Abstract
Mast cells (MCs) are central to asthma and other allergic diseases, and for responses to infection and tissue injuries. MCs arise from committed progenitors (PrMCs) that migrate from the circulation to tissues by incompletely characterized mechanisms, and differentiate in situ in perivascular connective tissues of multiple organs. PrMCs derived in vitro from human cord blood were examined for adhesion molecule expression and their ability to adhere to human umbilical vein endothelial cells (HUVECs) under conditions that mimic physiologic shear flow. The PrMCs expressed α4β1, low levels of β7, and the β2-integrins αLβ2 and αMβ2. The PrMCs also expressed PSGL-1, but not L-selectin. At low (0.5 dynes/cm2-1.0 dynes/cm2) shear stress, PrMCs attached and rolled on recombinant E-selectin and P-selectin and VCAM-1. An anti–PSGL-1 monoclonal antibody (mAb) blocked essentially all adhesion to P-selectin but reduced adhesion to E-selectin by only 40%, suggesting PrMCs express other ligands for E-selectin. PrMCs adhered strongly to tumor necrosis factor-α (TNF-α)–activated HUVECs, whereas adhesion to interleukin 4 (IL-4)–activated HUVECs was lower. PrMC adhesion to IL-4–activated HUVECs was totally α4-integrin– and VCAM-1–dependent. Adhesion to TNF-α–activated HUVECs was blocked by 50% by mAbs against α4-integrin, vascular cell adhesion molecule–1 (VCAM-1), E-selectin, or PSGL-1, whereas combinations of mAbs to α4-integrin plus PSGL-1, or VCAM-1 plus E-selectin, blocked adhesion by greater than 70%. Thus, PrMCs derived in vitro predominantly use α4-integrin, VCAM-1, PSGL-1, and other ligands that bind E-selectin for adhesion to cytokine-activated HUVEC monolayers. These observations may explain the abundance of MCs at sites of mucosal inflammation, where VCAM-1 and E-selectin are important inducible receptors.
Introduction
Mast cells (MCs) are critical effector cells of both innate1-3 and adaptive4 immunity that reside exclusively in tissues. MCs are constitutively located in perivascular connective tissues in the respiratory and gastrointestinal (GI) tract, skin, and several internal organs.5 Their prominence at interfaces with the external environment facilitates their key role in initiation of neutrophil recruitment in response to gram negative pathogens.1-3 Furthermore, increased numbers of MCs are frequently observed at foci of fibrosis6,7 and angiogenesis,8 in human atheroma located in coronary arteries,9,10 and in various circumstances of allergic mucosal inflammation.11-13 Both basal tissue MCs and the MCs that develop in foci of inflammation arise in situ from committed bone marrow–derived progenitors (PrMCs)14-19 that migrate from the circulation to the tissues by largely unexplored mechanisms. PrMCs are mononuclear cells that lack the characteristic secretory granules of their mature counterparts.18,19 In humans, PrMCs are found among a subset of mononuclear cells that express CD34, CD13 (a membrane aminopeptidase),20 and the receptor for stem cell factor (SCF), c-kit, but lack the CD1418 marker that is associated with monocytes, although mature cultured human MCs do express CD14.21 This same subset of circulating progenitors contains some cells with bipotent macrophage/MC colony-forming potential, as well as some that give rise to monocyte colonies, supporting an evolutionary relationship between the MCs and monocyte/macrophage lineages.20 The number of PrMCs is elevated in the peripheral blood of patients with asthma,22 which may relate to the increases in the numbers of MCs observed in the bronchial epithelial compartment of asthmatic patients compared with healthy controls.12 Since PrMCs represent only a very small subset (< 1:1000) of peripheral blood mononuclear cells, little is known regarding their expression of homing and adhesion receptors, and their utilization of these receptors for trafficking in vivo. Given the critical role for MCs in both innate and adaptive immunity and in several diseases, the mechanisms that control their distribution have broad and important implications.
For all circulating leukocytes, both basal homing and inflammation-induced recruitment into tissues involves specific receptor-mediated adhesive interactions with vascular endothelial cells. The initial attachment and rolling of leukocytes on vessel walls is mediated by selectins (E, P, and L) and their ligands (PSGL-1), reviewed in Kansas23 and Vestweber and Blanks.24 Firm adhesion of leukocytes prior to their transmigration into tissues is mediated by β2-integrins, which bind to intracellular adhesion molecule (ICAM)–1 or ICAM-2, or by the α4-integrins (α4β1, very late antigen [VLA]-4, and α4β7), which bind to vascular cell adhesion molecule (VCAM)–1 and mucosal addressin cell adhesion molecule-1 (MAdCAM-1), respectively. α4-integrin/VCAM-1 interactions can mediate both initial rolling and firm attachment, and are especially important for the recruitment of eosinophils, monocytes, and lymphocytes to sites of allergen-induced pulmonary inflammation in animal models of asthma.25-27 Interactions between α4β7 and MAdCAM-1 are critical for constitutive localization of lymphocytes to gut-associated lymphoid tissue.28 The wide tissue distribution of mature MCs, their increased numbers in several inflammatory tissues, and the fact that a pool of PrMCs is found under normal conditions in some tissues, particularly the intestine, each suggests that integrins and selectins may well be involved in the transit of PrMCs from blood to tissues. However, the specific requirements for PrMC adhesion to and egress through the blood vessel wall remain largely unexplored because of a lack of access to such cells.
We recently reported the use of an in vitro model system to derive PrMCs from umbilical cord blood mononuclear cells.29 When cultured in the presence of recombinant SCF, IL-6, and IL-10, the latter added to suppress the growth of monocyte/macrophages, these mononuclear cells give rise to a population of PrMCs, as defined by expression of c-kit and CD13, the lack of CD14 expression, and positive staining for chloroacetate esterase, a marker restricted in its expression to MCs and neutrophils. In the present study, we sought to determine the requirements for adhesion of these nontransformed PrMCs to immobilized recombinant endothelial cell adhesion molecules and to cytokine-activated vascular endothelial cells under flow conditions using a previously established in vitro model.
Materials and methods
Materials
Human recombinant TNF-α was obtained from Genzyme (Cambridge, MA) and was free of detectable endotoxins as reported previously.30 A concentration of 25 ng/mL for 24 hours induced expression of VCAM-1 and ICAM-1, whereas E-selectin expression had declined to approximately 50% of maximal expression as we have reported previously.31 Recombinant IL-4 (produced inEscherichia coli; working concentration was 25 ng/mL), IL-6, and SCF were obtained from R&D Systems (Minneapolis, MN). Recombinant IL-10 was purchased from Endogen (Woburn, MA). Hanks balanced salt solution (HBSS) with (HBSS+) or without Ca++ and Mg2+ (HBSS−), M199, RPMI-1640, Dulbecco phosphate-buffered saline (DPBS), with or without divalent cations were obtained from BioWhittaker (Walkersville, MD). Fetal bovine serum (FBS) was obtained from Hyclone (Urem, UT). All other chemicals were of the highest grade available from Baker Chemical (Phillipsburg, NJ). All buffers that came in contact with PrMC or endothelium were purchased commercially and all subsequent manipulations of cells were performed in sterile, disposable plasticware to minimize endotoxin contamination.
Monoclonal antibodies
All monoclonal antibodies (mAbs) were used as purified IgG at saturating concentrations as assessed by indirect immunofluorescence and flow cytometry. Murine mAbs directed to ICAM-1 (Hu5/3, IgG1, function-blocking), VCAM-1 (E1/6, function-blocking; Hu8/4, nonblocking, both IgG1), or E-selectin (7A9, IgG1, function-blocking) were used at 20 μg/mL.32Function-blocking mAbs TS1/18 (IgG1) or HP2/1 (IgG1) recognize human CD11/CD18 (common β2-integrin) and VLA-4 (α4-integrin) respectively, and were used at 20 μg/mL.32 KPL-1, which recognizes an epitope within the N-terminal tyrosine-sulfated motif of PSGL-1,33 was purchased from Pharmingen (San Diego, CA) and used at 10 μg/mL for all studies.32 Other mAbs used in flow cytometry have been described previously: c-kit (Biosource, Camarillo, CA, clone K69), CD13 (Pharmingen), CD14 (Pharmingen), and L-selectin (Lam1-14).32
Cell culture
Cord blood was obtained from human placentas after routine Caesarian section in accordance with established institutional guidelines. PrMCs were derived by the culture of the mononuclear cell fraction as previously described.29 Briefly, heparin-treated cord blood was mixed with a 4.5% dextran solution to sediment most erythrocytes and the resulting leukocyte-rich plasma was layered on Ficoll-Hypaque (Amersham-Pharmacia, Uppsala, Sweden) to isolate the mononuclear cell fraction. The mononuclear cells were suspended at a concentration of 106 cells/mL in RPMI 1640 medium (Gibco BRL, Gaithersburg, MD) containing 10% FBS, 2 mM L-glutamine, 0.1 mM nonessential amino acids, 100 U/mL penicillin, 100 mg/mL streptomycin, 2 μg/mL gentamycin (all from Sigma, St Louis, MO), and 0.2 M 2-mercaptoethanol (Gibco BRL). The medium was supplemented with 100 ng/mL SCF, 50 ng/mL IL-6, and 10 ng/mL IL-10. The nonadherent cells were transferred every week for up to 9 weeks into culture medium containing fresh cytokines. Cytospin preparations were examined weekly from samples of 2 × 104 cells utilizing a cytocentrifuge (Shandon, Pittsburgh, PA) and were stained with toluidine blue to assess metachromasia. Cells were harvested for study at 4 weeks due to their expression of chloroacetate esterase, c-kit, CD13, and lack of proliferative responses to IL-2, granulocyte colony-stimulating factor, and macrophage colony-stimulating factor at this time point, as previously reported.29 For experiments involving assessment of integrin and selectin functions, cultured crude PrMCs were depleted of CD14+ cells (contaminating monocytes and CD14+mature MCs) with CD14 magnetic cell separation microbeads used according to the manufacturer's instructions (Miltenyi Biotech, Sunnyvale, CA). Typically, 50% to 70% of the total cells were recovered, with an average yield of 2 × 107 to 4 × 107 CD14− PrMCs per 108 starting cells. Flow cytometry confirmed that more than 99% of the negative fraction lacked the CD14 marker after the column purification. The CD14− cells were stained for chloroacetate esterase activity and for metachromasia with toluidine blue as previously described29 prior to the adhesion studies.
Culture of human umbilical vein endothelial cells
Human umbilical vein endothelial cells (HUVECs) were isolated from 2 to 5 umbilical cord veins, pooled, and established as primary cultures in M199 containing 20% FBS.30 Primary HUVEC cultures were passed serially (1:3 split ratio) and maintained in M199 containing 10% FBS, endothelial cell growth factor (50 μg/mL; Biomedical Technologies, Stoughton, MA), porcine intestinal heparin (100 μg/mL, Sigma) and antibiotics. For use in the flow apparatus, HUVECs (passage 1) were plated at 80% confluence on 25 mm circular glass coverslips (no. 1 thickness; Carolina Biological Supply, Burlington, NC) previously precoated overnight with human fibronectin (2 μg/cm2). HUVECs were allowed to reach confluence and were used in experiments within 24 to 48 hours.
In vitro flow model
PrMC interactions with endothelial-cell or purified adhesion molecules under defined laminar flow were studied in a parallel plate flow chamber as previously described.30 Briefly, recombinant adhesion molecule proteins were immobilized to coverslips exactly as detailed previously.34 Confluent HUVEC monolayers were treated for 18 to 24 hours with HUVEC culture media alone or media containing 25 ng/mL TNF-α or IL-4 (25 ng/mL). PrMCs were suspended to 0.5 × 106 cells/mL in DPBS containing 0.2% (vol/vol) human serum albumin (HSA), 0.75 mM Ca++ and Mg2+, pH 7.4 and incubated for 15 minutes at room temperature (RT) with various mAbs. HUVEC monolayers were also incubated with appropriate test or control mAbs for 30 minutes at 37°C and then placed in the flow chamber. PrMCs were drawn through the chamber at a constant rate of 0.5 mL/min (estimated shear stress, 1.0 dynes/cm2) unless otherwise noted in the text. Leukocyte adhesion and transmigration were determined after 6 minutes of perfusion by analysis of 4 to 6 high power (×40 for adhesion and ×60 for transmigration) fields from videotape as detailed previously.30
Flow cytometry
PrMCs were preincubated with 0.1% human serum to block FcR and then incubated with primary mAbs for 30 minutes on ice, washed twice with RPMI-5% FBS, and then the primary mAb was detected with a fluorescein isothiocyanate (FITC)–labeled secondary goat F(ab′)2 antimouse mAb (Caltag Laboratories, Burlingame, CA).30 The stained cells were washed twice and fixed in 1% formaldehyde-PBS. A nonbinding primary control was used as a control. Fluorescence of 104 cells was detected using a Becton Dickinson FACS Calibur flow cytometer (San Jose, CA).
Statistical analyses
All results are expressed as the mean ± SD. Statistical analyses by unpaired t test were performed using Microsoft Excel 5.0 (Microsoft, Richmond, WA) and were considered statistically significant at P ≤ .05.
Results
Characteristics of PrMCs
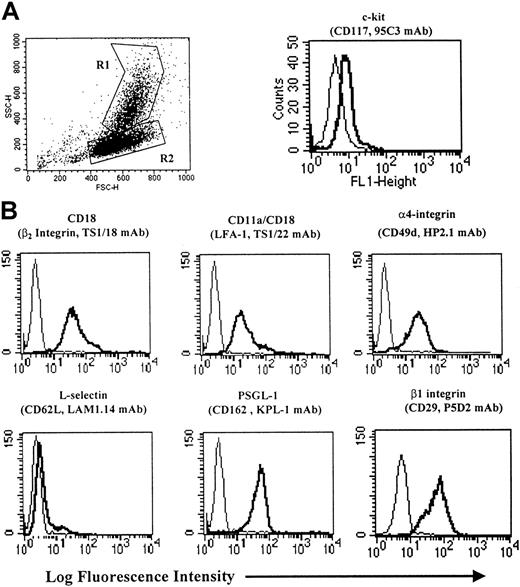
At 4 weeks of culture, typically between 20% to 40% of cells contained the metachromatic secretory granules characteristic of mature MCs. When analyzed by cytofluorographic indices of side angle light scatter (SSC), 2 populations of cells could be identified in most experiments: a group of relatively high SSC, designated R1 in Figure1A, and a group of lower SSC, designated R2 as reported previously.29 From our previous studies, the cell population exhibiting low SSC is predominantly CD14− PrMC. The 2 populations shown in Figure 1A could be separated from one another using the magnetic cell separation (MACS) CD14 Ag immunodepletion column as detailed in “Materials and methods.” The CD14− fraction (R2 in Figure 1A) contained few residual mature MCs (<5%) as determined by toluidine blue staining, but virtually all were positive for chloroacetate esterase staining (data not shown) and expressed low levels of c-kit. The remaining experiments were all performed using CD14-depleted PrMCs because of their relatively uniform phenotype from donor to donor. PrMCs expressed the CD18 (β2-integrin) subunit and CD11a/CD18 comprised most of the CD18 expression (Figure1B), whereas CD11b/CD18 was expressed at lower levels (data not shown). Both α4- and β1-integrins were highly expressed on PrMCs. L-selectin was not present on PrMCs, whereas PSGL-1 expression was robust.
Cytofluorographic phenotype of PrMCs.
(A) Flow cytometric analysis of in vitro–derived PrMCs and MCs was carried out as previously detailed24 to assess FSC and SSC as well as surface expression of c-kit. By light scatter, the PrMCs are identified as R2 and MCs as R1. The heavy line in the c-kit histogram identifies the low SSC population (PrMCs) and the thin line identifies the IgG control mAb. (B) Surface expression of various Ag on column-purified CD14− PrMCs. The mAbs used are identified in “Materials and methods.” The depicted results are representative of the 2 experiments performed.
Cytofluorographic phenotype of PrMCs.
(A) Flow cytometric analysis of in vitro–derived PrMCs and MCs was carried out as previously detailed24 to assess FSC and SSC as well as surface expression of c-kit. By light scatter, the PrMCs are identified as R2 and MCs as R1. The heavy line in the c-kit histogram identifies the low SSC population (PrMCs) and the thin line identifies the IgG control mAb. (B) Surface expression of various Ag on column-purified CD14− PrMCs. The mAbs used are identified in “Materials and methods.” The depicted results are representative of the 2 experiments performed.
PrMCs interact with E- and P-selectins and VCAM-1 under flow
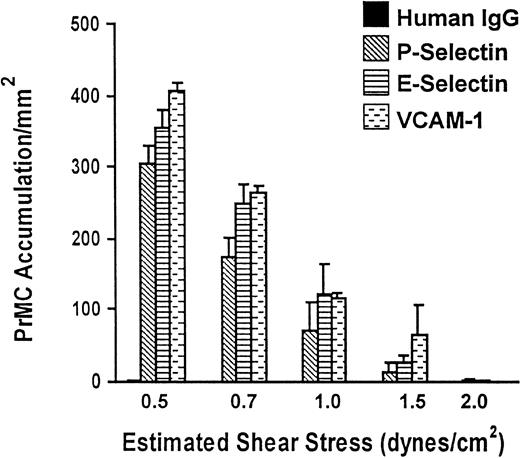
To determine whether PrMCs interact with P-selectin, E-selectin, or VCAM-1, saturating concentrations of these molecules were immobilized to glass coverslips and PrMCs were drawn through the flow chamber under various levels of shear flow stress at 37°C. PrMCs adhered to each molecule across a range of defined fluid shear stress (Figure 2), albeit at a lower level of fluid shear stress than we have observed for human peripheral blood neutrophils or monocytes.30 As expected, no adhesion occurred to control human IgG. The subsequent experiments were carried out at 0.7 dynes/cm2 to 1.0 dynes/cm2.
PrMC adhesion to immobilized P-selectin, E-selectin, and VCAM-1 under defined flow conditions.
Coverslips containing human recombinant E-selectin, P-selectin, or VCAM-1 at saturating concentrations were prepared and PrMCs (5 × 105/mL in perfusion buffer) were drawn through the flow chamber at various flow rates (resulting in estimated fluid shear stresses) for 3 minutes, as detailed in “Materials and methods.” Studies were initiated at 2.0 dynes/cm2 and subsequently the flow rate reduced to 1.5 dynes/cm2, 1.0 dynes/cm2, 0.7 dynes/cm2, and finally to 0.5 dynes/cm2 every 3 minutes. PrMC adhesion was determined at the end of each flow rate and reflects accumulation of adherent and rolling over time during the range of shear stress examined. The data represent mean ± SD from 2 separate experiments.
PrMC adhesion to immobilized P-selectin, E-selectin, and VCAM-1 under defined flow conditions.
Coverslips containing human recombinant E-selectin, P-selectin, or VCAM-1 at saturating concentrations were prepared and PrMCs (5 × 105/mL in perfusion buffer) were drawn through the flow chamber at various flow rates (resulting in estimated fluid shear stresses) for 3 minutes, as detailed in “Materials and methods.” Studies were initiated at 2.0 dynes/cm2 and subsequently the flow rate reduced to 1.5 dynes/cm2, 1.0 dynes/cm2, 0.7 dynes/cm2, and finally to 0.5 dynes/cm2 every 3 minutes. PrMC adhesion was determined at the end of each flow rate and reflects accumulation of adherent and rolling over time during the range of shear stress examined. The data represent mean ± SD from 2 separate experiments.
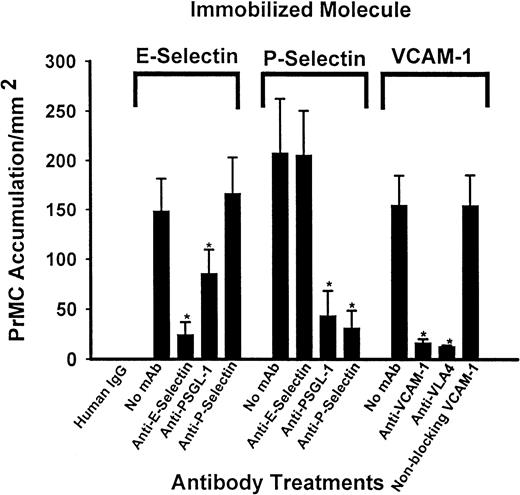
The specificity of PrMC adhesion to these molecules was assessed using blocking and control nonblocking murine mAbs (Figure3). Adhesion of PrMCs to E-selectin was significantly reduced (85%) by the anti–E-selectin 7A9 mAb and was also blocked (46%) in the presence of the anti–PSGL-1 Ab (KPL-1), but was not affected by the function-blocking mAb to P-selectin (HPDG2/3). Adhesion to P-selectin was blocked by over 80% in the presence of either anti–P-selectin or anti–PSGL-1 mAb (P < .01 for each, n = 3), but was not affected by anti–E-selectin mAb. Blockade of adhesion to VCAM-1 was nearly complete in the presence of either anti–VCAM-1 or anti–α4-integrin mAb, but not affected by a nonblocking anti–VCAM-1 mAb, Hu8/4. Anti-α4-integrin mAb did not interfere with adhesion to either E-selectin or P-selectin, whereas anti–PSGL-1 mAb did not affect binding of PrMCs to VCAM-1 under flow (data not shown), thus ruling out nonspecific effects of mAbs. Thus, PrMCs use α4-integrins exclusively for adhesion to VCAM-1, and use PSGL-1 for adhesion to both P- and E-selectins, although additional receptors are likely involved in the case of adhesion to E-selectin.
mAb inhibition of PrMC adhesion to immobilized adhesion molecules.
PrMCs (5 × 105/mL) were preincubated for 15 minutes at RT with blocking mAbs to α4-integrin (HP2/1) or PSGL-1 (KLP-1) or media alone and drawn across coverslips containing saturating concentrations of immobilized P-selectin, E-selectin, or VCAM-1 that had been incubated with media containing saturating concentrations of appropriate blocking or control mAb, or media alone. PrMC adhesion was assessed as described in “Materials and methods.” Data are mean ± SD from 3 separate experiments. The asterisk indicates a value statistically significant from media alone for each molecule tested (P < .05 unpaired ttest).
mAb inhibition of PrMC adhesion to immobilized adhesion molecules.
PrMCs (5 × 105/mL) were preincubated for 15 minutes at RT with blocking mAbs to α4-integrin (HP2/1) or PSGL-1 (KLP-1) or media alone and drawn across coverslips containing saturating concentrations of immobilized P-selectin, E-selectin, or VCAM-1 that had been incubated with media containing saturating concentrations of appropriate blocking or control mAb, or media alone. PrMC adhesion was assessed as described in “Materials and methods.” Data are mean ± SD from 3 separate experiments. The asterisk indicates a value statistically significant from media alone for each molecule tested (P < .05 unpaired ttest).
PrMC interactions with cytokine-activated vascular endothelium under flow
PrMC interactions with 24-hour TNF-α– or IL-4–activated HUVECs were examined in detail under various levels of defined laminar flow. Activation for 24 hours was used to mimic more chronically activated endothelium. PrMCs were perfused initially at 2.0 dynes/cm2 for 3 minutes and then the shear stress was reduced at 3-minute intervals. Adhesive interactions between PrMCs and TNF-α–activated HUVEC monolayers began at 1.5 dynes/cm2, with most accumulation occurring below 1.5 dynes/cm2 (Figure 4A). PrMCs were considered adherent after 20 seconds of stable contact with the monolayer and rolling cells were considered “adherent” for calculation of cell adhesion. Adhesion of PrMCs to IL-4–activated endothelium occured at a lower level of flow and the total accumulation was significantly lower as compared to TNF-α–activated endothelium. In contrast, control media–treated HUVEC monolayers did not support adhesion or rolling under flow. Further examination of experiments performed at 1.5 dynes/cm2 revealed that freely flowing PrMCs abruptly halted on TNF-α–activated endothelium and that of the total number of PrMCs in contact, 45% were rolling, either transiently or continuously, downstream on the apical endothelial cell surface (Figure 4B). Similar results were observed for PrMCs on IL-4–activated endothelial cell monolayers, although the total number of rolling cells was much lower. Stably arrested PrMCs did not form lines behind one another (not shown) on either of the activated endothelial monolayers, indicating secondary adhesions did not occur. By 8 to 10 minutes of assay, however, few if any PrMCs had transmigrated across either IL-4– or TNF-α–activated HUVEC monolayers as assessed using the fine focus and a ×60 phase contrast objective, which differs from the robust transmigration we had observed previously in this system with neutrophils or monocytes.30 32
PrMC adhesion to endothelial cell monolayers under defined flow conditions.
(A) Confluent endothelial cell monolayers treated with TNF-α or IL-4 for 24 hours were inserted into the flow chamber and PrMCs were drawn through the channel at various levels of flow as detailed in Figure 2. Studies were initiated at 2.0 dynes/cm2 and subsequently the flow rate reduced to 1.5 dynes/cm2, 1.0 dynes/cm2, 0.7 dynes/cm2, and finally to 0.5 dynes/cm2 every 3 minutes. PrMC adhesion was determined at the end of each flow rate and reflects accumulation of adherent and rolling over time during the range of shear stress examined. Data are mean ± SD, n = 2 experiments for each cytokine. (B) Cell rolling velocities were measured over a time period of 6 to 9 seconds in 2 different experiments using a customized image analysis program (Ed Marcus Laboratories, Brighton, MA54). PrMCs rolling at velocities of 0 μm/s to 2.0 μm/s were considered as adherent (black bar), cells rolling at velocities between 2 μm/s and 20 μm/s were considered rolling (light gray bars), and cells interacting transiently during the time of observation were considered transient rolling (dark gray). The number of cells analyzed for TNF-α and IL-4 were 127 and 32, respectively, from n = 2 separate experiments. (C) mAb inhibition of PrMC adhesion to 24-hour TNF-α–activated endothelial cell monolayers under flow. Confluent TNF-α–activated or control HUVEC monolayers were inserted into the flow apparatus for adhesion assays as detailed in “Materials and methods” and Figure 3. PrMCs were treated with various test or control mAbs on ice for 15 minutes, diluted 1:10 in perfusion buffer, and drawn through the chamber at 0.7 dynes/cm2. Adhesion was determined after 6 minutes of flow as detailed in “Materials and methods.” Data are mean ± SD for 4 paired experiments.
PrMC adhesion to endothelial cell monolayers under defined flow conditions.
(A) Confluent endothelial cell monolayers treated with TNF-α or IL-4 for 24 hours were inserted into the flow chamber and PrMCs were drawn through the channel at various levels of flow as detailed in Figure 2. Studies were initiated at 2.0 dynes/cm2 and subsequently the flow rate reduced to 1.5 dynes/cm2, 1.0 dynes/cm2, 0.7 dynes/cm2, and finally to 0.5 dynes/cm2 every 3 minutes. PrMC adhesion was determined at the end of each flow rate and reflects accumulation of adherent and rolling over time during the range of shear stress examined. Data are mean ± SD, n = 2 experiments for each cytokine. (B) Cell rolling velocities were measured over a time period of 6 to 9 seconds in 2 different experiments using a customized image analysis program (Ed Marcus Laboratories, Brighton, MA54). PrMCs rolling at velocities of 0 μm/s to 2.0 μm/s were considered as adherent (black bar), cells rolling at velocities between 2 μm/s and 20 μm/s were considered rolling (light gray bars), and cells interacting transiently during the time of observation were considered transient rolling (dark gray). The number of cells analyzed for TNF-α and IL-4 were 127 and 32, respectively, from n = 2 separate experiments. (C) mAb inhibition of PrMC adhesion to 24-hour TNF-α–activated endothelial cell monolayers under flow. Confluent TNF-α–activated or control HUVEC monolayers were inserted into the flow apparatus for adhesion assays as detailed in “Materials and methods” and Figure 3. PrMCs were treated with various test or control mAbs on ice for 15 minutes, diluted 1:10 in perfusion buffer, and drawn through the chamber at 0.7 dynes/cm2. Adhesion was determined after 6 minutes of flow as detailed in “Materials and methods.” Data are mean ± SD for 4 paired experiments.
To determine the adhesion mechanisms used by PrMCs for adhesion to TNF-α–activated HUVECs, blocking studies were carried out in the presence of combinations of antibodies at a shear stress of 0.7 dynes/cm2. This level of shear stress consistently provided similar levels of adhesion from donor to donor and gave more adhesion events to analyze as compared with the higher rate of 1.5 dynes/cm2 (see Figure 3). In all 3 experiments performed, PrMCs adhered to the TNF-α–activated HUVECs, with some interdonor variability (Figure 4C). Individual blocking Abs against VCAM-1 (58% inhibition, P < .05), E-selectin (53% inhibition, P < .05), α4-integrin (45% inhibition,P < .05), or PSGL-1 (56% inhibition,P < .05) each significantly reduced total accumulation. The combination of anti–α4-integrin plus anti–PSGL-1 (72% inhibition) and anti–E-selectin plus anti–VCAM-1 (80% inhibition) produced further blocking compared with the individual antibodies alone. The anti–VCAM-1 mAb (Hu8/4) failed to interfere with adhesion (4.8% inhibition, not significant), which is consistent with its non–function-blocking properties.32 54 Analysis of mAb inhibition of α4-integrin or PSGL-1 on PrMC rolling and arrest revealed that inhibition of PSGL-1 prevented essentially all rolling interactions of the cells that came in contact with the endothelium, with the caveat that overall accumulation decreased by 56% (Figure4C). PrMCs bound and arrested with little or no detectable rolling with anti–PSGL-1 mAb. mAb inhibition of α4-integrin also decreased accumulation (Figure 4C) and caused an elevation in rolling velocities (media treatment, 11.3 ± 7.7 μm/s [n = 30 cells examined] vs anti–α4-integrin mAb, 36.9 ± 30.6 μm/s [n = 54 cells],P ≤ .05). The blockade caused cells to transiently attach, roll a short distance, release to shear flow, and then repeat this sequence as the cells translated across the field of view. The net effect was a 3-fold increase in PrMC rolling velocities. We conclude that blockade of either α4-integrin or PSGL-1 reduces accumulation by almost 50% and when combined, their effect is additive (Figure 4C), and that α4-integrin is involved in stable arrest whereas PSGL-1 contributed more to initial attachment and rolling behavior. We note that the overall rolling fraction (rolling and transient rolling) for PrMC media control was less than or equal to 10% in both studies analyzed, which is less than observed in earlier studies reported above in Figure 4B.
The mechanisms underlying adhesion to IL-4–activated endothelial monolayers were totally dependent on the α4-integrin–VCAM-1 (> 90% inhibition with mAb to α4-integrin or VCAM-1). Blocking studies with mAb to CD18 or ICAM-1, alone or in combination, had no inhibitory effect on adhesion (data not shown).
Discussion
This study indicates that human PrMCs use VCAM-1 and E-selectin as the predominant ligands involved in their interactions with activated endothelial cells in an experimental model. PrMCs in vivo originate in bone marrow14 and exist as a constitutive cellular pool in some organs, such as the intestinal mucosa of mice,35 which permits a rapid expansion of gut MC numbers necessary for the elimination of adult worms in helminthic infections.4 Fully differentiated MCs are also found under basal conditions in the perivascular connective tissues of multiple organs, where they serve as sentinels of innate immunity.1-3 Increases in MC numbers are a common feature of asthma, multiple sclerosis, rheumatoid arthritis, pulmonary fibrosis, and other diseases, where their effector properties strongly contribute to disease pathogenesis.6-8,12 36-40 The likelihood that PrMCs respond in vivo to both constitutive and inducible adhesion signals from endothelial cells, the importance of MCs and their homing pathways in a wide array of biologic processes and diseases, and the lack of studies regarding the mechanisms utilized for PrMC homing led us to address these adhesion mechanisms using nontransformed PrMCs derived from human cord blood. Following immunomagnetic enrichment, the PrMCs retained c-kit and CD13, were uncontaminated by CD14+ monocytes, and were nearly uniformly chloroacetate esterase–positive, a marker that is restricted to PrMCs, MCs, and neutrophils. These cultured PrMCs thus provided the opportunity to characterize their adhesion receptor expression and function and potential mechanisms of recruitment using a well-characterized in vitro flow model.
As assessed by cytofluorographic criteria, the PrMCs expressed high levels of α4-integrin and β1-integrin, the 2 components of the VLA-4 heterodimer involved in inflammation-associated trafficking of lymphocytes, monocytes, and eosinophils. Fully differentiated MCs purified from human skin and uterus also express VLA-4.41,42 We also detected β7-integrin on PrMCs, although at lower levels than α4-integrin or β1-integrin. Like β1-integrin, the β7-integrin subunit pairs with α4-integrin, forming a heterodimer that can interact either with VCAM-1 or with MAdCAM on high endothelial venules. The latter interaction supports constitutive lymphocyte recruitment to the intestine and the development of Peyer Patches.43 Importantly, mice lacking β7-integrin have markedly subnormal levels of intestinal mucosal PrMCs,44 which based on antibody blockade use MAdCAM for constitutive intestinal homing. β7-integrin–deficient mice also fail to mount a normal mucosal MC hyperplasia when infected with the helminth Trichinella spiralis, even after adoptive transfer of normal congenic lymphocytes.45 The human PrMCs in our study expressed high levels of PSGL-1 (Figure 1B), which was not detected on mature MCs from lung or uterus or on the transformed human MC line HMC-1.46 Nonetheless, nontransformed immature mouse bone marrow MCs do express functional PSGL-1, and utilize P-selectin for rolling in vitro47 and in vivo.48 The PrMCs generated here also expressed the β2-integrin subunit, αL, and, to a lesser degree, αM. Whereas β2-integrins are critical determinants of homing for most leukocytes, their relevance for MC adhesion is unclear. Mice lacking the αM-integrin subunit had only modest decrements in peritoneal and skin MCs, while intestinal MC numbers were normal.49 MCs purified from human tissues lack β2-integrins, with the exception of uterine MCs, which express αXβ2.42 It is possible that PrMCs in vivo may express homing receptors, such as PSGL-1 and β2-integrin family members that participate in endothelial cell interactions, but that are modified or abolished in vivo with maturation in the target tissues.
The high expression of the VLA-4 subunits and PSGL-1 by PrMCs (Figure1B) suggested that they might interact with VCAM-1 and the selectins, respectively. Immobilized soluble VCAM-1, as well as both recombinant P- and E-selectins, supported the attachment of PrMCs across a range of flow conditions in vitro (Figure 2). We note here that this range of shear stress is lower than that observed previously for adhesion of monocytes or neutrophils, but is similar to that seen for T cells. The adhesion to VCAM-1 was blocked nearly completely by anti–VCAM-1 and anti–α4-integrin Abs, implying complete dependence on the α4β1- and/or α4β7-integrins for this event. Adhesion to P-selectin was essentially all PSGL-1–dependent because blocking mAbs to P-selectin or PSGL-1 had the same effect (> 90% inhibition). In contrast, about 40% of the E-selectin–mediated PrMC adhesion was mediated by PSGL-1 (Figure 3). Because PrMCs lack L-selectin and do not interact with already adherent PrMCs (ie, they lack secondary adhesion events typical of human neutrophils and monocytes),32 the blocking data indicate that PSGL-1 on PrMCs binds both P-selectin and E-selectin. In this context, previous studies showed that mAb KPL-1 recognized the consensus tyrosine sulfation motif of PSGL-1 that was essential for T-cell, monocyte, and neutrophil adhesion to P-selectin and L-selectin, but not to E-selectin.32,33 It is likely that the KPL-1 epitope for PSGL-1 on cultured human PrMCs is slightly different from that of other peripheral blood leukocytes because of its interaction with E-selectin. Further studies are necessary to define the basis for these differences. In addition to PSGL-1, cultured PrMCs express another as-yet-unidentified ligand(s) that mediates the bulk of their adhesion to E-selectin. A previous study reported an unknown 75-kd protein in extracts of mouse bone marrow MCs that bound E-selectin, as well as PSGL-1.47 Interestingly, the bone marrow MCs in that study bound to P-selectin, but not E-selectin, under both static and shear flow conditions, despite the fact that PSGL-1 extracted from these bone marrow MCs avidly bound both selectins ex vivo. P-selectin, but not E-selectin, was shown to be important for adhesion of bone marrow MCs in skin chambers in vivo, although it was not clear whether E-selectin was expressed on endothelium in this context.42 Memory B cells were recently shown to interact with E-selectin through a novel glycoprotein ligand.50 Our study indicates that human PrMCs do interact with both purified and endothelial cell–associated E-selectin, and that the interaction is mainly mediated by a ligand other than PSGL-1. Further, PrMCs in vivo can likely use both P-selectin and E-selectin, depending on the context.
We next assessed the functional significance of the integrins and selectins in the adhesion of PrMCs to cytokine-activated vascular endothelial monolayers. Treatment of HUVECs with IL-4 is an established stimulus for the induction of VCAM-1 expression.51 VCAM-1 is lacking on most endothelial cells in vivo, but is expressed in circumstances of allergen challenge,52 and at early fatty streak in nascent arterial lesions,53 and thus can provide an inducible mechanism for the recruitment of α4-integrin–expressing leukocytes, likely including PrMCs. The importance of VCAM-1/α4-integrin interactions in allergic inflammation is supported by the powerful inhibitory effects of blocking antibodies on allergen-induced pulmonary eosinophilia and bronchial hyperreactivity.25-27 TNF-α stimulation also induces VCAM-1 expression by endothelial cells, and also rapidly up-regulates E-selectin (by de novo synthesis) and ICAM-1, thus simultaneously promoting multiple adhesion pathways.48 The HUVEC monolayers used in these studies expressed significant levels of E-selectin at 24-hour cells and retained 50% of their peak expression of E-selectin determined at 4 hours by flow indirect immunofluorescence cytometry, and also expressed VCAM-1 and ICAM-1 (F.W.L., unpublished data, August 2001). Adhesion of PrMCs to IL-4–treated HUVECs was relatively weak and completely dependent on VCAM-1/α4-integrin interactions. Adhesion to TNF-α–activated HUVEC monolayers was more robust and involved not only VLA-4/VCAM-1, but also E-selectin interacting with PSGL-1 and other as-yet-undefined ligands on PrMCs. The E-selectin–specific Ab 7A9, which did not interfere with binding to P-selectin (Figure 3), inhibited the interaction of PrMCs with HUVECs by 54%. The combination of anti–VCAM-1 and anti–E-selectin Abs was greater that either mAb alone and inhibited more than 80% of the adhesion. The mAb to PSGL-1 blocked adhesion to the same extent as the mAb to E-selectin, which is somewhat unexpected because in other experiments presented in Figure 3, blockade of PSGL-1 blocked only 50% of adhesion to purified E-selectin. One explanation is that PSGL-1 is preferentially used during PrMC interactions with vascular endothelium, which express combinations of adhesion molecules, as compared with immobilized adhesion molecules studied singly and at saturating concentrations in isolation. Interestingly, although TNF-α is known to induce ICAM-1 expression by HUVECs, and despite the expression of β2-integrins (CD11a/CD18 and CD11b/CD18) by PrMCs, the ICAM-1/β2-integrin pathway did not contribute significantly to PrMC adhesion under these circumstances. This may well reflect the avidity state of these integrins in the absence of appropriate endogenous chemoattractants from HUVECs.54 Since TNF-α–primed HUVECs do not express P-selectin, their contribution to PrMC/endothelial cell interactions could not be assessed in this model.
Although prior studies have not addressed requirements for adhesion of nontransformed human PrMCs, studies of adhesion performed using neutrophils and eosinophils reveal differential cell lineage–specific requirements, as well as differences that depend on the nature of the immune response in question. For example, human neutrophils tether preferentially to E-selectin rather than to P-selectin on rabbit mesenteric venular endothelial cells, whereas eosinophils did not adhere except at subphysiologic levels of shear stress.55In another study, human peripheral blood eosinophils adhered to P-selectin, but not efficiently to E-selectin, under shear flow conditions.56 Antibodies against E-selectin or PSGL-1 abolished primary tethers to TNF-α–activated HUVECs in this study. An in vivo study in mouse skin revealed that acute recruitment of eosinophils in response to injection of chemoattractants mainly involves P-selectin, while both P-selectin and E-selectin are involved in eosinophil recruitment in the late phase of an active cutaneous anaphylactic reaction. Furthermore, α4-integrins, but not selectins, are dominant in delayed-type hypersensitivity reactions.57Our study indicates that PrMCs can utilize either P-selectin or E-selectin, that the former is largely PSGL-1–dependent, and the relative importance of each selectin for PrMC trafficking may depend on the tissue vascular bed and stimulus. Indeed, we speculate that P-selectin may provide a ligand for constitutive PrMC/endothelial cell interactions in providing baseline PrMC populations, whereas E-selectin and VCAM-1 may provide inducible adhesion pathways under conditions of inflammation. Both pathways carry implications for the role of MCs in host defense and disease. Constitutive tissue MCs are critical for initiating TNF-α–mediated neutrophil recruitment in response to bacterial infection, using the innate recognition receptors CD48, toll-like receptor 4, and complement receptors.58-60Constitutive intestinal PrMCs give rise to mucosal MCs that are essential for eliminating helminthic parasites.4,44Moreover, MC-deficient mouse strains require adoptive transfer of PrMCs in order to develop characteristic tissue pathology in antigen-induced models of asthma61 and multiple sclerosis,39and MCs are implicated in the pathogenesis of the corresponding human diseases.12,22,38 Combined with our prior observations that PrMCs express 4 functional chemokine receptors (CXCR2, CCR3, CXCR4, and CCR5),25 the present study clearly suggests that PrMCs are endowed with homing determinants that would permit increased recruitment for subsequent MC hyperplasia in a wide array of inflammatory disease states, as well as those that ensure constitutive pools of PrMCs in some tissues.
Supported by National Institutes of Health grants HL-36028, HL-53993, HL-56985, and HL-65090 (F.W.L.), and AI-01305, AI-31599, AI-22531, and HL-36110 (J.A.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joshua A. Boyce, Division of Rheumatology, Immunology, and Allergy, Brigham and Women's Hospital, Smith Research Building, Room 616C, 1 Jimmy Fund Way, Boston, MA 02115.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal