Abstract
Using polymerase chain reaction–coupled subtractive hybridization, we have isolated genes expressed during the in vitro differentiation of murine embryonic stem cells into endothelial cells (ECs). Among the genes obtained, we identified one gene that was inducible by vascular endothelial growth factor (VEGF) in the murine EC line MSS31. Analysis of the nucleotide and deduced amino acid sequences revealed that the protein was composed of 930 amino acids, including an HEXXH(X)18E consensus sequence of the M1 aminopeptidase family, and is thought to be a mouse orthologue of puromycin-insensitive leucyl-specific aminopeptidase (mPILSAP). The recombinant protein hydrolyzed N-terminal leucyl and methionyl residues from synthetic substrates. Immunohistochemical analysis revealed that mPILSAP was expressed in ECs during postnatal angiogenesis. Specific elimination of mPILSAP expression by antisense oligodeoxynucleotide (AS-ODN) attenuated VEGF-stimulated proliferation, migration, and network formation of ECs in vitro. Moreover, AS-ODN to mPILSAP inhibited angiogenesis in vivo. These results suggest a novel function of mPILSAP, which is expressed in ECs and plays an important role in angiogenesis.
Introduction
The vascular system is the first functional organ to develop in the embryo. During the formation of the first vascular system (vasculogenesis), a subset of mesodermal cells, termed hemangioblasts, aggregate and differentiate into an external layer of endothelial cells (ECs) and an inner core of blood cells. In the ensuing process of angiogenesis, new vessels are generated from the primary capillary plexus by sprouting and intussuception and become distributed throughout the entire embryo. In the final process of vascular development, mesenchymal cells surround the blood vessels and differentiate into smooth muscle cells or pericytes, and as a result, mature, stabilized vessels are established.1 In contrast to embryonic vessels, blood vessels in the adult are composed of ECs and mural cells and are stabilized. Angiogenesis in the adult commences with the detachment of pre-existing mural pericytes from pre-existing microvessels (vascular destabilization), followed by vascular remodeling, which includes sprouting and intussuception and vascular stabilization by reattachment of pericytes.2
A number of molecules have been reported to act on ECs and regulate vascular development in the embryo and angiogenesis postnatally. These factors include the following: vascular endothelial growth factor (VEGF)3,4 and its receptors fms-like tyrosine kinase 1 and fetal liver kinase 1 (Flk-1)5,6; angiopoietins and their receptor tyrosine kinase with immunoglobulinlike loops and epidermal growth factor homology domain 2;7-9 ephrinB2 and its receptor, EphB4;10,11 transforming growth factor-β and its receptors12-14; and sphingosine-1-phosphate and its receptor, endothelial differentiation gene 1.15 Among these factors, VEGF and its receptors are thought to be the principal stimulator of angiogenesis in the adult.16 However, the precise molecular mechanisms of vascular development in the embryo and of angiogenesis in the adult have not been clarified.
In vitro systems of EC differentiation from totipotent embryonic stem (ES) cells are useful for analyzing vascular development at both the cellular and the molecular level.17-19 The most popular system is one involving EC-derived embryoid body formation.17,20 However, the existence of a variety of cell types other than ECs in embryoid bodies makes it difficult to analyze, in detail, the cellular and molecular events occurring in ECs during vascular development. To overcome these difficulties, Nishikawa et al21 and Hirashima et al22 recently developed a system that allows ES cells to differentiate into ECs under 2-dimensional culture conditions. Flk-1+/vascular endothelial (VE)–cadherin− EC progenitors were derived from ES cells cultured on type-IV collagen-coated dishes. When Flk-1+/VE-cadherin− cells were sorted and cultured on either type-IV collagen–coated dishes or an OP9 feeder cell layer, these cells gave rise to sheetlike clusters, with concomitant expression of VE-cadherin concentrated at cell-cell junctions. These Flk-1+/VE-cadherin+ cells are thought to represent ECs.
To explore the molecular mechanisms of vascular development and angiogenesis, we isolated genes expressed exclusively during EC differentiation in this ES cell culture system. Polymerase chain reaction (PCR)–coupled subtractive hybridization was used to identify genes abundantly expressed in the Flk-1+/VE-cadherin+ cell population compared with Flk-1+/VE-cadherin− cells. One gene we have isolated appears to be a mouse orthologue of puromycin-insensitive leucyl-specific aminopeptidase (PILSAP). Murine PILSAP (mPILSAP) was induced in ECs following VEGF stimulation and is expressed in ECs during angiogenesis in vivo. Antisense oligodeoxynucleotide (AS-ODN) to mPILSAP attenuated VEGF-mediated EC proliferation, migration, and network formation in vitro and angiogenesis in vivo. These results suggest a novel function of mPILSAP in angiogenesis.
Materials and methods
Materials
The following materials were used: VEGF (R&D Systems, Minneapolis, MN); basic fibroblast growth factor-2 (bFGF) and growth factor–reduced Matrigel (Collaborative Research, Bedford, MA); α-minimum essential medium (αMEM), Opti-MEM, Lipofectin, Superscript II reverse transcriptase, and oligo (dT)12-18 primer (all from Gibco BRL, Rockville, MD); Dulbecco modified Eagle medium (DMEM) (Nissui Pharmaceutical, Tokyo, Japan); anti–von Willebrand factor (vWF) antibody (Ab) (Santa Cruz Biotechnology, Santa Cruz, CA); Isogen (Nippon Gene, Toyama, Japan); Hybond N+ membrane and nitrocellulose membranes (Amersham, Buckinghamshire, United Kingdom); fumagillin (Calbiochem, Darmstadt, Germany); actinonin (Biomol Research Laboratories, Plymouth Meeting, PA); aminoacyl-4-methylcoumaryl-7-amide (aminoacyl-MCA) (Peptide Institute, Osaka, Japan). Other chemicals were purchased from Sigma (St Louis, MO).
The ES cell line CCE was maintained and induced to differentiate into ECs as described previously.22 Mouse EC line MSS31,23 a spontaneously immortalized cell line from spleen ECs, was cultured in αMEM supplemented with 10% fetal calf serum (FCS) as described previously.24 COS-7 cells were grown in DMEM containing 10% FCS.
Preparation of complementary DNAs from Flk-1+/VE-cadherin− cells and Flk-1+/VE-cadherin+ cells
Flk-1+/VE-cadherin− cells were purified from ES cells that had differentiated in collagen IV–coated dishes.22 The same cells were further cultured on the OP9 stromal cell layer for 3 days, and Flk-1+/VE-cadherin+ cells were sorted. Total RNA was prepared from sorted cells by using Isogen. RNA was reverse transcribed with Superscript II reverse transcriptase and oligo (dT)12-18 primer according to the manufacturer's instructions.
PCR-coupled subtractive complementary DNA cloning
With the complementary DNAs (cDNAs) from Flk-1+/VE-cadherin− cells as driver and from Flk-1+/VE-cadherin+ cells as tester, PCR-coupled cDNA subtraction was performed with a PCR-select cDNA subtraction kit (Clontech, Palo Alto, CA), used according to the manufacturer's instructions. The cDNAs abundant in Flk-1+/VE-cadherin+ cells were inserted into pCR2.1-TOPO vector by means of a TOPO TA cloning kit (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The nucleotide sequences of approximately 250 clones were determined on both strands by means of a DNA sequencing kit (Applied Biosystems, Foster City, CA) and an ABI Prism 310 DNA sequencer (Applied Biosystems) and was analyzed by the BLAST program. With the cDNA from MSS31 cells as template, full-length cDNA was obtained by both 5′ and 3′ rapid amplification of cDNA ends (RACE) with a Marathon cDNA amplification kit (Clontech), used according to the manufacturer's instructions. For the amplification of the 5′ part of mPILSAP cDNA, a specific reverse primer (5′-AAACCCAGTGGGTTATCCATTGGCCTGGAAG-3′) and AP1 primer supplied in the kit were used. For amplification of the 3′ region of mPILSAP cDNA, a specific reverse primer (5′-ACGTGCTCTGGTGGAAAACTGGTCTGTTGTT-3′) and AP1 primer were used. PCR products were subcloned, sequenced, and analyzed as above.
Reverse transcription–PCR analysis
We amplified 500 ng cDNA from Flk-1+/VE-cadherin− cells or Flk-1+/VE-cadherin+ cells with an Advantage 2 PCR Enzyme system kit (Clontech) according to the manufacturer's instructions. The conditions of PCR were as follows: initial denaturation at 95°C for 5 minutes; 35 cycles of denaturation at 95°C for 1 minute, annealing at 60°C for 1 minute, and extension at 72°C for 1 minute; and a final extension at 72°C for 10 minutes. The following primers were synthesized and used for amplification: for mPILSAP, forward primer corresponding to exon 12, 5′-GAT- GATGGATGGGCTTCTCT-3′, and reverse primer corresponding to exon 15, 5′-GGCTTTTCTCAGTACTAGAC-3′; and for mouse glyceraldehyde-3-phosphate dehydrogenase (G3PDH), forward primer 5′-ACCACAGTCCATGCCATCAC-3′ and reverse primer 5′-TCCACCACCCTGTTGCTGTA-3′. PCR products were loaded and electrophoretically separated on 2% agarose gels containing 0.5 μg/mL ethidium bromide solution.
Northern blot analysis
Northern blot analysis was carried out as described previously.24 Briefly, total RNA was extracted by Isogen according to the manufacturer's instructions. Total RNA was separated on a 1% agarose gel containing 2.2 M formaldehyde and transferred to Hybond N+ membrane. The membrane was hybridized with32P-labeled full-length mPILSAP cDNA probe in hybridization solution at 42°C for 24 hours. After hybridization, the membrane was washed once with 0.1% sodium dodecyl sulfate (SDS) in 2 × SSC at room temperature and then 3 times with 0.1% SDS in 0.2 × SSC at 65°C. Autoradiography was carried out on an imaging plate and analyzed by FLA 2000 (Fuji Film, Tokyo, Japan).
Assay for aminopeptidase activity
The EcoRI fragment of the mPILSAP cDNA from pCR2.1-TOPO/mPILAP was subcloned into the expression vector pcDNA4/HisMax (Invitrogen). COS-7 cells (4 × 106 cells per 100-mm dish) were transfected according to the manufacturer's instructions with 10 μg pcDNA/mPILSAP mixed with Lipofectin. Transfected COS-7 cells were cultured in 10% FCS/DMEM for 48 hours, washed twice with phosphate-buffered saline (PBS), collected by centrifugation at 500g for 10 minutes, and suspended in PBS. Cells were homogenized by ultrasonication and lysates centrifuged for 15 minutes at 10 000g at 4°C. Supernatant was loaded onto a nickel-chelated resin column, and the histidine-tagged protein was purified by means of an Xpress System Protein Purification Kit (Invitrogen) according to the manufacturer's instructions. We added 1 μg purified protein to 100 μL 25 mM Tris-HCl (pH 7.5), and reactions were initiated by adding an equal volume of Tris-HCl containing 0.5 mM aminoacyl-MCA. After incubation at 37°C for 30 minutes, aminopeptidase activity was measured with a Fluoroskan Ascent (Dai Nippon-Pharmaceuticals, Osaka, Japan) operated at an excitation wavelength of 355 nm and an emission wavelength of 460 nm. For inhibitor assays, the indicated substances were preincubated with the enzyme on ice for 5 minutes, and the reaction was started by adding 0.5 mM leucyl-4-MCA.
Subcellular fractionation
COS-7 cells transiently transfected with mPILSAP expression vector were suspended in buffer A (30 mM Hepes [pH 7.4], 2 mM EDTA, 5 mM ethyleneglycotetraacetic acid); homogenized in a Dounce-homogenizer; and centrifuged for 10 minutes at 800g. The resulting cell lysates were loaded onto a sucrose cushion (40% sucrose in buffer A) and centrifuged at 100 000g for 60 minutes at 4°C. The supernatant was collected as the cytosolic fraction; the interface, as the plasma membrane (PM) fraction; and the pellet, as the microsomal fraction. The PM fraction was pelleted by centrifugation at 100 000g for 30 minutes at 4°C and solubilized in solubilization buffer (20 mM Tris-HCl, [pH 7.5], 150 mM NaCl, 5 mM EDTA, 0.5% Triton X-100, 1 mM phenylmethylsulfonyl fluoride). Protein concentration was determined by use of the DC protein assay (Bio-Rad, Hercules, CA). We subjected 40 μg each fraction to Western blot analysis.
Western blot analysis
Polyclonal rabbit antibody against a synthetic peptide corresponding to the C-terminal 15 amino acids of mPILSAP, including a cysteine linker (CYQSSLSSTEKSQIE), was prepared (anti-mPILSAP Ab). For the detection of mPILSAP in MSS31 cells, cells were starved for 24 hours in 0.1% FCS/αMEM and then stimulated with 100 ng/mL VEGF for 8 hours. The protein extracted from the cells was separated by SDS–polyacrylamide gel electrophoresis on a 7.5% separating gel and transferred to nitrocellulose membranes. The membranes were blocked for 1 hour at room temperature with Tris-HCl–buffered saline (TBS), pH 7.4, containing 5% skim milk, and then incubated for 1 hour at room temperature in TBS containing 0.05% Tween 20 (T-TBS), 1% bovine serum albumin (BSA), and anti-mPILSAP Ab diluted to 1/2000. The filter was washed 4 times with T-TBS and incubated for 1 hour with horseradish peroxidase–conjugated protein G (Bio-Rad) diluted to 1/3000 in T-TBS. After the filter had been washed 3 times with T-TBS, blots were detected by an enhanced chemiluminescence method by means of an ECL Western blotting detection kit (Amersham). The results were visualized by using the LAS-1000 (Fuji Film).
ODN transfection
MSS31 cells were rinsed with Opti-MEM and incubated for 6 to 24 hours with synthetic phosphorothioate ODNs in Lipofectin containing Opti-MEM at a fixed ratio of ODNs versus Lipofectin (1:3; eg, 500 nM oligo per 1500 nM Lipofectin). Thereafter, ODN-containing medium was replaced with normal 0.1% FCS/αMEM with or without VEGF. The sequences of ODNs used in this study were as follows: AS-ODN, 5′-TATGTGGTCAGTCCCCAGTT-3, which is complementary to the messenger RNA (mRNA) region in mPILSAP; sense (S-ODN), 5′-AACTGGGGACTGACCACATA-3′; mismatched (MIS-ODN), 5′-TTTGTGGACAGTCGCCACTT-3′, comprising AS-ODN with 4 base pairs substituted; and scrambled (SCR-ODN), 5′-CTGTATCAGTCAGTGTTCGC-3′, comprising all the base pairs of the antisense codons in random order. AS-ODN was not homologous to any genes checked against the reported DNA sequences present in the BLAST program.
Cell proliferation
MSS31 cells were inoculated at a density of 2 × 103 per well into 96-well plates and cultured in 10% FCS/αMEM for 24 hours. Cells were subsequently treated for 24 hours with or without 500 nM ODN. Following this, the medium was changed to 0.1% FCS/αMEM with or without 100 ng/mL VEGF, and the cells were incubated for the desired period. We added 10 μL TetraColor ONE (Seikagaku, Tokyo, Japan) to each well, and proliferation was quantified by means of a multiple-plate reader (Tosoh, Tokyo, Japan) set at 492 nm.
Cell migration
Cell migration was examined as described previously.25 Briefly, confluent monolayers of MSS31 cells were treated for 6 hours with or without 500 nM ODN, and then wounded with a razor blade. After rinsing with PBS, medium was changed to 0.1% FCS/αMEM with or without 30 ng/mL VEGF, and the cells were then incubated for 24 hours. The number of cells that had migrated across the wound edge was counted.
Network formation
MSS31 cells were cultured in 0.1% FCS/αMEM for 24 hours and were subsequently treated for 6 hours with or without 1000 nM ODNs. Cells were harvested with 0.25% trypsin and 1 mM EDTA, resuspended in 0.1% FCS/αMEM with or without 100 μg/mL VEGF in a final volume of 300 μL, replated (2 × 105 cells per well) onto 24-well plates coated with Matrigel (250 μL per well), and incubated at 37°C for 4 hours. Cells were observed by phase-contrast microscopy. The lengths of network structures were quantified with microcomputer-assisted NIH Image (National Institutes of Health, Bethesda, MD).
Mouse angiogenesis model
A mouse angiogenesis model was prepared as described previously.24 Briefly, male C57BL/6 mice at 4 weeks of age were used. We injected 300 μL growth factor–reduced Matrigel containing VEGF (100 ng/mL) or bFGF (100 ng/mL) plus heparin (32 U/mL) in liquid form at 4°C into the abdominal subcutaneous tissue at the midperitoneal area of each mouse. In some experiments, 50 μM AS-ODN or S-ODN was mixed with the Matrigel. On day 6 after injection, mice were killed and gels recovered. The gels were fixed in 4% paraformaldehyde in PBS and embedded in paraffin. We subjected 3-μm sections of the gels to hematoxylin and eosin staining and immunohistochemical analysis.
Immunohistochemical analysis
Immunohistochemistry of the sections of the gel was performed as described previously.26 Briefly, anti-mPILSAP Ab, anti-vWF Ab, and nonimmune rabbit immunoglobulin (Ig)–G were used as primary antibody. They were diluted 200-fold with PBS containing 1% BSA. The secondary antibody was biotin-conjugated antirabbit antibody diluted 200-fold with the same diluent. Each reaction was conducted for 30 minutes at room temperature. Coupling of streptavidin to biotin from an ABC kit (Vector Laboratories, Burlingame, CA) was performed for 45 minutes at room temperature. Immunocomplexes were visualized by means of 3,3′-diamidobenzidine tablet sets (Sigma).
Quantification of angiogenesis in the Matrigel
We measured the hemoglobin contents of the Matrigel from each group; each group contained 4 animals according to the method of Majima et al27 with the following modifications as a parameter for angiogenesis. Briefly, the Matrigels were weighed and homogenized in distilled water (10 μL/mg wet weight). The hemoglobin concentration in the supernatant after centrifugation at 5000gfor 5 minutes was determined by a hemoglobin B-test kit (Wako, Osaka, Japan).
Calculations and statistical analysis
The statistical significance of differences in the data were evaluated by use of unpaired analysis of variance, and Pvalues were calculated by the unpaired Student t test.P < .05 was accepted as statistically significant.
Results
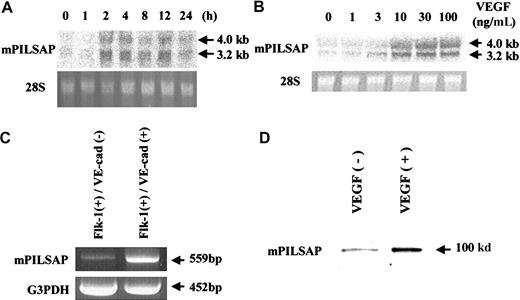
Murine ES cells spontaneously differentiate into ECs in vitro, in a process that can be monitored by the sequential expression of Flk-1 followed by VE-cadherin.21 22 We defined Flk-1+/VE-cadherin− cells as EC progenitors, and Flk-1+/VE-cadherin+ cells as ECs, and purified these populations by cell sorting. PCR-coupled subtractive hybridization was then used to identify genes expressed abundantly by the Flk-1+/VE-cadherin+ cell population compared with their expression in the Flk-1+/VE-cadherin− cell population. Approximately 250 clones were initially obtained, and their nucleotide sequences were determined. To isolate genes that may be involved in angiogenesis, we tested the inducibility of the gene in response to VEGF in the murine EC line MSS31. Northern blot analysis revealed that one of the isolated genes was significantly induced by VEGF. This induction reached a peak at 2 hours (Figure1A). The induction was dependent on the concentration of VEGF, with the maximal effect observed above 30 ng/mL VEGF (Figure 1B). In contrast, bFGF had little effect on the expression of this gene in MSS31 cells (data not shown). Reverse transcription (RT)–PCR analysis confirmed that expression of this gene was enhanced in the Flk-1+/VE-cadherin+ cell population as compared with the Flk-1+/VE-cadherin−cell population (Figure 1C). Furthermore, Western blot analysis confirmed that VEGF augmented the synthesis of the product of this gene in MSS31 cells (Figure 1D).
Expression of mPILSAP.
(A) MSS31 cells were preincubated for 24 hours in 0.1% FCS/αMEM and then stimulated with 100 ng/mL VEGF for the indicated period. (B) After preincubation, MSS31 cells were stimulated with the indicated concentrations of VEGF for 4 hours. Total RNA was obtained and Northern blot analysis performed. As a control, 28S RNA in the ethidium bromide–stained gel is also shown. (C) Total RNAs were obtained from Flk-1+/VE-cadherin− and Flk-1+/VE-cadherin+ cells. RT-PCR was performed as described in “Materials and methods.” Mouse G3PDH was used as internal control. (D) After preincubation, MSS31 cells were stimulated with the indicated concentrations of VEGF for 8 hours. Western blot analysis was performed as described in “Materials and methods.”
Expression of mPILSAP.
(A) MSS31 cells were preincubated for 24 hours in 0.1% FCS/αMEM and then stimulated with 100 ng/mL VEGF for the indicated period. (B) After preincubation, MSS31 cells were stimulated with the indicated concentrations of VEGF for 4 hours. Total RNA was obtained and Northern blot analysis performed. As a control, 28S RNA in the ethidium bromide–stained gel is also shown. (C) Total RNAs were obtained from Flk-1+/VE-cadherin− and Flk-1+/VE-cadherin+ cells. RT-PCR was performed as described in “Materials and methods.” Mouse G3PDH was used as internal control. (D) After preincubation, MSS31 cells were stimulated with the indicated concentrations of VEGF for 8 hours. Western blot analysis was performed as described in “Materials and methods.”
We constructed the full-length cDNA of this gene by the RACE method for sequencing. The nucleotide sequence and the deduced amino acid sequence are shown in Figure 2. The first ATG triplet was considered to be the initiation codon, as the 5′-untranslated region contained an in-frame stop codon preceding this initiation codon. The cDNA contained a 2790–base pair open reading frame encoding a protein with 930 amino acids. The deduced amino acid sequence contained a consensus sequence typical of the zinc-aminopeptidase family; this sequence bears an essential zinc-binding site HEXXH(X)18E of the peptidase at amino acid residues 342 through 346 with a second glutamic acid separated by 18 amino acids (marked by the box in Figure 2). This motif is found in aminopeptidase sequences and is a criterion for classification in the M1 family of aminopeptidases.28 Homology search of the amino acid sequence revealed that it was 93.4% homologous to rat29 and 84.1% homologous to human PILSAP,30respectively (Figure 3). Therefore, this molecule is proposed as a murine orthologue of PILSAP (mPILSAP).
Nucleotide and deduced amino acid sequence of mPILSAP.
Nucleotide residues are numbered from 5′ to 3′, with the first residue of the ATG codon encoding the putative initiating methionine. The deduced amino acid sequence is displayed below the nucleotide sequence as a sequence of one-letter codes starting from the methionine. The HEXXH(X)18E consensus sequence is boxed. * indicates the terminal codon.
Nucleotide and deduced amino acid sequence of mPILSAP.
Nucleotide residues are numbered from 5′ to 3′, with the first residue of the ATG codon encoding the putative initiating methionine. The deduced amino acid sequence is displayed below the nucleotide sequence as a sequence of one-letter codes starting from the methionine. The HEXXH(X)18E consensus sequence is boxed. * indicates the terminal codon.
Comparison of mPILSAP protein with rat and human PILSAPs.
The deduced amino acid sequence of mPILSAP is aligned with the sequences of rat and human PILSAPs.
Comparison of mPILSAP protein with rat and human PILSAPs.
The deduced amino acid sequence of mPILSAP is aligned with the sequences of rat and human PILSAPs.
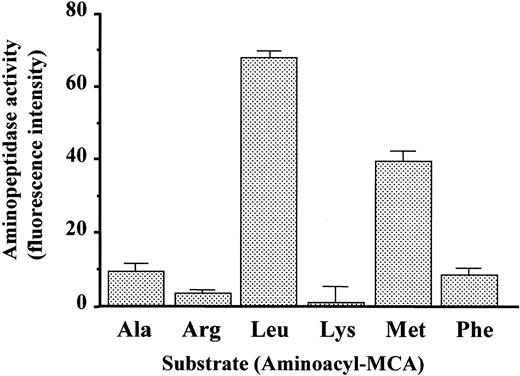
Next, we examined whether this protein exhibits aminopeptidase activity. The protein isolated from COS-7 cells transfected with this gene was measured for relative hydrolytic activity toward various aminoacyl-MCAs. As shown in Figure 4, the protein was shown to have aminopeptidase activity. Leucyl-MCA was the most efficiently hydrolyzed, followed by methionyl-MCA, but little activity was observed toward other aminoacyl-MCAs. To further characterize the enzymatic activity of mPILSAP, we measured aminopeptidase activity in the presence of various inhibitors (Table 1). The mPILSAP was highly sensitive to leucinethiol and zinc ions. General aminopeptidase inhibitors, such as bestatin and amastatin, significantly inhibited the enzymatic activity of mPILSAP. Puromycin (a puromycin-sensitive aminopeptidase inhibitor), 1,10-phenanthroline (a chelating agent), leupeptin (a serine/cysteine protease inhibitor), aprotinin (a serine protease inhibitor), and pepstatin (an aspartic acid protease inhibitor) had little effect. Interestingly, fumagillin, an inhibitor of angiogenesis, inhibited the enzymatic activity of mPILSAP. The analysis of subcellular localization revealed that mPILSAP was present in cytosolic and microsomal fractions but not in the membrane fraction (data not shown). Immunostaining and confocal microscopic observation further confirmed that mPILSAP was localized to the cytoplasm (data not shown).
Aminopeptidese activity of mPILSAP.
Purified mPILSAP protein from transfected COS-7 cells was incubated with various aminoacyl-MCA substrates to assess peptidase activity. Values are means and SDs of triplicate samples.
Aminopeptidese activity of mPILSAP.
Purified mPILSAP protein from transfected COS-7 cells was incubated with various aminoacyl-MCA substrates to assess peptidase activity. Values are means and SDs of triplicate samples.
Effect of various inhibitors on mPILSAP activity
| Inhibitor . | Concentration (μM) . | Activity (%) . |
|---|---|---|
| None | 100 | |
| Bestatin | 1 | 84.3 ± 5.2* |
| 10 | 66.9 ± 4.3† | |
| Amastatin | 1 | 85.0 ± 6.3 |
| 10 | 58.1 ± 3.4† | |
| Leucinethiol | 1 | 60.0 ± 1.6† |
| 10 | 17.4 ± 2.2† | |
| Fumagillin | 1 | 79.5 ± 5.4* |
| 10 | 60.1 ± 5.5† | |
| Puromycin | 10 | 97.5 ± 3.8 |
| 1,10-Phenanthroline | 10 | 92.6 ± 5.4 |
| ZnCl2 | 10 | 27.8 ± 4.9† |
| Leupeptin | 10 | 94.8 ± 2.9 |
| Aprotinin | 10 | 100.9 ± 3.7 |
| Pepstatin | 10 | 95.5 ± 5.0 |
| Inhibitor . | Concentration (μM) . | Activity (%) . |
|---|---|---|
| None | 100 | |
| Bestatin | 1 | 84.3 ± 5.2* |
| 10 | 66.9 ± 4.3† | |
| Amastatin | 1 | 85.0 ± 6.3 |
| 10 | 58.1 ± 3.4† | |
| Leucinethiol | 1 | 60.0 ± 1.6† |
| 10 | 17.4 ± 2.2† | |
| Fumagillin | 1 | 79.5 ± 5.4* |
| 10 | 60.1 ± 5.5† | |
| Puromycin | 10 | 97.5 ± 3.8 |
| 1,10-Phenanthroline | 10 | 92.6 ± 5.4 |
| ZnCl2 | 10 | 27.8 ± 4.9† |
| Leupeptin | 10 | 94.8 ± 2.9 |
| Aprotinin | 10 | 100.9 ± 3.7 |
| Pepstatin | 10 | 95.5 ± 5.0 |
P < .05 versus control.
P < .005 versus control.
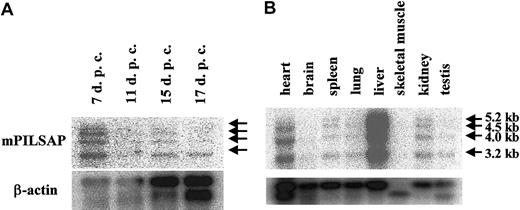
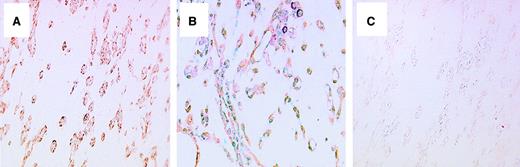
By Northern blot analysis using poly(A)+ RNA to examine the expression of mPILSAP in mouse embryonic and adult tissues, we detected 3.2-kilobyte (kb), 4.0-kb, 4.5-kb, and 5.2-kb transcripts (Figure5). Expression of mPILSAP in embryonic development peaked at 7 days after coitus (dpc) and decreased thereafter (Figure 5A). In the adult, mPILSAP was constitutively and abundantly expressed in both the liver and the heart. The expression was also observed in spleen, lung, kidney, and testis, whereas expression was very faint in skeletal muscle and brain (Figure 5B). As mPILSAP was inducible in ECs by VEGF, we examined whether mPILSAP was expressed in ECs during angiogenesis. When Matrigel containing VEGF was inoculated into mouse subcutaneous tissue, new vessels invaded the gel. Immunohistochemistry with anti–mPILSAP Ab revealed positive staining of mPILSAP protein in ECs of new vessels, whereas nonimmune rabbit IgG gave a negative reaction (Figure 6A,C). ECs of vessels surrounding the Matrigel were faintly stained with anti-mPILSAP Ab (data not shown). Immunostaining with anti-vWF Ab confirmed that almost all cells in Matrigel were ECs (Figure 6B).
Expression of mPILSAP mRNA in embryo and adult tissues.
Northern blot filters (Clontech) of mouse embryonic (panel A) and adult (panel B) tissues were probed with 32P-labeled full-length cDNA probe of mPILSAP. As a control, mouse β-actin is shown below.
Expression of mPILSAP mRNA in embryo and adult tissues.
Northern blot filters (Clontech) of mouse embryonic (panel A) and adult (panel B) tissues were probed with 32P-labeled full-length cDNA probe of mPILSAP. As a control, mouse β-actin is shown below.
Immunohistochemistry of mPILSAP protein at the site of angiogenesis in vivo.
Mouse angiogenesis assay was performed as described in “Materials and methods.” Immunostaining of sectioned Matrigel was performed with anti-mPILSAP Ab (panel A), anti-VWF Ab (panel B), or nonimmune rabbit IgG (panel C). Magnification, × 200.
Immunohistochemistry of mPILSAP protein at the site of angiogenesis in vivo.
Mouse angiogenesis assay was performed as described in “Materials and methods.” Immunostaining of sectioned Matrigel was performed with anti-mPILSAP Ab (panel A), anti-VWF Ab (panel B), or nonimmune rabbit IgG (panel C). Magnification, × 200.
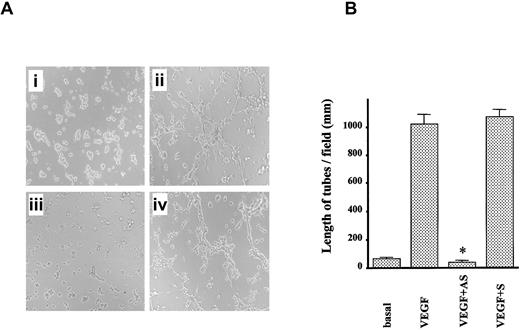
Next, we constructed ODNs to examine whether mPILSAP was required for angiogenesis. Northern blot analysis revealed that one AS-ODN effectively and selectively inhibited mPILSAP expression in MSS31 cells in response to VEGF stimulation. Corresponding controls of S-ODN, MIS-ODN, and SCR-ODN did not affect the induced expression of mPILSAP (Figure 7A). The synthesis of mPILSAP protein was also inhibited by AS-ODN (Figure 7A). Proliferation and migration are principal properties of ECs in angiogenesis, and VEGF augments the degree to which they occur. Therefore, we tested whether AS-ODN would affect these properties. As shown in Figure 7B, AS-ODN inhibited the proliferation of MSS31 cells at days 1, 2, and 3, whereas S-ODN, MIS-ODN, and SCR-ODN exhibited no effect. AS-ODN inhibited the proliferation in a dose-dependent manner over the range of 1 to 1000 nM (data not shown). Likewise, AS-ODN inhibited the migration of MSS31 cells, whereas S-ODN, MIS-ODN, and SCR-ODN had no effect (Figure 7C). Again, AS-ODN showed a dose-dependent inhibition of cell migration, over the range of 1 to 1000 nM (data not shown). We further examined the effect of AS-ODN on angiogenesis. We used network formation of MSS31 cells as an in vitro model of angiogenesis. AS-ODN inhibited the network formation of MSS31 cells, whereas S-ODN, MIS-ODN, and SCR-ODN had no effect (Figure 8A), and this was further confirmed by quantitative analysis (Figure 8B).
Role of mPILSAP in VEGF-stimulated proliferation and migration of MSS31 cells.
The mPILSAP is required for VEGF-stimulated proliferation and migration of MSS31 cells. MSS31 cells were preincubated in 0.1% FCS/αMEM for 24 hours and then treated with mPILSAP ODNs for 6 hours. Thereafter, cells were stimulated with 30 ng/mL VEGF for 12 hours. (A) Northern blot analysis showed that AS-ODN specifically eliminated expression of mPILSAP mRNA. AS indicates AS-ODN; S, S-ODN; MIS, MIS-ODN; SCR, SCR-ODN. As a control, 28S RNA from the ethidium bromide–stained gel is also shown. Western blot analysis also showed that AS-ODN specifically down-regulated mPILSAP protein level of VEGF-stimulated cells. (B) Cell proliferation was examined as described in “Materials and methods.” AS-ODN specifically inhibited VEGF-stimulated proliferation of MSS31 cells. Values are means and SDs of triplicate samples. *Significant difference compared with the VEGF-stimulated group (P < .001). (C) Cell migration was examined as described in “Materials and methods.” AS-ODN specifically inhibited VEGF-stimulated migration of MSS31 cells. Values are means and SDs of 4 fields. *Significant difference compared with the value for the corresponding VEGF-stimulated group (P < .001).
Role of mPILSAP in VEGF-stimulated proliferation and migration of MSS31 cells.
The mPILSAP is required for VEGF-stimulated proliferation and migration of MSS31 cells. MSS31 cells were preincubated in 0.1% FCS/αMEM for 24 hours and then treated with mPILSAP ODNs for 6 hours. Thereafter, cells were stimulated with 30 ng/mL VEGF for 12 hours. (A) Northern blot analysis showed that AS-ODN specifically eliminated expression of mPILSAP mRNA. AS indicates AS-ODN; S, S-ODN; MIS, MIS-ODN; SCR, SCR-ODN. As a control, 28S RNA from the ethidium bromide–stained gel is also shown. Western blot analysis also showed that AS-ODN specifically down-regulated mPILSAP protein level of VEGF-stimulated cells. (B) Cell proliferation was examined as described in “Materials and methods.” AS-ODN specifically inhibited VEGF-stimulated proliferation of MSS31 cells. Values are means and SDs of triplicate samples. *Significant difference compared with the VEGF-stimulated group (P < .001). (C) Cell migration was examined as described in “Materials and methods.” AS-ODN specifically inhibited VEGF-stimulated migration of MSS31 cells. Values are means and SDs of 4 fields. *Significant difference compared with the value for the corresponding VEGF-stimulated group (P < .001).
Role of mPILSAP in angiogenesis in vitro.
The mPILSAP is required for angiogenesis in vitro. (A) Network formation by MSS31 cells was examined as described in “Materials and methods.” AS-ODN inhibited VEGF-induced network formation (×40 magnification). (B) Values are means and SDs of 6 fields. *Significant difference compared with the value for the corresponding VEGF-stimulated group (P < .001).
Role of mPILSAP in angiogenesis in vitro.
The mPILSAP is required for angiogenesis in vitro. (A) Network formation by MSS31 cells was examined as described in “Materials and methods.” AS-ODN inhibited VEGF-induced network formation (×40 magnification). (B) Values are means and SDs of 6 fields. *Significant difference compared with the value for the corresponding VEGF-stimulated group (P < .001).
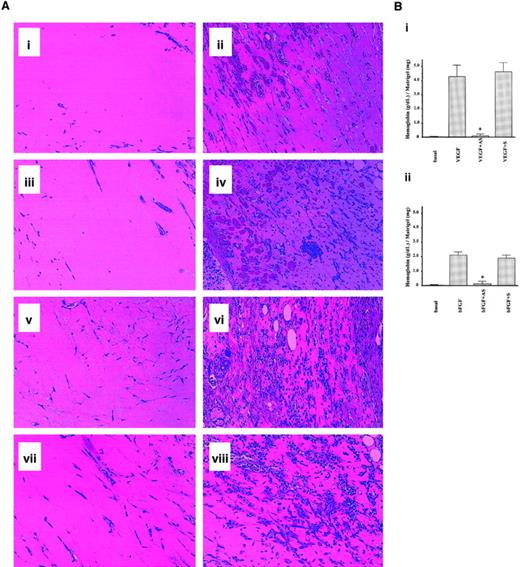
Finally, we used a mouse model of angiogenesis to confirm the requirement of mPILSAP in angiogenesis in vivo. The Matrigel plug assay is widely accepted as an in vivo model of angiogenesis, and the direct application of antisense phosphothioate oligonucleotides is easily achieved in this assay.31 Matrigel containing VEGF and either AS or S-ODN was inoculated into mouse subcutaneous tissue. Invasion of new vessels into the gel was enhanced by VEGF (Figure9A; panel 9Ai versus 9Aii). AS-ODN but not S-ODN inhibited this process (Figure 9Aiii,9Aiv). Similar results were obtained when bFGF was used as an angiogenic factor (Figure 9Av-9Aviii). Quantitative analysis confirmed that AS-ODN significantly inhibited VEGF- and bFGF-stimulated angiogenesis in vivo, whereas S-ODN exhibited no inhibition (Figure 9Bi,9Bii).
Role of mPILSAP in angiogenesis in vivo.
The mPILSAP is required for angiogenesis in vivo. (A) Mouse angiogenesis assay and histological analysis were performed as described in “Materials and methods.” (i) Matrigel alone. (ii) Matrigel containing VEGF and heparin. (iii) Matrigel containing VEGF, heparin, and AS-ODN. (iv) Matrigel containing VEGF, heparin, and S-ODN. (v) Matrigel alone. (vi) Matrigel containing bFGF. (vii) Matrigel containing bFGF and AS-ODN. (viii) Matrigel containing bFGF and S-ODN (×200 magnification). (B) Quantification of angiogenesis was performed as described in “Materials and methods.” Values are means and SDs of 4 animals from each group. *Significant difference compared with the value for the corresponding Matrigel containing VEGF (panel Bi) and bFGF (panel Bii) (P < .001). Hematoxylin-eosin staining was used.
Role of mPILSAP in angiogenesis in vivo.
The mPILSAP is required for angiogenesis in vivo. (A) Mouse angiogenesis assay and histological analysis were performed as described in “Materials and methods.” (i) Matrigel alone. (ii) Matrigel containing VEGF and heparin. (iii) Matrigel containing VEGF, heparin, and AS-ODN. (iv) Matrigel containing VEGF, heparin, and S-ODN. (v) Matrigel alone. (vi) Matrigel containing bFGF. (vii) Matrigel containing bFGF and AS-ODN. (viii) Matrigel containing bFGF and S-ODN (×200 magnification). (B) Quantification of angiogenesis was performed as described in “Materials and methods.” Values are means and SDs of 4 animals from each group. *Significant difference compared with the value for the corresponding Matrigel containing VEGF (panel Bi) and bFGF (panel Bii) (P < .001). Hematoxylin-eosin staining was used.
Discussion
We found that mPILSAP expression was augmented during in vitro differentiation of ECs from murine ES cells. VEGF induced mPILSAP in ECs in vitro, and the expression of mPILSAP was confirmed in ECs during postnatal angiogenesis in vivo. Moreover, the specific elimination of mPILSAP expression by AS-ODN abrogated proliferation and migration of ECs in vitro, as well as angiogenesis in vivo. These results indicate that mPILSAP plays an important role in angiogenesis. Although bFGF did not significantly induce mPILSAP in ECs in vitro, the angiogenic activity of bFGF in vivo was also significantly inhibited by AS-ODN to mPILSAP. The reason for this inhibition is not clear at the moment. The angiogenic activity of bFGF in vivo is reported to be dependent on the endogenous VEGF.31 Thus, it is possible that AS-ODN to mPILSAP inhibited bFGF-stimulated angiogenesis by inhibiting the effect of endogenous VEGF.
Aminopeptidases catalyze the sequential removal of amino acids from unblocked N-termini of peptides and proteins and play important roles in various biological processes, such as maturation, activation, modulation, and degradation of bioactive peptides, and in the determination of protein stability.32 The mPILSAP belongs to the M1 family of aminopeptidases, which contain an HEXXH(X)18E motif and a central Zn2+ ion essential for their enzymatic activity. Eight aminopeptidases of this subfamily have been identified to date and are subdivided into membrane-bound and cytoplasmic enzymes. Membrane-bound ectoenzymes include aminopeptidase N (APN)/CD13,33 aminopeptidase A,34thyrotropin-releasing-hormone–degrading ectoenzyme,35 and insulin-regulated membrane aminopeptidase/human placental leucine aminopeptidase/oxytocinase.36 Cytoplasmic enzymes include aminopeptidase B,37 leukotriene-A4 hydrolase,38 puromycin-sensitive aminopeptidase,39 and PILSAP.29,30 We confirmed that mPILSAP was present in cytoplasmic and microsome fractions but not in the membrane fraction when expressed in COS-7 cells. The mPILSAP is not the only member of the M1 aminopeptidase thought to play a role in angiogenesis. APN/CD13 is expressed in ECs of tumor vessels. Anti-APN antibodies as well as the aminopeptidase inhibitor bestatin inhibit angiogenesis in vivo.40APN/CD13 belongs to the membrane-bound ectoenzyme group of aminopeptidases, whereas mPILSAP is present in the cytoplasm. Therefore, APN/CD13 and mPILSAP are thought to play distinct functions. However, the mechanism by which aminopeptidases regulate angiogenesis is currently unknown.
Methionine aminopeptidase 1 (MetAP1) and MetAP2 belong to another subset of aminopeptidases, which contain a central Co2+ ion for their enzymatic activity and do not belong to the M1 family of aminopeptidases.41,42 Fumagillin and its derivative TNP-470 are potent inhibitors of EC proliferation and angiogenesis.43,44 The target molecule of fumagillin and of its derivative TNP-470 was recently shown to be MetAP2.45 However, the expression of MetAP2 is not restricted to ECs, and selective inhibition of EC proliferation by fumagillin is not related to differential expression of MetAP2.46 Therefore, it is not clear whether fumagillin and TNP-470 exhibit antiangiogenic activity via the specific inhibition of MetAP2. We observed that fumagillin inhibited the enzymatic activity of mPILSAP. Therefore, it is possible that fumagillin and TNP-470 exert their antiangiogenic activity by inhibiting other aminopeptidases including mPILSAP in ECs. Further study is required to explore whether fumagillin and TNP-470 can bind to mPILSAP and other aminopeptidases besides MetAP2.
As mPILSAP was isolated during EC differentiation from ES cells in vitro, we assume that mPILSAP is involved not only in postnatal angiogenesis but also in embryonic vascular development. The expression of mPILSAP in the mouse embryo peaked at 7 dpc, and declined thereafter (Figure 6A). The high expression of VEGF in the mouse embryo is associated with vasculogenesis, whereas its low expression is associated with angiogenesis.47 Thus, mPILSAP expression may correlate with VEGF expression. In contrast, mPILSAP is constitutively and highly expressed in limited organs, such as heart and liver in the adult (Figure 6B). These findings also suggest that the biological function of mPILSAP is not restricted to the vascular system. Further studies, including the targeted disruption and overexpression of the mPILSAP gene in mice, are currently underway to clarify the biological significance of mPILSAP in embryonic vascular development as well as in other organs.
We thank Dr Stuart T. Fraser for critical reading of this manuscript. We are indebted to Ms Hiroko Oikawa for her excellent technical assistance.
Supported by the Japan Society of the Promotion of Science Research for the Future (99L01304) and by the Japanese Ministry of Education, Science, Sports, and Culture.
The nucleotide sequence reported in this paper has been submitted to the DDBJ/EMBL/GenBank with accession number AB047552.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yasufumi Sato, Dept of Vascular Biology, Institute of Development, Aging and Cancer, Tohoku University, 4-1 Seiryo-machi, Aoba-ku, Sendai 980-8575, Japan; e-mail: y-sato@idac.tohoku.ac.jp.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal