via a median laparotomy, and a neurosurgical vascular clip (Braun Medical, Sheffield, England) was applied for 15 seconds on 2 occasions, 30 seconds apart, to a segment of the vena cava. A 5/0 Prolene thread was then placed alongside the vena cava and a stenosis produced by tying a 4/0 silk suture around the vena cava and the Prolene thread. The thread was removed to allow blood flow to resume. The abdomen was closed and the animal allowed to recover. After 4 hours, the mice were reanesthetized and a 1 cm portion of the inferior vena cava (between the point of ligature and iliac bifurcation) was excised, washed in 10% phosphate-buffered saline, and soaked overnight in 1% paraformaldehyde. Vessel segments were embedded in paraffin wax, and 7-μm transverse sections were cut at 0.5-mm intervals from the ligature down.
Thrombus assessment
Sections were stained by hematoxylin and eosin, Martius Scarlet Blue (MSB), and a rabbit antiplatelet antibody (Accurate Chemical & Scientific, Westbury, NY). MSB stains fresh fibrin red or mature fibrin blue/gray, red cells yellow, and collagen bright blue.
Thrombus size was measured by scoring the 7 sections for the presence of thrombus, giving a score of 1 for the presence and 0 for the absence of thrombus in each. Scores were then added up for each animal. The investigators performing the operations and the microscopic analyses were blinded toward treatment groups.
Thrombus formation in wild-type mice treated with mAb-LE2E9
Thrombosis was induced in 2 groups of 8 wild-type mice 16 hours following subcutaneous injection of 150 μg mAb-LE2E9 or saline. In additional experiments, 2 groups of 6 wild-type C57BL/6 mice were treated with an intravenous injection of 10 μg mAb-LE2E9 or saline 30 minutes before thrombus induction.
Effect of mAb-LE2E9 antibody on thrombus formation in FVIII−/− mice reconstituted with rhFVIII
Following initial experiments to establish optimal conditions, 2 series of controlled experiments were carried out.
In the first experiments, venous thrombosis was induced in a group of 18 FVIII−/− mice 30 minutes after reconstitution with either 7.5 IU rhFVIII (350 IU/kg) (n = 9) or 7.5 IU human rhFVIII preincubated for 2 hours in vitro with 10 μg mAb-LE2E9 (350 IU rhFVIII per kilogram, 450 μg mAb-LE2E9 per kilogram) (n = 9).
In the second set of experiments, a cohort of 28 FVIII−/−mice were reconstituted with 7.5 IU rhFVIII (350 IU/kg) followed 15 minutes later by an intravenous injection of either 10 μg mAb-LE2E9 antibody (450 μg/kg) (n = 14) or saline (n = 14). Venous thrombi were induced 30 minutes later as described above.
All animals were killed after 4 hours, and 1 cm of the vena cava, between the point of ligature and iliac bifurcation, was harvested and processed for histology.
Effect of mAb-LE2E9 on bleeding after tail cutting in wild-type mice
The risk of severe bleeding associated with high concentrations of mAb-LE2E9 in plasma was evaluated in a tail-cutting experiment. This assay is based on the observation that sectioning of the distal portion of the tail results in important blood loss leading to death in most FVIII−/− mice14 whereas normal mice should survive. This procedure allows an in vivo evaluation of FVIII activity. Twelve wild-type mice were injected subcutaneously with either 150 μg mAb-LE2E9 (n = 6) or saline (n = 6). Tail cutting was also performed in 5 FVIII−/− mice in a control experiment. A 7-mm section of the tail was then cut 16 hours later and survival rate monitored over the subsequent 24 hours.
Statistical analysis
The statistical significance of differences between groups was evaluated on the presence or absence of thrombus using the Fisher exact test (2-sided). The effect on thrombus size was tested by comparing thrombus scores using the Mann-Whitney Utest.15
Results
In vitro inhibition of FVIII by mAb-LE2E9 in plasma from different species
To identify animal models suitable to evaluate the antithrombotic activity of mAb-LE2E9, inhibition of FVIII was measured in plasma of different strains. Rat and rabbit FVIII appeared to be resistant to inhibition by mAb-LE2E9. By contrast, mouse FVIII activity was dose-dependently inhibited, although with a lower efficacy, evidenced by a shift to the right of FVIII inhibition curve. Twenty times more mAb-LE2E9 was required to achieve maximal FVIII inhibition for murine than for human plasma.
In vitro inhibition of rhFVIII was evaluated by incubating different concentrations of rhFVIII spiked in human plasma containing mAb-LE2E9. At rhFVIII concentrations between 1 and 8 IU/mL, the proportion of FVIII activity inhibited by 20 μg/mL mAb-LE2E9 was identical: the residual FVIII activity increased by 0.11 ± 0.03 IU/mL (mean ± SD) per international unit of rhFVIII added to plasma. Similarly, in presence of 1 μg/mL mAb-LE2E9, the increase in FVIII activity was 0.12 ± 0.02 IU/mL (mean ± SD) per international unit of rhFVIII added to plasma, when rhFVIII was added at concentrations lower or equal to 4 IU/mL.
Pharmocokinetics and pharmacodynamics of mAb-LE2E9 in wild-type mice
Because mouse FVIII was inhibited by mAb-LE2E9, this animal strain was selected for in vivo analysis of mAb-LE2E9 activity. Injection of 100 μg mAb-LE2E9 in wild-type mice, which constitutes at least 100-fold molar excess over murine FVIII, was followed by a plasma disappearance rate with a half-life of about 3 days. The murine endogenous FVIII decreased to roughly 10% of baseline for 7 days when the mAb-LE2E9 level had decreased by two thirds, after which FVIII gradually rose to reach baseline levels at 20 days, concomitant with further clearance of the antibody. No significant differences were observed between intravenous (Figure 1A) and subcutaneous (Figure 1B) injection of the antibody.
Pharmacokinetics and pharmacodynamics of mAb-LE2E9 in wild-type mice.
Wild-type mice were injected intravenously (A) or subcutaneously (B) with 100 μg mAb-LE2E9. Blood samples were harvested for 3 weeks, starting 5 hours after intravenous administration and 24 hours following subcutaneous administration. The concentrations of mAb-LE2E9 (open circle) are expressed as percentage of concentration in the first blood sample. FVIII activity is expressed as percentage of FVIII activity before mAb-LE2E9 injection (closed circle). Results are expressed as mean ± SD.
Pharmacokinetics and pharmacodynamics of mAb-LE2E9 in wild-type mice.
Wild-type mice were injected intravenously (A) or subcutaneously (B) with 100 μg mAb-LE2E9. Blood samples were harvested for 3 weeks, starting 5 hours after intravenous administration and 24 hours following subcutaneous administration. The concentrations of mAb-LE2E9 (open circle) are expressed as percentage of concentration in the first blood sample. FVIII activity is expressed as percentage of FVIII activity before mAb-LE2E9 injection (closed circle). Results are expressed as mean ± SD.
Characteristics of mouse model of venous thrombosis
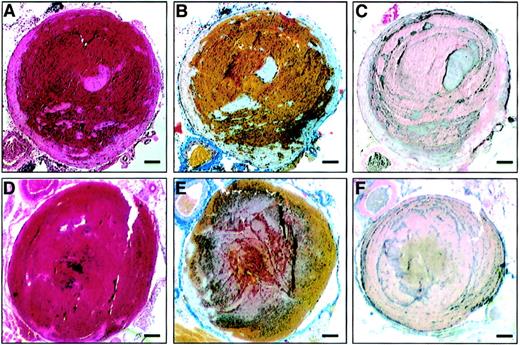
A new model was developed to evaluate the development of deep vein thrombosis in mice. Vascular wall damages and partial stenosis of the vena cava were induced by short time compression and ligation of the vein. Experimental thrombi that occluded up to 100% of the vessel were produced by 4 hours in 70% to 80% of wild-type mice and in FVIII−/− mice reconstituted with rhFVIII. The procedure was well tolerated (no evidence of hemorrhage and no postoperative deaths). Thrombi had a classical coralline structure with red cells trapped between layers of platelets, leukocytes, and fibrin, indicating that they were formed under flow conditions (Figure2A-C). This structure resembles that of human venous thrombi.16
Vena cava thrombus in wild-type C57BL/6 mice and FVIII−/−mice reconstituted with rhFVIII.
Inferior vena cava thrombosis was induced by ligation of the vein and endothelial damage resulting from short-term localized compression of the vessel. The surgical procedure was performed in wild-type mice (A-C) and FVIII−/− mice reconstituted with 7.5 IU rhFVIII (D-F). Four hours following surgery, mice were killed and thrombi analyzed histologically. (A,D) Hematoxylin/eosin-stained microscopic slide of a transverse section. (B,E) MSB stain with red cells, yellow; fresh fibrin, red; mature fibrin, blue/gray. (C,F) Immunostaining of platelets forming dark gray lines of Zahn. Magnification bar: 100 μm.
Vena cava thrombus in wild-type C57BL/6 mice and FVIII−/−mice reconstituted with rhFVIII.
Inferior vena cava thrombosis was induced by ligation of the vein and endothelial damage resulting from short-term localized compression of the vessel. The surgical procedure was performed in wild-type mice (A-C) and FVIII−/− mice reconstituted with 7.5 IU rhFVIII (D-F). Four hours following surgery, mice were killed and thrombi analyzed histologically. (A,D) Hematoxylin/eosin-stained microscopic slide of a transverse section. (B,E) MSB stain with red cells, yellow; fresh fibrin, red; mature fibrin, blue/gray. (C,F) Immunostaining of platelets forming dark gray lines of Zahn. Magnification bar: 100 μm.
Effect of mAb-LE2E9 on thrombosis in wild-type mice
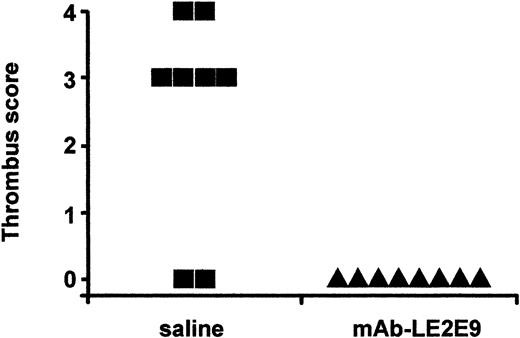
Two groups of 8 mice were injected subcutaneously with either 150 μg mAb-LE2E9 or saline 16 hours before induction of thrombosis. Six of 8 mice injected with saline developed a thrombus, compared with 0 of 8 animals pretreated with mAb-LE2E9 (P = .007, Figure3). By contrast, no significant reduction of thrombus was seen following intravenous administration of 10 μg mAb-LE2E9 into wild-type mice 30 minutes before induction of thrombosis (4 of 6 mice injected with saline and 3 of 6 mice treated with mAb-LE2E9 developed a thrombus).
Effect of mAb-LE2E9 on vena cava thrombosis in mice.
Thrombus was induced in the inferior vena cava 16 hours after subcutaneous administration of 150 μg mAb-LE2E9 or saline. Animals were killed after 4 hours. Seven transverse segments at 0.5-mm intervals through the infrarenal vena cava were scored 1 if thrombus was present or zero if absent, and the scores were summed.
Effect of mAb-LE2E9 on vena cava thrombosis in mice.
Thrombus was induced in the inferior vena cava 16 hours after subcutaneous administration of 150 μg mAb-LE2E9 or saline. Animals were killed after 4 hours. Seven transverse segments at 0.5-mm intervals through the infrarenal vena cava were scored 1 if thrombus was present or zero if absent, and the scores were summed.
Vena cava thrombosis in FVIII−/− mice
Because the dose-dependent inhibition of mice and human FVIII by mAb-LE2E9 is different, we investigated the ability of mAb-LE2E9 to prevent thrombosis in FVIII−/− mice reconstituted with rhFVIII. We first determined whether induction of deep vein thrombosis in FVIII−/− mice required administration of FVIII. The surgical procedure for induction of vena cava thrombus was applied to 6 FVIII−/− mice. All animals showed signs of significant blood loss and deterioration of their physical condition by 4 hours after surgery, and none produced a thrombus. Therefore, FVIII−/− mice reconstituted with rhFVIII were explored as an alternative model to their wild-type counterpart.
Pharmacokinetics of rhFVIII and rhFVIII/mAb-LE2E9 complex in FVIII−/− mice
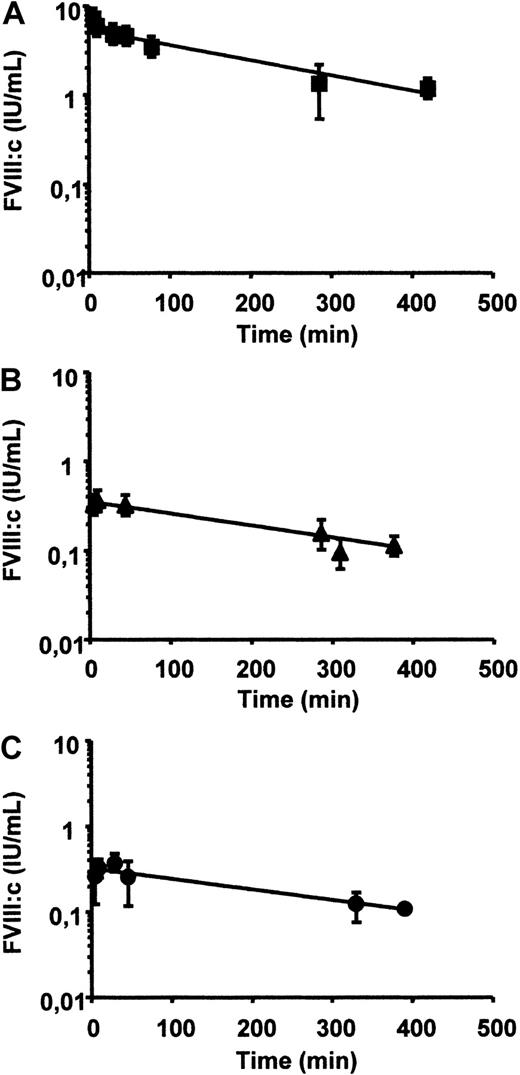
The in vivo recoveries and plasma disappearance rates in FVIII−/− mice were determined for rhFVIII, for rhFVIII complexed in vitro with mAb-LE2E9, and for rhFVIII followed by injection of mAb-LE2E9, as described in “Materials and methods.” Injection of 7.5 IU rhFVIII produced FVIII levels of 4.6 ± 1.2 IU/mL (mean ± SD) at 30 minutes, which was cleared with a plasma half-life of approximately 3 hours to reach levels of 1.3 ± 0.8 IU/mL after 4.5 hours (Figure 4A). Injection of 100 μL of a mixture of 7.5 IU rhFVIII, preincubated for 30 minutes with 10 μg mAb-LE2E9 (resulting in plateau inhibition), produced FVIII levels of 0.32 ± 0.02 IU/mL at 30 minutes, which was cleared with a plasma half-life of approximately 4 hours (Figure 4B). Finally, injection of 7.5 rhFVIII followed 15 minutes later by injection of 10 μg mAb-LE2E9 produced FVIII levels at 15 minutes of 0.36 ± 0.04 IU/mL, which was cleared with a plasma half-life of approximately 4 hours (Figure 4C).
Pharmacokinetics of rhFVIII and rhFVIII/mAb-LE2E9 complex in FVIII−/− mice.
FVIII activity was measured in FVIII−/− mice following administration of rhFVIII in presence or absence of mAb-LE2E9. (A) A total of 7.5 IU rhFVIII (350 IU/kg) was injected intravenously; (B) 100 μL of a mixture containing 7.5 IU rhFVIII and 10 μg mAb-LE2E9 was preincubated for 30 minutes in vitro and administered intravenously; (C) 100 μL containing 7.5 IU rhFVIII was injected intravenously followed after 15 minutes by 100 μL containing 10 μg mAb-LE2E9. FVIII levels were measured in plasma samples taken at indicated periods of time in groups of 3 mice. Results are expressed as mean ± SD.
Pharmacokinetics of rhFVIII and rhFVIII/mAb-LE2E9 complex in FVIII−/− mice.
FVIII activity was measured in FVIII−/− mice following administration of rhFVIII in presence or absence of mAb-LE2E9. (A) A total of 7.5 IU rhFVIII (350 IU/kg) was injected intravenously; (B) 100 μL of a mixture containing 7.5 IU rhFVIII and 10 μg mAb-LE2E9 was preincubated for 30 minutes in vitro and administered intravenously; (C) 100 μL containing 7.5 IU rhFVIII was injected intravenously followed after 15 minutes by 100 μL containing 10 μg mAb-LE2E9. FVIII levels were measured in plasma samples taken at indicated periods of time in groups of 3 mice. Results are expressed as mean ± SD.
Vena cava thrombosis in FVIII −/− mice reconstituted with rhFVIII
Thrombus formation was induced in FVIII−/− mice following administration of 7.5 IU rhFVIII, which resulted in a concentration of FVIII higher than 1 IU/mL for more than 4 hours. Thrombi formed in FVIII−/− mice reconstituted with rhFVIII were microscopically similar to those formed in wild-type animals (Figure 2D-F and 2A-C, respectively).
Effect of mAb-LE2E9 on thrombosis in FVIII−/− mice reconstituted with rhFVIII
The effect of mAb-LE2E9 was evaluated in 2 comparative studies. One study compared rhFVIII with rhFVIII/mAb-LE2E9 complex preincubated 2 hours in vitro (Figure 5A). This experimental procedure was selected to allow for a prolonged incubation period between mAb-LE2E9 and rhFVIII. Indeed, the half-life of rhFVIII in mice is relatively short (Figure 4A), which precludes a long incubation period in vivo before initiation of the surgical procedure. The other procedure compared rhFVIII followed by saline with rhFVIII followed by mAb-LE2E9 administered 30 minutes before initiation of the surgical procedure (Figure 5B).
Effect of mAb-LE2E9 on vena cava thrombosis in FVIII−/− mice reconstituted with rhFVIII.
Inferior vena cava thrombus induction was evaluated in FVIII−/− mice following reconstitution with rhFVIII in presence or absence of mAb-LE2E9. (A) Two groups of mice (n = 9) were reconstituted with rhFVIII (7.5 IU) or with a mixture of rhFVIII (7.5 IU) and mAb-LE2E9 (10 μg) preincubated for 30 minutes in vitro. The surgical procedure for thrombus induction was started 30 minutes following reconstitution. (B) Comparison of thrombus induction in 2 groups of mice (n = 14) treated with rhFVIII (7.5 IU intravenously) followed after 15 minutes with intravenous administration of saline of mAb-LE2E9 (10 μg). The surgical procedure was initiated 30 minutes following administration of the antibody. Animals were killed after 4 hours. Thrombus scores were evaluated as in Figure 3.
Effect of mAb-LE2E9 on vena cava thrombosis in FVIII−/− mice reconstituted with rhFVIII.
Inferior vena cava thrombus induction was evaluated in FVIII−/− mice following reconstitution with rhFVIII in presence or absence of mAb-LE2E9. (A) Two groups of mice (n = 9) were reconstituted with rhFVIII (7.5 IU) or with a mixture of rhFVIII (7.5 IU) and mAb-LE2E9 (10 μg) preincubated for 30 minutes in vitro. The surgical procedure for thrombus induction was started 30 minutes following reconstitution. (B) Comparison of thrombus induction in 2 groups of mice (n = 14) treated with rhFVIII (7.5 IU intravenously) followed after 15 minutes with intravenous administration of saline of mAb-LE2E9 (10 μg). The surgical procedure was initiated 30 minutes following administration of the antibody. Animals were killed after 4 hours. Thrombus scores were evaluated as in Figure 3.
Recombinant human FVIIII preincubated with mAb-LE2E9.
Thrombus was observed in 7 of 9 mice treated with rhFVIII only, compared with 1 of 9 mice in the rhFVIII/mAb-LE2E9 complex–treated group (P = .015) (Figure 5A). The median thrombus score in the rhFVIII group was 4 (interquartile range 1 to 7), compared with 0 (interquartile range 0 to 0) in the rhFVIII/mAb-LE2E9 group (P < .001).
Recombinant human FVIIII followed by mAb-LE2E9.
Thrombus was found in 10 of 14 mice in the rhFVIII-treated group but in only 1 of 14 mice treated with the rhFVIII followed by mAbLE2E9 (P < .001) (Figure 5B). The median thrombus scores were 6 (interquartile range 0 to 7) and 0 (interquartile range 0 to 0), respectively (P < .01).
Effect of mAb-LE2E9 on bleeding after tail cutting in wild-type mice
The risk of severe bleeding associated with high concentrations of mAb-LE2E9 in plasma was evaluated in a tail-cutting experiment. This assay is based on the observation that sectioning of the distal portion of the tail results in important blood loss leading to death in most FVIII−/− mice14 whereas normal mice survive. This procedure therefore allows an in vivo evaluation of FVIII activity. Thus, 6 mice were injected subcutaneously with 150 μg mAb-LE2E9 16 hours before tail cutting. No death was recorded in control wild-type mice or wild-type mice treated with mAb-LE2E9. In a control group made of 5 FVIII−/− mice, all animals died within 24 hours after tail cutting.
Discussion
In this study, we sought evidence that the partially inhibitory human monoclonal antibody against FVIII, mAb-LE2E9, might constitute a novel approach to anticoagulant therapy without the risk of overdosing or causing spontaneous bleeding.
The possibility of using mAb-LE2E9 as an antithrombotic was derived from 2 main observations. (1) Patients with mild hemophilia A (FVIII levels between 5% and 40%) do not experience bleeding except after severe trauma or surgery.17 (2) Venous thrombosis is very rare in these patients.18 This observation supports a key role for FVIII in venous thrombosis.7-10 Partial inhibition of FVIII by mAb-LE2E9 seems to mimic mild hemophilia A. Reducing FVIII activity may limit coagulation while avoiding the risk of spontaneous hemorrhage.
Some patients with hemophilia A develop an immune response toward FVIII following administration of FVIII concentrates. The anti-FVIII antibodies produced typically only partially inhibit FVIII activity in presence of VWF, whereas in the absence of the latter, the inhibition of FVIII is complete.19 By contrast, mAb-LE2E9 inhibits FVIII activity only partially even in absence of VWF.11 The mechanism responsible for the limited FVIII inactivation is still currently investigated. Surface plasmon resonance analysis indicated that mAb-LE2E9 has a high affinity for FVIII (kass = 2.9 × 105M−1 s−1;kdiss = 1.5 × 10−4s−1; calculated Kd= 0.5 × 10−9 M) (R. Lavend'homme, unpublished data, 2001). In excess of mAb-LE2E9, it can therefore be calculated that nearly all FVIII molecules should be complexed to the antibody. Recent analysis indicated that mAb-LE2E9 does not prevent FVIII binding to phospholipid membranes similar to those expressed at the surface of activated platelets (R. Lavend'homme, unpublished data, 2001). Interestingly, a recent evaluation of the role of the FVIII C1 domain suggested that this domain enhances the binding of the FVIII C2 domain to FIXa and FX.20 Given that mAb-LE2E9 recognizes an epitope located in the FVIII C1 domain, it can be hypothesized that the antibody alters interaction of FVIIIa with FIXa or FX, thereby partially reducing FVIII cofactor activity in the tenase complex.
The evaluation of mAb-LE2E9 as an antithrombotic in existing experimental rat and rabbit animal models has been hampered by the species specificity of the human monoclonal antibody. Both rat and rabbit FVIII are resistant to mAb-LE2E9. Mouse FVIII reacts, although with lower affinity than human FVIII. The latter species has the advantage that FVIII−/− animals have been generated by inactivation of the FVIII gene via homologous recombination and that rhFVIII can be used for “rescuing” the FVIII deficiency and thereby humanising the FVIII−/− mice with respect to FVIII. A model of venous thrombosis previously used to induce laminar thrombosis in the rat13 was therefore adapted for the mouse to study the antithrombotic effects of mAb-LE2E9.
The experimental thrombi produced in this model consisted mainly of red cells and were typically laminated with platelets, fibrin, and leukocytes and were similar to those seen in the rat and in humans.13,15 The thrombosis model also replicates several features relevant to the development of deep vein thrombosis in man, including endothelial disturbance, low flow, and hypercoagulability (high levels of FVIII).7-10 Thrombus developed in about 70% to 80% of wild-type and FVIII−/− mice reconstituted with rhFVIII. This probably reflects the variation in flow at the ligation site and/or the extent of vascular wall trauma.
Experiments were conducted both in wild-type mice and in FVIII−/− animals reconstituted with human FVIII because there was a marked difference between the inhibition of murine and human rhFVIII by mAb-LE2E9. A 4-hour observation period was selected because this was compatible with a 3- to 4-hour half-life of both rhFVIII and rhFVIII/mAb-LE2E9 complex in the mouse, avoiding repeated FVIII administration. Injection of 7.5 IU/kg rhFVIII resulted in FVIII levels of about 3.5 to 5.0 IU/mL 30 minutes after administration. At the end of the experiment, mean residual FVIII levels were 1.3 IU/mL. These conditions resemble the human situation in which FVIII levels above 1.5 IU/mL have a significant prothrombotic risk.7-10
The results from this study show that in FVIII−/− mice reconstituted with rhFVIII, partial neutralization of rhFVIII by in vitro complex formation with mAb-LE2E9 had a highly significant antithrombotic effect. Similarly, administration of mAb-LE29 (10 μg per animal) to FVIII−/− mice previously reconstituted with rhFVIII significantly prevented thrombus development. In wild-type mice, high doses of mAb-LE2E9 (150 μg per animal) significantly inhibited thrombus production. By contrast, low amounts of mAb-LE2E9 (10 μg per animal) had no significant effect, in agreement with the lower efficacy of mAb-LE2E9 to inhibit mice FVIII than human FVIII.
The surgical procedure did not cause excessive bleeding in animals treated with mAb-LE2E9, while the FVIII−/− animals that were not reconstituted with rhFVIII died from uncontrolled hemorrhage. Similarly, tail cutting in FVIII−/− mice resulted in death of all animals within 24 hours, whereas in wild-type mice treated with mAb-LE2E9 at a concentration required to prevent thrombosis, all animals survived. This indicates that the threshold of FVIII inactivation provided by mAb-LE2E9 is sufficient to prevent thrombosis while preserving hemostasis. In clinical practice, the level of inhibition of the coagulation cascade can be adjusted according to the thrombotic disorder. Accordingly, it may be interesting to investigate whether other anti-FVIII antibodies inhibiting FVIII to various levels could provide a better safety/efficacy ratio.
The data in this study support the concept that anticoagulant agents targeting the intrinsic pathway of the coagulation cascade could be potent antithrombotic agents while maintaining a sufficient residual coagulation activity to preserve hemostasis. Several new anticoagulant agents targeting FIX have recently been developed.21,22Anti-FIX antibodies also proved to be efficient in animal models of thrombosis while inducing only minor bleeding.23,24However, mAb-LE2E9 has several advantages over these and other antithrombotic therapies. FVIII has the lowest plasma concentration of all the coagulation factors. A small amount of mAb-LE2E9 antibody will therefore be required for its inhibition. Monoclonal antibodies are currently used for treatment of chronic diseases, such as allergy.25,26 In the latter studies, the humanized antibody omalizumab was administered every other week for months at doses up to 300 mg per injection. Given the kinetics of FVIII inactivation by mAb-LE2E9, at least 10-fold less antibody would be required for patients with thrombotic disorders. IgG4 antibodies, such as mAb-LE2E9, have a half-life of 3 weeks in humans,27 which would reduce the frequency of administration and provide very stable plasma FVIII levels. Strong FVIII inhibition can be obtained while avoiding overdosing because, even in large excess, FVIII inhibition is only partial. Finally, administration of FVIII could be used to restore a normal coagulation level if required in case of trauma or surgery.
In conclusion, our data show that a human monoclonal antibody that partially inhibits FVIII activity may represent a novel type of potent antithrombotic agent. This observation may have important implications for the development of efficient, easy, and safe strategies for the prevention and treatment of venous thrombosis.
Supported in part by grant G.0378.01 from the Flemish Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marc Jacquemin, Center for Molecular and Vascular Biology, University of Leuven, Campus Gasthuisberg, Herestraat 49, B-3000 Leuven, Belgium; e-mail: marc.jacquemin@med.kuleuven.ac.be.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal