Abstract
In contrast to the tumor cells (L&H cells) of lymphocyte predominant Hodgkin disease (LPHD), Hodgkin and Reed-Sternberg (HRS) cells of classical Hodgkin disease (cHD) are unable to transcribe immunoglobulin, despite the presence of rearranged immunoglobulin genes. Although initial studies have suggested crippling immunoglobulin gene mutations to be the cause of absent immunoglobulin expression in cHD, recent work of our group has demonstrated an impaired activation of the immunoglobulin promoter as a superior mechanism. As immunoglobulin transcription is mainly regulated by the B-cell transcription factors Oct2 and BOB.1/OBF.1, we analyzed 35 cases of LPHD, 32 cases of cHD, and 2 Hodgkin disease cell lines for the expression of these transcription factors and also in parallel for immunoglobulin expression. Our results demonstrate an absence of Oct2 and/or BOB.1/OBF.1 in cHD and a striking overexpression of Oct2 in LPHD. Immunoglobulin expression was lacking in cHD but present in LPHD. Furthermore, the reintroduction of BOB.1/OBF.1 and Oct2 into cultured HRS cells restored the activity of cotransduced immunoglobulin promoter constructs. Our findings dismiss the concept that the different immunoglobulin expression in cHD and LPHD is due to disrupting mutations of immunoglobulin V genes in cHD but is most likely due to a down-regulation of Oct2 and/or BOB.1/OBF.1. This study further revealed Oct2 as a new and valuable marker for the identification of L&H cells and their distinction from HRS cells. The impairment of immunoglobulin transcription with a down-regulated synthesis of Oct2 and BOB.1/OBF.1 is the first established general recurrent defect found in HRS cells.
Introduction
Hodgkin disease (HD) is one of the most common categories of malignant lymphoma in Europe and North America. Two distinct types, the rare lymphocyte predominant (LPHD) form and the common classical form (cHD), have been distinguished. LPHD and cHD have both common features and marked differences. The features that they have in common are (1) a few large tumor cells in an abundant admixture of non-neoplastic inflammatory cells, (2) T-cell rosettes around the tumor cells, (3) a high proliferation rate, and (4) the presence of clonal immunoglobulin gene rearrangements in the tumor cells (5) with a high load of somatic mutations. The features that differentiate them mainly concern the gene expression of the tumor cells. In LPHD the tumor cells (designated L&H cells) constantly express B-cell–associated molecules (eg, CD20, CD79a, J-chain, and immunoglobulin transcripts in the absence of CD30 and CD15), whereas the tumor cells of cHD (termed Hodgkin and Reed-Sternberg [HRS] cells) carry CD30 and CD15 but lack CD20, CD79a, J-chain, and immunoglobulin transcripts in most or all instances (reviewed by Stein et al1 and Anagnostopoulos et al2). The different immunoglobulin expression has raised particular interest because the tumor cells of both LPHD and cHD contain rearranged immunoglobulin genes.3-7 This difference was explained by Rajewsky and colleagues7,8 by the presence of intact immunoglobulin gene coding sequences in L&H cells and crippled immunoglobulin genes in HRS cells. In contrast to this, our studies have shown that the immunoglobulin gene coding capacity is intact not only in LPHD but also in the majority of cHD cases,4prompting the conclusion that the absent immunoglobulin expression in HRS cells is due to a defect in the transcription machinery rather than to a disruption of the immunoglobulin genes themselves. This was confirmed by our recent transfection studies which showed that immunoglobulin gene promoter luciferase reporter constructs exhibited no activity in cultured HRS cells.4
The transcription factors Oct1 and Oct2 and their coactivator BOB.1/OBF.1 regulate immunoglobulin gene transcription. Oct1 and Oct2 bind to the immunoglobulin gene promoter octamer motif and activate immunoglobulin gene transcription when the coactivator BOB.1/OBF.1 is recruited into the transcription complex.9,10 BOB.1/OBF.1 was also shown to act as a molecular clamp that holds together the POU subdomains of Oct1 or Oct2.11 Finally, according to knockout experiments performed, Oct1 and Oct2 can replace each other, whereas BOB.1/OBF.1 is unconditionally required for the activation of the immunoglobulin gene promoter.12 This implies that Oct1 and Oct2 or their coactivator BOB.1/OBF.1 are regularly expressed in L&H cells but are absent from, or are defective in, HRS cells. This paper reports on studies designed to check this claim. The results obtained confirm the claim concerning the absence of Oct2 and/or BOB.1/OBF.1 in HRS cells with the additional interesting observation that Oct2 is enormously overexpressed by the L&H cells. These findings, in association with our demonstration of the restoration of the immunoglobulin promoter activity by cotransfection of BOB.1/OBF.1 and Oct2 into cultured HRS cells, strengthen our hypothesis that the non-expression of immunoglobulin by HRS cells is caused by a down-regulated synthesis of immunoglobulin transcription factors required for immunoglobulin transcription rather than by crippling mutations of immunoglobulin genes. Our findings also add more knowledge to the molecular differences found between LPHD and cHD. Finally, the overexpression of Oct2 in L&H cells is a new and valuable feature for their distinction from classical HRS cells.
Materials and methods
Tissues and cell lines
Paraffin-embedded tissue samples from 35 cases of LPHD, 32 cases of cHD, 6 of which had previously been investigated by single-cell analysis,4 and 8 cases of follicular center lymphoma were taken from the files of the Institute of Pathology, University Hospital Benjamin Franklin of the Free University of Berlin. All cases were diagnosed according to the Revised European-American Lymphoma Classification. The HD-derived cell lines L428 and KM-H213and the control B-cell lines B-JAB, Raji, Jok-1, and DG75 were grown in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), antibiotics, glutamate, and 50 mM β-mercaptoethanol at 37°C and 5% CO2.
Immunohistochemistry
Five-micrometer thick sections of paraffin-embedded tissue blocks were immunostained by the immunoalkaline-phosphatase (APAAP) method.14 The following antibody panel was used: CD20 (L26), CD79a (JCB117), CD30 (Ber-H2), CD15 (C3D1) and anti-LMP1 (CS1-4), polyclonal CD3, anti-immunoglobulin M (IgM), anti-IgD, anti-IgG (DAKO, Glostrup, Denmark), and the anti–J-chain antibody (Biogenex, San Ramon, CA). The monoclonal antibody to Oct1 (clone 12F11) and polyclonal antibodies to Oct2 and BOB.1/OBF.1 were purchased from Santa Cruz Biotechnology, Santa Cruz, CA. All these primary antibodies were applied to dewaxed sections that were pretreated by heating in citrate buffer (10 mMol, pH 6.0, 2 minutes) in a pressure cooker. For the detection of the bound antibodies either the APAAP method or the streptavidin-biotin method was employed.
In situ hybridization
The detection of messenger RNA (mRNA) for Igμ, Igδ, Igγ, Igκ, Igλ, and Oct2 was carried out, using a highly sensitive radioactive in situ hybridization method as previously described.4 15 The Oct2 probe was prepared from complementary DNA (cDNA) that had been amplified by reverse transcriptase-polymerase chain reaction (RT-PCR) from a B-cell line and cloned into the appropriate plasmids. After linearization of the plasmids with restriction enzymes, anti-sense and sense (control) transcripts were generated, using T7 or SP6 RNA-polymerase with the incorporation of [35S]-dUTP. Cells with a 4-fold signal intensity, as compared to background signal, were scored positive. Hybridization with sense-transcripts showed only very few homogeneously distributed background signals.
Northern and Western blot analyses
Cell culture and transfection
For electroporation, 5 × 106 cultured cells were pelleted and resuspended in a final volume of 300 μL RPMI medium supplemented with 10% FCS. Fifteen micrograms of the respective luciferase reporter plasmid (details about the wild-type immunoglobulin octamer enhancer and the synthetic multimerized octamer reporter constructs can be found in Laumen et al12 and Pfisterer et al19 20) were added to the cells. The mixture was transferred to a cuvette with a gap of 0.4 cm and electroporated at 230 V and 950 μF with a Gene Pulser (Biorad, Munich, Germany). The cells were immediately transferred to a Petri dish containing 10 mL RPMI/10% FCS and incubated at 37°C in 5% CO2 for 20 hours. The cells were harvested, and the lysates were analyzed with a dual-luciferase reporter assay system (Promega, Mannheim, Germany). All luciferase values were calculated against the renilla-luciferase values to correct for different transfection efficiencies.
Results
Expression of immunoglobulin in cHD and LPHD
The expression of immunoglobulin in classical HRS cells and L&H cells was investigated by radioactive in situ hybridization and immunohistochemistry. As in a previous study,4 there were no immunoglobulin-specific transcripts demonstrable in the HRS cells of 25 cases of cHD (Table 1). This result held true even when the exposure time was prolonged considerably, and the internal control cells (plasma cells and B cells) became very intensely positive. Immunostaining with immunoglobulin-specific antibodies often produced a cytoplasmic labeling of many HRS cells. The staining, however, spared the nuclear space of most HRS cells although this space was strongly positive in the bystander B lymphocytes and plasma cells in all instances. As revealed by Northern blotting and immunohistochemistry, the cell lines L428 and KM-H2 derived from HRS cells of cHD cases were devoid of immunoglobulin-specific transcripts and protein (Table 2).
Expression of immunoglobulin and immunoglobulin transcription factors in the tumor cells of classical and lymphocyte predominant Hodgkin disease
| . | HRS cells of classical HD . | L&H cells of LPHD . | Follicular lymphoma . |
|---|---|---|---|
| CD30 | 32/32 | 0/35 | 0/8 |
| CD20 | 6/32 | 35/35 | 8/8 |
| J-chain | 4*/32 | 31/35 | 6/8 |
| Immunoglobulin mRNA | 0/25† | 25/27 | 8/8 |
| Immunoglobulin protein | 0/25‡ | 26/32 | 8/8 |
| Oct2-mRNA | 21-153/11 | 6/6 | 4/4 |
| Oct2-protein | 41-154/321-155 | 35/35 | 8/8 |
| BOB.1 | 8/321-155 | 35/35 | 8/8 |
| . | HRS cells of classical HD . | L&H cells of LPHD . | Follicular lymphoma . |
|---|---|---|---|
| CD30 | 32/32 | 0/35 | 0/8 |
| CD20 | 6/32 | 35/35 | 8/8 |
| J-chain | 4*/32 | 31/35 | 6/8 |
| Immunoglobulin mRNA | 0/25† | 25/27 | 8/8 |
| Immunoglobulin protein | 0/25‡ | 26/32 | 8/8 |
| Oct2-mRNA | 21-153/11 | 6/6 | 4/4 |
| Oct2-protein | 41-154/321-155 | 35/35 | 8/8 |
| BOB.1 | 8/321-155 | 35/35 | 8/8 |
HRS cells indicates Hodgkin and Reed-Sternberg cells; HD, Hodgkin disease; LPHD, lymphocyte predominant Hodgkin disease; L&H cells, lymphocytic/histiocytic tumor cells of LPHD; Ig, immunoglobulin; mRNA, messenger RNA.
Very weak labeling.
One case with very weak signals for Igκ in some HRS cells.
Most cases showed in some or many HRS cells immunoreactivity with antibodies to IgG, Igκ, and Igλ. However, in the majority of the cases the immune reaction did not include the nuclear space of the HRS cells in contrast to the B cells and plasma cells that showed a strong nuclear space staining for one IgL chain type in all instances; in a few cases there was a nuclear space staining in some of the HRS cells.
These cases also expressed Oct2 at the protein level.
In only one case all HRS cells were positive.
There were no classical HD cases in which HRS cells expressed both Oct2 and BOB.1/OBF.1.
Expression of immunoglobulin, Oct1, Oct2, and BOB.1/OBF.1 in classical Hodgkin disease–derived cell lines and B-cell lines
| Transcripts and proteins . | L428 . | KM-H2 . | B-cell lines* . |
|---|---|---|---|
| Immunoglobulin mRNA† | − | − | + |
| Immunoglobulin protein‡ | − | − | + |
| Oct1 mRNA†,2-153 | + | + | + |
| Oct1 protein‡ | + | + | + |
| Oct2 mRNA†,2-153 | − | − | + |
| Oct2 protein‡,2-154 | − | − | + |
| BOB.1 mRNA†,2-153 | − | − | + |
| BOB.1 protein2-154 | − | − | + |
| Transcripts and proteins . | L428 . | KM-H2 . | B-cell lines* . |
|---|---|---|---|
| Immunoglobulin mRNA† | − | − | + |
| Immunoglobulin protein‡ | − | − | + |
| Oct1 mRNA†,2-153 | + | + | + |
| Oct1 protein‡ | + | + | + |
| Oct2 mRNA†,2-153 | − | − | + |
| Oct2 protein‡,2-154 | − | − | + |
| BOB.1 mRNA†,2-153 | − | − | + |
| BOB.1 protein2-154 | − | − | + |
mRNA indicates messenger RNA.
B-JAB, Namalwa, Raji, Jok-1, and DG-75.
Northern blot analysis.
Immunocytology.
Reverse transcriptase-polymerase chain reaction.
Western blot analysis.
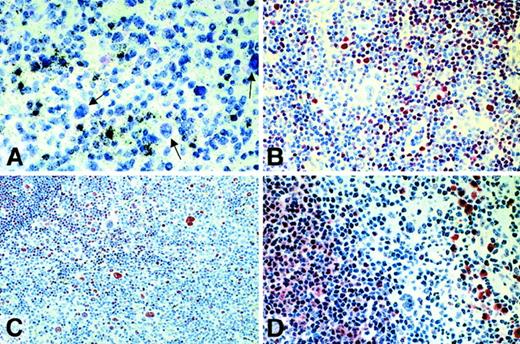
In contrast, L&H cells of all but 2 cases were labeled with immunoglobulin-specific probes with an intensity between that generally seen in plasma cells and B lymphocytes (Table 1, Figure1A). Immunostaining with antibodies directed at immunoglobulin isotypes revealed a very strong labeling of the nuclear space and the cytoplasm of the L&H cells in 26 of 32 cases (Figure 1B-D,F; Table 1). The tumor cells of 8 cases of follicular lymphoma studied for control purposes showed positive labeling for immunoglobulin mRNA and immunoglobulin protein in all instances (Table1). The positive immunoglobulin staining included the nuclear space in all 8 cases. The frequency with which the different IgH classes and the IgL types were found to be expressed by the L&H cells is shown in Table3.
Lymphocyte predominant Hodgkin disease.
(A,E) Radioactive in situ hybridization with probes specific for Igγ and Igλ mRNA, respectively. The L&H cells (white arrows) are positive with an intensity between that seen in plasma cells and B cells (black arrows). (B-D,F) Immunostaining for Igγ, -μ, -δ, and -λ, using a avidin-biotin-peroxidase method. The L&H cells display a strong labeling.
Lymphocyte predominant Hodgkin disease.
(A,E) Radioactive in situ hybridization with probes specific for Igγ and Igλ mRNA, respectively. The L&H cells (white arrows) are positive with an intensity between that seen in plasma cells and B cells (black arrows). (B-D,F) Immunostaining for Igγ, -μ, -δ, and -λ, using a avidin-biotin-peroxidase method. The L&H cells display a strong labeling.
Expression of immunoglobulin Isotypes by L&H cells of lymphocyte predominant Hodgkin disease
| Immunoglobulin isotype . | No. cases studied . | Cases with labeled L&H cells . | |
|---|---|---|---|
| mRNA . | Protein . | ||
| Igμ | 34 | 3 | 3 |
| Igδ | 34 | 3 | 3 |
| Igγ | 34 | 21 | 9 |
| Igκ | 27 | 18 | 16 |
| Igλ | 27 | 2 | 2 |
| Immunoglobulin isotype . | No. cases studied . | Cases with labeled L&H cells . | |
|---|---|---|---|
| mRNA . | Protein . | ||
| Igμ | 34 | 3 | 3 |
| Igδ | 34 | 3 | 3 |
| Igγ | 34 | 21 | 9 |
| Igκ | 27 | 18 | 16 |
| Igλ | 27 | 2 | 2 |
For L&H definition, see Table 1; mRNA, messenger RNA; Ig, immunoglobulin.
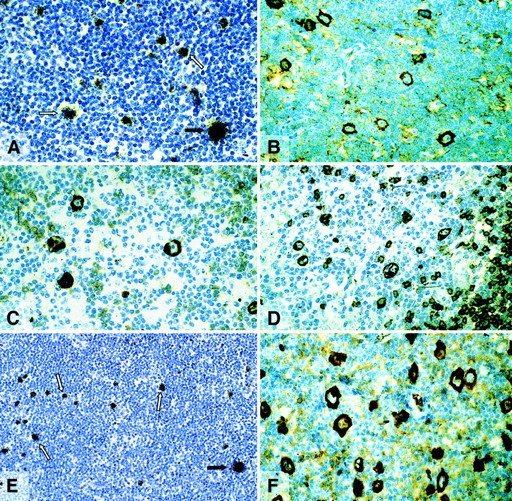
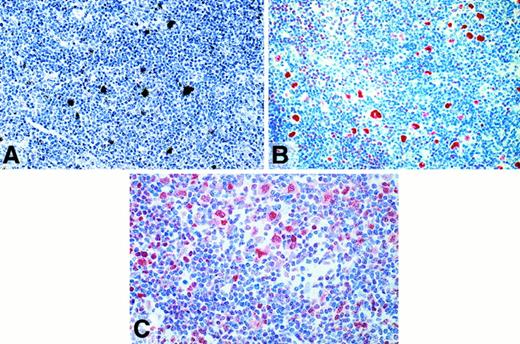
Expression of BOB.1/OBF.1 and Oct2 in cHD and LPHD
The expression of BOB.1/OBF.1 and Oct2 was studied by immunohistochemistry. BOB.1/OBF.1 and Oct2 were completely absent from the HRS cells of 24 (75%) and 28 (87.5%) of the 32 cHD cases, respectively (Table 1; Figure 2B,D). In the remaining cases only a small proportion of the HRS cells showed a weak labeling for these proteins (Figure 2C) with the exception of one case in which all HRS cells showed a strong labeling for Oct2 protein. To check the validity of the negative labeling for Oct2 in HRS cells, 11 cases were examined by in situ hybridization with a radioactive Oct2 probe. In 9 cases there were no signals over the HRS cells, although internal control cells were labeled (Table 1, Figure 2A). In difference to cHD, expression of BOB.1/OBF.1 and Oct2 was demonstrable in all L&H cells of all LPHD cases studied (Table 1, Figure3A). The immunostaining of L&H cells for BOB.1/OBF.1 was as strong as in normal and neoplastic germinal center cells. However, the labeling for Oct2 proved to be much more intense in the L&H cells when compared with normal cells and the tumor cells of follicular lymphoma (Figure 3A,B).
Classical Hodgkin disease.
(A) Radioactive in situ hybridization with a probe specific for Oct2 mRNA. In contrast to bystander B cells, HRS cells remained completely unlabeled (arrows). (B) Immunostaining for Oct2. All HRS cells are unlabeled. (C) Immunostaining for Oct2. A proportion of HRS cells is positive. (D) Immunostaining for BOB.1/OBF.1. HRS cells are negative. All immunolabeling reactions were performed with the APAAP technique.
Classical Hodgkin disease.
(A) Radioactive in situ hybridization with a probe specific for Oct2 mRNA. In contrast to bystander B cells, HRS cells remained completely unlabeled (arrows). (B) Immunostaining for Oct2. All HRS cells are unlabeled. (C) Immunostaining for Oct2. A proportion of HRS cells is positive. (D) Immunostaining for BOB.1/OBF.1. HRS cells are negative. All immunolabeling reactions were performed with the APAAP technique.
Lymphocyte predominant Hodgkin disease.
(A) Radioactive in situ hybridization with a probe specific for Oct2. L&H cells show dense signals. (B) Immunostaining (APAAP) for Oct2. The nuclei of the L&H cells are strongly labeled. (C) Immunostaining (APAAP) for BOB.1/OBF.1. The nuclei and the cytoplasma (less intense) of the L&H cells display a moderately strong labeling.
Lymphocyte predominant Hodgkin disease.
(A) Radioactive in situ hybridization with a probe specific for Oct2. L&H cells show dense signals. (B) Immunostaining (APAAP) for Oct2. The nuclei of the L&H cells are strongly labeled. (C) Immunostaining (APAAP) for BOB.1/OBF.1. The nuclei and the cytoplasma (less intense) of the L&H cells display a moderately strong labeling.
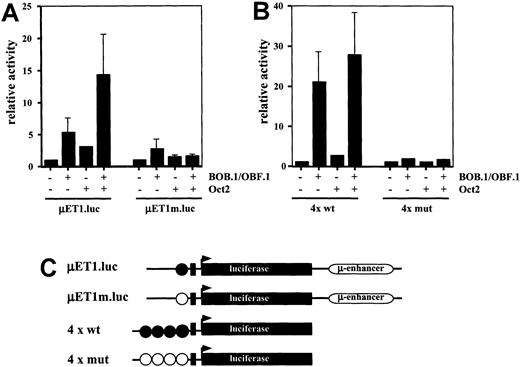
Co-transfection of cultured HRS cells with immunoglobulin promoter-reporter constructs and Oct2 and/or BOB.1/OBF.1
The above findings prompted the question as to whether direct evidence can be provided showing that the lack of the Oct2 transcription factor and the BOB.1/OBF.1 coactivator is decisive for deficient immunoglobulin gene transcription in HRS cells. For this purpose we cotransfected the HD-derived cell line L428 with wild-type and mutated (point mutation in the promoter octamer motif) immunoglobulin reporter constructs either with BOB.1/OBF.1 or Oct2 expression vectors individually or with a combination of both. As shown in Figure 4A, the wild-type immunoglobulin reporter was slightly stimulated by BOB.1/OBF.1 and to a lower extent induced by Oct2. The combination of both factors, however, resulted in a significantly higher stimulation of immunoglobulin reporter gene activity. This activity was virtually identical to that obtained in a normal B-cell line expressing endogenous levels of these factors (data not shown).
Transient cotransfections of HD-derived L428 cells.
(A) L428 cells were cotransfected with immunoglobulin promoter/enhancer-driven luciferase reporters and expression vectors for BOB.1/OBF.1 and/or Oct2 as indicated. μET1.luc represents reporter wild type promoter element extending from +19 to −131, μEtm.luc contains the same promoter element with point mutations in the octamer motif (see C). Relative activity is shown and the cotransfections with empty expression vectors were arbitrarily set to 1. (B) L428 cells were cotransfected with wild type (4 × wt) or mutant (4 × mut) octamer-dependent luciferase reporters together with expression vectors for BOB.1/OBF.1 and/or Oct2 as indicated. Relative activity is shown and the cotransfections with empty expression vectors were arbitrarily set to 1. (C) Schematic representation of the reporter constructs used in A and B. Wild-type octamer motifs in the immunoglobulin promoter or synthetic promoter are indicated as filled circles; mutant octamer motifs are shown as open circles. Details about the reporter constructs can be found in Laumen et al12 (for μET1.luc and μET1m.luc) or Pfisterer et al19 (for 4 × wt and 4 × mut).
Transient cotransfections of HD-derived L428 cells.
(A) L428 cells were cotransfected with immunoglobulin promoter/enhancer-driven luciferase reporters and expression vectors for BOB.1/OBF.1 and/or Oct2 as indicated. μET1.luc represents reporter wild type promoter element extending from +19 to −131, μEtm.luc contains the same promoter element with point mutations in the octamer motif (see C). Relative activity is shown and the cotransfections with empty expression vectors were arbitrarily set to 1. (B) L428 cells were cotransfected with wild type (4 × wt) or mutant (4 × mut) octamer-dependent luciferase reporters together with expression vectors for BOB.1/OBF.1 and/or Oct2 as indicated. Relative activity is shown and the cotransfections with empty expression vectors were arbitrarily set to 1. (C) Schematic representation of the reporter constructs used in A and B. Wild-type octamer motifs in the immunoglobulin promoter or synthetic promoter are indicated as filled circles; mutant octamer motifs are shown as open circles. Details about the reporter constructs can be found in Laumen et al12 (for μET1.luc and μET1m.luc) or Pfisterer et al19 (for 4 × wt and 4 × mut).
These results were confirmed by transfection of L428 with immunoglobulin reporter constructs that contain multimerized octamer motifs upstream of a minimal promoter consisting of only a TATA box (Figure 4B). Transfection of the multimerized immunoglobulin reporter construct with BOB.1/OBF.1 resulted in a strong stimulation of wild-type octamer promoter activity, whereas transfection with Oct2 displayed only a marginal effect on immunoglobulin reporter activity. The BOB.1/OBF.1-induced immunoglobulin promoter activity could be increased further by cotransfection of BOB.1/OBF.1 together with Oct2 (Figure 4B). Mutated multimerized immunoglobulin reporter constructs showed no significant activity under all conditions tested (Figure 4B).
Discussion
In this study we have analyzed how consistent cHD and LPHD differ from each other in their expression of immunoglobulin and which molecular mechanisms might account for this difference. We have confirmed our previous findings that HRS cells of all (or nearly all) cases of cHD lack transcripts specific for IgL and IgH chains4 and have found that the L&H cells of LPHD overexpress immunoglobulin at the protein and/or transcriptional level in most instances when compared with normal follicular mantle cells and follicular lymphoma cells. We have in addition found that the octamer transcription factor Oct2 is highly overexpressed in L&H cells but totally absent from 87.5% of the cases or only partially (9.4%) present in classical HRS cells. There was only one exception to this in which all HRS cells expressed Oct2 at a relatively high level. BOB.1/OBF.1, the coactivator of Oct2, proved to be consistently expressed in the L&H cells at a level equivalent to that seen in reactive and neoplastic germinal center B cells, whereas there was no (75% of the cases) or only very little expression (25%) of BOB.1/OBF.1 in classical HRS cells. Co-transfection experiments performed revealed that the reintroduction of BOB.1/OBF.1 and Oct2 into cultured HRS cells can restore the transcription activity of the immunoglobulin promoter. On the basis of these findings, we conclude that the difference in the immunoglobulin expression between LPHD and cHD does not lie in the absence of crippling immunoglobulin gene mutations in L&H cells and their presence in HRS cells—as recently assumed—but in a differing expression of the octamer transcription factor Oct2 and/or its coactivator BOB.1/OBF.1.
In the past, many studies21-25 have been performed to analyze the immunoglobulin production by L&H cells and HRS cells. However, in none of these previous studies has a larger series of cases of LPHD and cHD been investigated in parallel with a highly efficient antigen retrieval procedure and a highly sensitive radioactive in situ hybridization method. Furthermore, all previous in situ hybridization studies that were applied to LPHD cases were restricted to IgL chains.3,26-28 To clarify how frequently and at which level the immunoglobulin synthesis in the tumor cells of cHD is disturbed, it is necessary to obtain representative results to investigate the same collection of cases not only for the immunoglobulin light chain types κ and λ but also for the heavy chain isotypes Igμ, Igδ, and Igγ at the protein and RNA levels. Another important reason for studying cases of cHD for both immunoglobulin proteins and immunoglobulin transcripts in parallel lies in the fact that the immunostaining of paraffin sections for immunoglobulin protein often leads—due to uptake of immunoglobulin from interstitial fluids—to a falsely positive labeling of larger cells.1,13,24,25 The tumor cells of cHD are particularly susceptible to immunoglobulin uptake during the formol fixation process.24 Falsely positive labeling for immunoglobulin transcripts due to uptake does not occur because immunoglobulin mRNA—in difference to immunoglobulin proteins—is not secreted and thus is not present in the extracellular fluid. Because of these reasons and to establish criteria by which endogenous immunoglobulin protein can be distinguished from uptaken immunoglobulin in immunostains, we subjected a larger series of cases of cHD and of LPHD to immunohistological staining for IgH and IgL proteins and corresponding transcripts. The comparison of the immunohistological findings with those obtained by in situ hybridization showed that there are high congruent labeling results if the evaluation of the immunoglobulin protein labeling is restricted to the perinuclear space, indicating that immunoglobulin that is immunohistochemically demonstrated in the nuclear space represents endogenous immunoglobulin rather than uptaken immunoglobulin proteins. Classical HRS cells did not usually show a staining of the nuclear space for immunoglobulin. Significant signals for IgH- or IgL-chain-specific mRNA were not obtained in any case. In contrast, there was a positive labeling of L&H cells for immunoglobulin transcripts and proteins in most cases. The immunoglobulin protein labeling was usually very intense and in density comparable to that seen in plasma cells. The labeling included the perinuclear space in all instances, which is difficult to recognize in the photographs because of the density of the label. The density of the immunoglobulin transcript signals was stronger than that of normal B cells, suggesting that increased immunoglobulin transcription contributes to the overexpression of immunoglobulin in L&H cells. In the majority of the cases the L&H cells proved to be derived from class-switched germinal center B cells as revealed by the immunoglobulin isotypes expressed.
In contrast to other reports7,8,29 we were recently able to demonstrate that in most instances the rearranged immunoglobulin genes are not only functional in L&H cells but also in classical HRS cells, prompting the conclusion that the absent expression of immunoglobulin molecules in HRS cells is not caused by crippling mutations of the rearranged immunoglobulin genes but by silencing the immunoglobulin promoter.4 We confirmed the lack of immunoglobulin promoter activity in HRS cells by analyzing the HD cell lines L428 and KM-H2 in transient transfection experiments. An immunoglobulin promoter/enhancer reporter construct showed virtually no activity in these cells in contrast to control B-cell lines.4 We hypothesized that the loss of immunoglobulin promoter activity in the HRS cells might be due to an absence of Oct2 and/or BOB.1/OBF.1 and, if true, that HRS cells should not only differ from L&H cells in immunoglobulin expression but also in the expression of Oct2 and/or BOB.1/OBF.1. To verify this hypothesis we investigated a larger series of cHD and LPHD cases by immunohistochemistry for Oct2 and BOB.1/OBF.1 protein expression and correlated the results with the presence of immunoglobulin protein and/or transcripts. For comparison we included 8 cases of follicular lymphoma with monotypic IgH and IgL expression and 3 non-neoplastic tonsils (data not shown). Our investigations revealed that L&H cells—like follicular lymphoma cells and non-neoplastic germinal center B cells—consistently express Oct2 and BOB.1/OBF.1 with the only difference that Oct2 proved to be highly overexpressed in the L&H cells in all instances. This makes Oct2 the most valuable marker for the identification of L&H cells. In contrast to L&H cells, HRS cells proved to be devoid of both Oct2 and BOB.1/OBF.1 in most cases. In the few cases in which only one of the 2 factors was missing it could be shown that the expression of the demonstrable factor was low and restricted to a usually small proportion of HRS cells. There was no case in which both Oct2 and BOB.1/OBF.1 were simultaneously expressed in the HRS cells.
The absence of the Oct2 protein from classical HRS cells came as a surprise because in one study30 its constant presence in primary HRS cells and HD cell lines, including the L428 and the KM-H2 lines, was reported. This discrepancy prompted us to examine the validity of our immunohistological results by investigating 11 cases of cHD, 4 cases of follicular lymphoma, and 3 hyperplastic tonsils for Oct2 mRNA with our very sensitive radioactive in situ hybridization procedure. In contrast to L&H cells, follicular lymphoma cells, and normal germinal center B cells, the HRS cells consistently failed to give rise to any Oct2-specific transcript signals. We also re-evaluated Oct2 expression in the HD-derived cell lines L428 and KM-H2, using various RNA and protein detection methods. We were unable to detect any Oct2-specific signals with any of the methods employed.
In light of the highly positive correlation between immunoglobulin production and expression of Oct2 and BOB.1/OBF.1, it is tempting to assume that the absent transcription of immunoglobulin genes in HRS cells is due to the lack of Oct2 and/or BOB.1/OBF.1 in these cells. To strengthen or dismiss this conclusion, we performed cotransfection experiments in which we reintroduced BOB.1/OBF.1 and/or Oct2 into cultured HRS cells (L428) in association with immunoglobulin promoter-reporter constructs. The results obtained demonstrate that the lack of these transcription factors is indeed the likely cause for the defective immunoglobulin gene transcription in HRS cells. The strongest effect was seen when both the BOB.1/OBF.1 coactivator and the Oct2 transcription factor were cotransduced. However, the transfection of BOB.1/OBF.1 by itself also showed a significant effect. This observation is consistent with previous studies demonstrating that BOB.1/OBF.1 can also function in the absence of Oct2 as a coactivator because the function of Oct2 can be replaced by the ubiquitous Oct1 transcription factor and vice versa.12 31 In line with this assumption, Oct1 is expressed in the cultured HRS cells used for our transfection experiments (data not shown).
The finding that the effect of BOB.1/OBF.1 is augmented by Oct2 in the cultured HRS cells suggests that in more advanced differentiated B cells—as the HRS cells are7,32,33—Oct1 cannot fully replace the function of Oct2. This conclusion is in harmony with more recent work showing that in later-stage B cells Oct2 is more important for immunoglobulin transcription than Oct1.34 The stronger role of Oct2 in later-stage B cells might also explain the absence of immunoglobulin transcription in the few cases of cHD in which the HRS cells were negative for Oct2 but partially positive for BOB.1/OBF.1. We, therefore, speculate that the down-regulated expression of BOB.1/OBF.1 as well as that of Oct2 are both critical events for the loss of immunoglobulin transcription through HRS cells.
Because the strong overexpression of Oct2 correlates to the high level of immunoglobulin in LPHD in the majority of cases, it is suggestive that the enormous amount of Oct2 in L&H cells might be responsible for, or contribute to, the frequent overexpression of immunoglobulin by these cells. However, what is more difficult to understand are the 2 cases of LPHD in which Oct2 is overexpressed and BOB.1/OBF.1 is normally expressed, but no immunoglobulin production is detectable. This points to the possibility that a further as yet unidentified factor (or factors) is involved in the regulation of immunoglobulin expression.
Acknowledgments
We thank H. Protz, A. Foerster, E. Berg, E. Seibt, and L. Oehring for their excellent technical assistance as well as L. Udvarhelyi for his help with the preparation of the manuscript.
Supported by grants of the Deutsche Forschungsgemeinschaft and Deutsche Krebshilfe, AIRC (Associazione Italiana per la Ricerca sul Cancro), and MURST.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Harald Stein, Institute of Pathology, Benjamin Franklin University Hospital, Free University Berlin, Hindenburgdamm 30, 12200 Berlin, Germany; e-mail: stein@ukbf.fu-berlin.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal