Abstract
Immunoglobulin transcription is impaired in Hodgkin and Reed-Sternberg (HRS) cells of classical Hodgkin disease (cHD). We recently demonstrated that defective immunoglobulin promoter transcription correlates with the down-regulation of the B-cell transcription factors Oct2 and BOB.1/OBF.1. These results prompted us to investigate whether immunoglobulin enhancer activity is also impaired in HRS cells and whether as yet unidentified factors could be necessary for immunoglobulin enhancer activity in HRS cells of cHD. Here we analyzed 30 cases of cHD for expression of the Ets family member PU.1 that is known to collaborate with multiple transcription factors and to regulate expression of immunoglobulin genes. We show that PU.1 is not expressed in primary and cultured HRS cells. Reintroduction of PU.1 and Oct2 in cultured HRS cells restored the activity of cotransduced immunoglobulin enhancer constructs. Our study identifies PU.1 deficiency as a recurrent defect in HRS cells that might contribute to their impairment of immunoglobulin transcription.
Introduction
Absent immunoglobulin expression despite the presence of clonal immunoglobulin gene rearrangements is a hallmark of tumor cells of classical Hodgkin disease (cHD).1 Recently, we showed that crippling mutations in the immunoglobulin coding region and in the octamer motif of the immunoglobulin promoter are rare events in cHD.1 We further demonstrated that defective immunoglobulin promoter transcription is most likely due to the down-regulated expression of B-cell–specific transcription factors such as Oct2 and BOB.1/OBF.1.1-3 In this study, we investigated whether immunoglobulin enhancer activity is also impaired in Hodgkin and Reed-Sternberg (HRS) cells and whether as yet unidentified factors could be necessary for immunoglobulin enhancer activity in HRS cells of cHD. We, therefore, analyzed the expression of the Ets family transcription factor PU.1/SPI-1 that is known to essentially contribute to the regulation of immunoglobulin-μ, -κ, and -λ gene expression.4
PU.1 is a tissue-restricted transcriptional regulator within the hematopoietic system that is essential for the development of both B cells and macrophages.4-9 Furthermore, several DNA-binding studies revealed that PU.1 binds to a large number of promoters and regulates the expression of genes that are required for terminal differentiation of B cells.9,10 The list includes genes encoding immunoglobulin light (κ or λ) and heavy chain genes, RAG-1, immunoglobulin α (Ig-α), Ig-β, Vpre-B, λ5, CD19, and J-chain.5,10-14 Thereby, PU.1 requires interactions with other transcription factors to regulate transcription and plays an architectural role in forming higher-order complexes.15-17
Here we show that PU.1 is not detectable in cultured and primary HRS cells of cHD. We demonstrate that cotransfection of PU.1 and Oct2 in transient transfection studies activates immunoglobulin heavy chain (IgH) intronic enhancer expression in cultured HRS cells. Together with the absence of Oct2 and BOB.1/OBF.1, PU.1 deficiency is an important recurrent defect of HRS cells probably contributing to defective immunoglobulin gene expression in cHD.
Study design
Formalin-fixed and paraffin-embedded lymph node specimens from 30 patients with the diagnosis of cHD of nodular sclerosis or mixed cellularity subtype were retrieved from the files of the Reference Center for Lymph Node Pathology and Hematopathology at the Institute of Pathology, Klinikum Benjamin Franklin, Free University of Berlin, Berlin, Germany. Activated lymphoid tissue from palatinal tonsils served as control. Tissue sections (4 μm) were deparaffinized and subjected to a brief high-temperature pretreatment with the use of a pressure cooker and 0.01 M citrate buffer pH 6.0. The sections were incubated with the anti-PU.1 monoclonal antibody (clone G148-74 purchased from Becton Dickinson Biosciences, Heidelberg, Germany). Bound antibody was visualized by using the EnVision Kit (DAKO, Glostrup, Denmark).
Cell extracts were prepared and quantitated as described.18 The primary antibodies were anti-PU.1 (Santa Cruz, CA) and anti-Spi-B (M.C. Simon, Howard Hughes Medical Institute, Philadelphia, PA) antibodies.
Electroporation was performed as recently described.2,3The luciferase reporter plasmids driven by conalbumin promoter with or without the MluI-HpaI fragment of the human IgH gene intronic enhancer (pEcona-Luc and pcona-Luc) were provided by Dr T. Watanabe (Kyusyu University, Fukuoka, Japan19). Murine PU.1 complementary DNA was provided by Dr D. Tenen (Harvard Medical School, Boston, MA) and cloned in a pCDNA-3.1 expression plasmid (Invitrogen, Groningen, The Netherlands).
Results and discussion
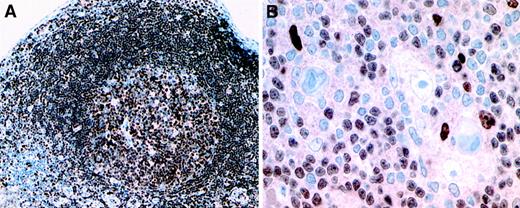
In this study we investigated the role of the B-cell transcription factor and Ets family member PU.1 in defective immunoglobulin gene expression in HRS cells of cHD. For this purpose we analyzed 30 cases of cHD for expression of PU.1 by immunohistochemistry. In all cases PU.1 expression was totally absent from HRS cells, whereas we found, as expected, PU.1 protein levels in reactive B cells, granulocytes, and macrophages surrounding HRS cells (Figure1B). PU.1 expression was further detected in the various B-cell compartments of reactive lymphoid tissues that served as a positive control (Figure 1A). In contrast to our analysis of the transcription factor Oct2 and its coactivator BOB.1/OBF.1, that were partially present at low levels in 9.4% and 25% of cases of cHD respectively,2 PU.1 expression was not detectable in any case. PU.1 deficiency, therefore, is a recurrent defect of a B-cell transcription factor in HRS cells of cHD, underlining our hypothesis that absent immunoglobulin expression in cHD is probably due to defects in the transcriptional machinery. These data are in accordance with our recent results, showing that neither crippling mutations within the V region gene nor in the octamer motif of the immunoglobulin promoter or other regulatory sequences can be the general cause for the defective immunoglobulin transcription in cHD.1-3
Expression pattern of PU.1 in reactive lymphoid tissue and in classical Hodgkin disease.
(A) Uniform nuclear expression of PU.1 by germinal center and follicular mantle B cells in tonsillar lymphoid tissue. (B) Hodgkin and Reed-Sternberg cells show absent PU.1 expression, whereas small B cells and occasional histiocytes express the protein in the nuclei (immunostains using a PU.1 monoclonal antibody and the EnVision method using diaminobenzidine as chromogen, brown reaction product). Isotype-matched irrelevant antibodies did not show positive staining patterns on the same tissues. Original magnification A, × 100; B, × 250.
Expression pattern of PU.1 in reactive lymphoid tissue and in classical Hodgkin disease.
(A) Uniform nuclear expression of PU.1 by germinal center and follicular mantle B cells in tonsillar lymphoid tissue. (B) Hodgkin and Reed-Sternberg cells show absent PU.1 expression, whereas small B cells and occasional histiocytes express the protein in the nuclei (immunostains using a PU.1 monoclonal antibody and the EnVision method using diaminobenzidine as chromogen, brown reaction product). Isotype-matched irrelevant antibodies did not show positive staining patterns on the same tissues. Original magnification A, × 100; B, × 250.
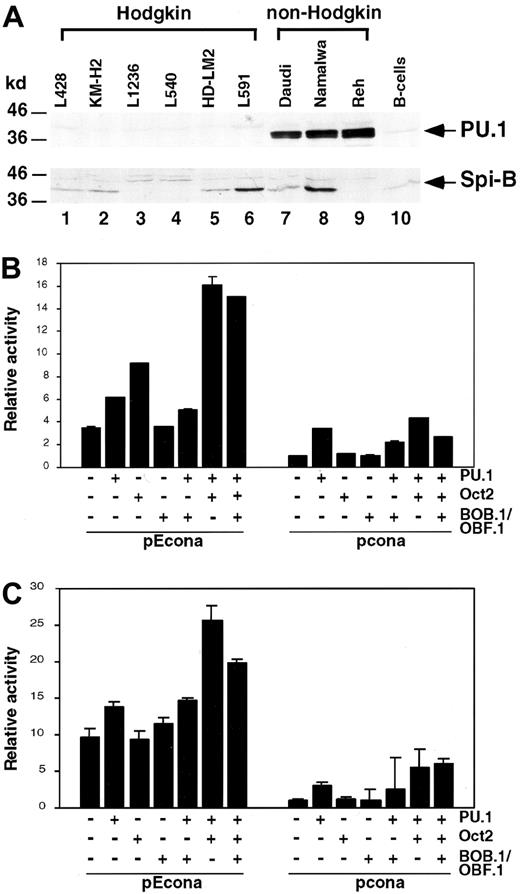
To verify PU.1 deficiency in cultured HRS cells, we performed Western blot analysis by using PU.1-specific antibodies (Figure2A). As expected, all Hodgkin cell lines (lanes 1-6) did not show PU.1 protein levels in contrast to other B-cell–derived non-Hodgkin cell lines (lanes 7-9) and CD19+ B cells (lane 10). Correlating with the absence of PU.1 protein levels, we did not detect any PU.1-specific transcripts in the Hodgkin cell lines by Northern blot analysis (data not shown). Whereas PU.1 messenger RNA was readily detectable in the non-Hodgkin cell lines tested (data not shown).
PU.1 expression in Hodgkin cell lines and transient cotransfections of the Hodgkin cell lines L428 and KM-H2 with immunoglobulin enhancer constructs.
(A) Western blot analysis of cell lysate proteins from Hodgkin (lanes 1-6), non-Hodgkin (Burkitt lymphoma: Daudi, Namalwa; pre-B cell line: Reh; lanes 7-9) cell lines and mature CD19+ B cells (B cells; lane 10), showing bands of PU.1 and Spi-B. Left margin, size markers in kilodalton. L428 (B) and KM-H2 (C) cells were transfected with luciferase reporter plasmids driven by conalbumin promoter with or without the MluI-HpaI fragment of the human IgH gene intronic enhancer (pEcona and pcona). Expression vectors for PU.1, Oct2, and BOB/OBF.1 were cotransfected as indicated. Relative luciferase activity is shown and transfections with empty expression vectors were arbitrarily set to 1.
PU.1 expression in Hodgkin cell lines and transient cotransfections of the Hodgkin cell lines L428 and KM-H2 with immunoglobulin enhancer constructs.
(A) Western blot analysis of cell lysate proteins from Hodgkin (lanes 1-6), non-Hodgkin (Burkitt lymphoma: Daudi, Namalwa; pre-B cell line: Reh; lanes 7-9) cell lines and mature CD19+ B cells (B cells; lane 10), showing bands of PU.1 and Spi-B. Left margin, size markers in kilodalton. L428 (B) and KM-H2 (C) cells were transfected with luciferase reporter plasmids driven by conalbumin promoter with or without the MluI-HpaI fragment of the human IgH gene intronic enhancer (pEcona and pcona). Expression vectors for PU.1, Oct2, and BOB/OBF.1 were cotransfected as indicated. Relative luciferase activity is shown and transfections with empty expression vectors were arbitrarily set to 1.
However, Spi-B, another member of the Ets family of DNA-binding proteins,20,21 was expressed in several Hodgkin and non-Hodgkin cell lines (Figure 2A; lanes 1, 2, 5-8) and in CD19+ B cells (lane 10). Our data indicate that in contrast to the Ets family member Spi-B, PU.1 is not expressed in cultured HRS cells. All tested Hodgkin cell lines, therefore, resemble tumor cells in vivo with respect to PU.1 expression. The correspondence between our in vitro and in vivo data is particularly important, because recent studies of our group showed that there might be discrepant results studying B-cell transcription factors in cultured and primary HRS cells. High expression of Oct2, shown in cultured HRS cells by electromobility shift assays, could subsequently neither be detected in primary nor in cultured tumor cells by our very sensitive in situ hybridization analysis.2 22 This discrepancy could possibly be explained by cell culture conditions that might have transitorily induced Oct2 levels in the Hodgkin cell lines.
There is abundant evidence that PU.1 is important for heavy and light chain immunoglobulin gene transcription.10-14 PU.1 stimulates, for example, the activity of the immunoglobulin-κ enhancer by playing an architectural role in the assembly of a higher-order protein-DNA complex.17 Thereby binding of phosphorylated PU.1 to its target sequence in the 3′ enhancer results in the cooperative recruitment of additional transcription factors such as Pip, E2A, and AP-1 to adjacent DNA sites.17 However, none of the enhancer binding proteins alone can significantly activate the 3′ enhancer.17 To investigate whether PU.1 can contribute to immunoglobulin enhancer activity in cultured HRS cells, we performed cotransfection experiments in which we reintroduced PU.1 and Oct2 as well as the Oct2 coactivator BOB.1/OBF.1 into L428 and KM-H2 cells (Figure 2B,C). By using luciferase reporter constructs driven by conalbumin promoter with the human IgH intronic enhancer, we showed that in L428 and KM-H2 cells PU.1, Oct2, and BOB.1/OBF.1 alone only slightly activated reporter constructs. However, reintroduction of PU.1 together with Oct2 (L428 cells, Figure 2B; KM-H2 cells, Figure 2C) significantly enhanced reporter activity. This activity was identical to that obtained in the Burkitt lymphoma cell lines Daudi and Namalwa (data not shown), expressing endogenous levels of these factors (Figure2A and data not shown). Our data indicate that in HRS cells PU.1 can cooperate with Oct2 in activation of the human IgH gene intronic enhancer. These data are in line with a previous report, suggesting that PU.1 plays a general role for the activation of immunoglobulin gene transcription.10 According to these data PU.1 can bind to pyrimidine-rich motifs that are functionally important, in particular for those regulatory elements that have only imperfect octamer sites. PU.1 and Oct2 bind concomitantly to these elements and thereby activate immunoglobulin gene transcription.10Because there are binding sites in the IgH intronic enhancer for PU.1 and Oct2, it seems conceivable that they regulate enhancer activity in our reporter constructs.
In our recent study we used reporter constructs with the wild-type or mutated immunoglobulin promoter octamer motif.2 We demonstrated a high transcriptional activation of the immunoglobulin promoter construct after cotransfection of Oct2 and/or its coactivator BOB.1/OBF.1 in L428, KM-H2, and L1236 cells.2 3 We conclude from our recent and present results that in HRS cells dependent on the immunoglobulin promoter or enhancer context, Oct2, BOB.1/OBF.1, and PU.1 can contribute to their transcriptional activation.
In summary, our data suggest that the loss of expression of several important B-cell transcription factors such as Oct2, BOB.1/OBF.1, and PU.1 is responsible for the inactivity of immunoglobulin promoters and enhancers in HRS cells of cHD.
Supported by the Deutsche Forschungsgemeinschaft through JU 426/1-1.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Franziska Jundt, Charité, Robert-Rössle-Klinik, Humboldt University of Berlin, D-13125 Berlin, Germany; e-mail: fjundt@mdc-berlin.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal